
Efficacy and outcome analysis: monotherapy and combination therapy of S-1 for patients with advanced non-small cell lung cancer
Highlight box
Key findings
• Both S-1 monotherapy and combination therapy demonstrated favorable efficacy and tolerability as second-line or subsequent treatments for patients with advanced non-small cell lung cancer (NSCLC). The objective response rate was 28.8%, with a median progression-free survival of 2.7 months and a median overall survival of 9.5 months.
What is known and what is new?
• The efficacy of second-line or later-line treatments for advanced NSCLC remains suboptimal. Both S-1 monotherapy and combination therapy have shown promising anti-tumor activity in patients at advanced stages of disease progression, with mild adverse effects.
• In our study, we first assessed the efficacy and safety of combining S-1 with other therapies in advanced NSCLC patients. In patients with late-stage NSCLC lacking driver gene mutations, compromised physical capacity, multiple comorbidities, and elevated Eastern Cooperative Oncology Group performance status scores, which have led to limited therapeutic alternatives, S-1 demonstrated potential as a viable option. Both S-1 monotherapy and combination therapy exhibited encouraging efficacy and were well-tolerated as second-line or subsequent-line interventions for advanced NSCLC patients.
What is the implication, and what should change now?
• Future research should focus on conducting large-scale, multicenter studies to further investigate the potential benefits of S-1 therapy. Additionally, new investigations could explore the efficacy of combining S-1 with immunotherapy. The role of S-1 as a therapeutic option for advanced NSCLC warrants further elucidation.
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide. There were an estimated 2.3 million new cases and 1.2 million deaths in the United States in 2024, representing approximately 11.7% of cancers diagnosed and 20.4% of deaths (1). Non-small cell lung cancer (NSCLC) constitutes approximately 80% of all lung cancer cases in humans and is frequently detected at an advanced stage, resulting in limited treatment options (2). Although targeted and immunotherapies have advanced in recent years, advanced NSCLC still has a poor prognosis. Patients whose disease has advanced following initial treatment face a scarcity of alternative medications. For individuals with NSCLC lacking oncogenic driver mutations who have experienced progression after initial therapy, the standard treatment options include immune checkpoint inhibitor (ICI) or gemcitabine and docetaxel monotherapy or docetaxel plus ramucirumab (3), with a median progression-free survival (PFS) of only 3 months (4,5). However, not all patients can tolerate subsequent chemotherapy regimens, such as patients with a performance status (PS) ≥2. Moreover, significant adverse effects such as bone marrow suppression and fatigue are observed in patients treated with gemcitabine or docetaxel. Additionally, the incidence of grade ≥3 pneumonitis is found to be elevated in relation to the combined treatment of docetaxel and ramucirumab. In the context of the ALTER-0303 trial, anlotinib demonstrated enhanced efficacy as a third-line or subsequent therapy for patients with advanced NSCLC (6). Nevertheless, there was a significant increase in grade 3 or higher adverse reactions with anlotinib (61.9% vs. 37.1%). The therapeutic effects in the second-line or later-line treatment remained unsatisfactory, therefore highly efficient and low-toxic therapeutic strategies are urgently required.
S-1, an oral prodrug of fluorouracil, is administered in combination with tegafur, 5-chloro-2,4-dihydroxypyridine (CDHP), and potassium oxonate at specific molar ratios (1:0.4:1) (7). CDHP increases 5-fluorouracil (5-FU) levels in tumor tissues and plasma by generating 5-FU in the blood. By inhibiting the phosphorylation of 5-FU in the gastrointestinal tract, oxonate reduces the gastrointestinal toxicity of 5-FU. The comparative effectiveness of S-1 monotherapy vs. a docetaxel regimen was assessed in two randomized phase III trials to establish non-inferiority (8,9). Neither PFS nor overall survival (OS) differed significantly between S-1 oral and docetaxel regimens, indicating non-inferiority of S-1. It was found that compared with docetaxel, S-1 had similar anti-tumor activity but milder hematologic adverse effects. Furthermore, S-1 combination therapy exhibited favorable anti-tumor activity in patients with advanced NSCLC at later stages of disease progression (10-12). Given the limited treatment options available for late-stage NSCLC patients with compromised physical functions, multiple underlying diseases, and high PS scores, the potential of S-1 as a breakthrough therapy is significant (13). Furthermore, NSCLC treatment with S-1 was approved in Japan in 2004.
This study sought to investigate the efficacy and safety of S-1 combination therapy in advanced NSCLC patients as a second-line or later treatment option, as well as comparing its effectiveness with S-1 monotherapy. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-940/rc).
Methods
Patients
All patient-related procedures adhered to the principles outlined in the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Zhejiang Cancer Hospital (No. IRB-2023-615). Individual consent for this retrospective analysis was waived. A retrospective analysis was conducted on the medical records of patients diagnosed with advanced NSCLC at tumor-node-metastasis (TNM) classification stage IIIB/IV, who received S-1 as second-line or subsequent therapy at Zhejiang Cancer Hospital in Hangzhou, China, between January 2018 and August 2023. Enrollment criteria in this study were as follows: (I) patients with histologically or cytologically confirmed NSCLC; (II) Eastern Cooperative Oncology Group (ECOG) PS scores 0–2; (III) following first-line chemotherapy, the patient exhibited recurrent tumor growth and demonstrated resistance to the first-line therapeutic approach; (IV) patients with unresectable stage IIIB/IV NSCLC; and (V) patients not harboring epidermal growth factor receptors (EGFRs) mutation or anaplastic lymphoma kinases (ALKs) rearranged. Exclusion criteria in this study were as follows: (I) patients with other types of malignancy; and (II) patients lacking response evaluation due to insufficient follow-up of less than 4 weeks or loss to follow-up. Patients’ data were collected including age, gender, histological subtype, clinical TNM stage (8th edition of the American Joint Committee on Cancer Tumor-Node-Metastasis staging system) (14), smoking status, ECOG PS scores, liver metastases, brain metastases, pleural effusion, programmed cell death 1-ligand 1 (PD-L1) expression, date of treatment start, treatment regimens, lines of treatment regimens, best of efficacy, previous radiotherapy, previous surgical resection, date of disease progression, date of death, adverse events (AEs).
Treatments
Based on their treatment regimen, patients were categorized into groups receiving either S-1 monotherapy or S-1 combination therapy. S-1 were selected according to body surface area (BSA): BSA <1.25 m2, 40 mg twice daily (b.i.d.); BSA ≥1.25 m2, but <1.5 m2, 50 mg b.i.d.; and BSA ≥1.5 m2, 60 mg b.i.d. S-1 was administered orally at a daily dose in two divided doses after a meal for a duration of 4 weeks, followed by a drug-free interval of 2 weeks (one cycle). The chemotherapy regimens involved gemcitabine, which was administered at a dose of 1,250 mg/m2 on days 1 and 8 every 3 weeks. ICIs involved camrelizumab, tislelizumab, sintilimab, nivolumab, toripalimab, sugemalimab. Camrelizumab, tislelizumab, sintilimab at a dose of 200 mg by intravenous (iv) infusion every 3 weeks. Nivolumab at a dose of 360 mg via iv infusion every 3 weeks. Toripalimab at a dose of 240 mg by iv infusion every 3 weeks. Sugemalimab at a dose of 1,200 mg by iv infusion every 3 weeks. Antiangiogenic drugs included bevacizumab, anlotinib, and endostar. Bevacizumab was administered at a dose of 15 mg/kg by iv infusion every 3 weeks. Anlotinib was administered at varying doses (12 mg/10 mg/8 mg) via iv infusion over 2 weeks, with a 1-week break, dependent on patient tolerance levels. Endostar was administered at a dose of 7.5 mg/m2 by iv infusion daily for 2 weeks and stopped for 1 week.
Assessment of response and toxicity
Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 were used to evaluate tumor responses (15). After starting the S-1 therapy, a routine evaluation was conducted every 6–8 weeks. OS was the primary endpoint, and it was measured from the date of the initial S-1 treatment to the date of death. The secondary endpoints were PFS, objective response rate (ORR), disease control rate (DCR), and AEs. PFS was defined as the period from the initiation of S-1 treatment to disease progression or death. ORR refers to the proportion of patients who had a complete response (CR) or partial response (PR), and the DCR refers to that of patients who had a CR or PR or stable disease (SD).
AEs were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 developed by the National Cancer Institute.
Statistical analysis
All statistical analyses were performed using GraphPad Prism software version 9.5 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS statistical software version 25.0 (IBM Corp., Armonk, NY, USA). Baseline characteristics were compared in the two groups using Chi-squared and Fisher’s exact tests. OS and PFS were assessed using Kaplan-Meier survival curves, with differences between groups detected using log-rank tests. A multivariate logistic regression analysis was conducted to identify independent prognostic factors associated with OS, focusing on variables with P values below 0.2 in the univariate regression analysis. Two-sided P values <0.05 were considered statistically significant.
Results
Patient characteristics
Between January 2018 and August 2023, 396 patients received S-1 in our cancer center. There were 237 patients excluded for other tumor types, and 36 patients were excluded for ECOG PS 3–4. There were 123 patients with stage IIIB, IV, or recurrent NSCLC treated with S-1. Fourteen patients who received S-1 as first-line therapy, 42 patients without response evaluation, and 10 patients with positive EGFR mutations or ALK rearranged were excluded from the study. The follow-up rate was 90.4%, and five patients were lost to follow-up (Figure 1).

As a result, a total of 52 patients who received S-1 as a second-line or later therapy were included in the analysis. The monotherapy group consisted of 13 patients, while the combination group consisted of 39 patients. A total of 75.0% of patients were in the combination group. One (1.9%) patient received S-1 plus chemotherapy, 28 (53.8%) patients received S-1 plus immunotherapy, 10 (19.2%) patients received S-1 plus antiangiogenic therapy. As shown in Table 1, most variables, except histologic features, were similar between the two groups at baseline. The rate of adenocarcinoma patients was 23.1% in the combination group and 53.8% in the monotherapy therapy group. Among the two groups, the mean age was 64 years, ranging from 58 to 70 years. A total of 35 (67.3%) patients exhibited a PS of 1, while 17 patients (32.7%) displayed a PS of 2. Nine (17.3%) patients were stage IIIB, while 43 (82.7%) patients were stage IV. Fourteen (26.9%) patients were never smokers. Thirty-six (69.2%) patients had squamous cell carcinoma on histopathology of their biopsy specimen, while 16 (30.7%) patients had adenocarcinoma. Twenty-five (48.1%) patients had received second-line therapy, and 27 (51.9%) patients had received third-line or later therapy. Eight (15.4%) patients had developed liver metastases and 4 (7.7%) patients had developed brain metastases. A total of 17 (32.7%) patients had pleural effusion. Among the 52 patients, 6 (11.5%) patients had positive PD-L1 expression. Seven (13.5%) patients received previous radiotherapy, while 12 (23.1%) patients received previous surgical resection. Twenty-two (42.3%) patients received albumin paclitaxel + carboplatin/cisplatin/nedaplatin ± immunotherapy as previous first-line chemoimmunotherapy regimen, 20 (38.5%) patients received pemetrexed + carboplatin/cisplatin/nedaplatin ± immunotherapy/bevacizumab as previous first-line chemoimmunotherapy regimen, 8 (15.4%) patients received gemcitabine + carboplatin/cisplatin/nedaplatin ± immunotherapy as previous first-line chemoimmunotherapy regimen, while 2 (3.8%) patients received immunotherapy monotherapy as previous first-line regimen.
Table 1
| Items | Total (n=52) | S-1 monotherapy (n=13) | S-1 combination therapy (n=39) | P value |
|---|---|---|---|---|
| Age (years) | 0.86 | |||
| Median [range] | 64 [58, 70] | 62 [58, 68] | 64 [58, 70] | |
| ≥70 | 13 (25.0) | 3 (23.1) | 10 (25.6) | |
| Sex | 0.42 | |||
| Male | 47 (90.4) | 11 (84.6) | 36 (92.3) | |
| Female | 5 (9.6) | 2 (15.4) | 3 (7.7) | |
| TNM stage | 0.83 | |||
| IIIB | 9 (17.3) | 3 (5.8) | 6 (15.4) | |
| IV | 43 (82.7) | 10 (19.2) | 33 (84.6) | |
| Smoking status | 0.72 | |||
| NA | 14 (26.9) | 4 (30.8) | 10 (25.6) | |
| Current/former | 38 (73.1) | 9 (69.2) | 29 (74.4) | |
| ECOG PS score | 0.13 | |||
| 1 | 35 (67.3) | 11 (84.6) | 24 (61.5) | |
| 2 | 17 (32.7) | 2 (15.4) | 15 (38.5) | |
| Histologic features | 0.04 | |||
| Squamous cell carcinoma | 36 (69.2) | 6 (46.2) | 30 (76.9) | |
| Adenocarcinoma | 16 (30.7) | 7 (53.8) | 9 (23.1) | |
| Liver metastases | >0.99 | |||
| No | 44 (84.6) | 11 (84.6) | 33 (84.6) | |
| Yes | 8 (15.4) | 2 (15.4) | 6 (15.4) | |
| Brain metastases | 0.23 | |||
| No | 48 (92.3) | 13 (100.0) | 35 (89.7) | |
| Yes | 4 (7.7) | 0 (0.0) | 4 (10.3) | |
| Pleural effusion | 0.87 | |||
| No | 35 (67.3) | 9 (69.2) | 33 (84.6) | |
| Yes | 17 (32.7) | 4 (30.8) | 6 (15.4) | |
| PD-L1 expression | 0.14 | |||
| Negative/NA | 46 (88.5) | 13 (100.0) | 33 (84.6) | |
| Positive | 6 (11.5) | 0 (0.0) | 6 (15.4) | |
| Previous radiotherapy | 0.82 | |||
| No | 45 (86.5) | 11 (84.6) | 34 (87.2) | |
| Yes | 7 (13.5) | 2 (15.4) | 5 (12.8) | |
| Previous surgical resection | >0.99 | |||
| No | 40 (76.9) | 10 (76.9) | 30 (76.9) | |
| Yes | 12 (23.1) | 3 (23.1) | 9 (23.1) | |
| Previous first-line chemoimmunotherapy regimen | – | |||
| Albumin paclitaxel + carboplatin/cisplatin/nedaplatin ± immunotherapy | 22 (42.3) | – | – | |
| Pemetrexed + carboplatin/cisplatin/ nedaplatin ± immunotherapy/bevacizumab | 20 (38.5) | – | – | |
| Gemcitabine + carboplatin/cisplatin/nedaplatin ± immunotherapy | 8 (15.4) | – | – | |
| Immunotherapy monotherapy | 2 (3.8) | – | – | |
| Treatment regimen | – | |||
| S-1 monotherapy | 13 (25.0) | – | – | |
| S-1 + chemotherapy | 1 (1.9) | – | – | |
| S-1 + immunotherapy | 28 (53.8) | – | – | |
| S-1 + antiangiogenic therapy | 10 (19.2) | – | – | |
| Number of lines | 0.23 | |||
| Median [IQR] | 3 [2, 4] | 4 [2.5, 4] | 2 [2, 3] | |
| Second-line therapy | 25 (48.1) | 3 (23.1) | 22 (56.4) | |
| ≥ Third-line treatment | 27 (51.9) | 10 (76.9) | 17 (43.6) |
Data are presented as n (%). TNM, tumor-node-metastasis; NA, not available; ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death-ligand 1; IQR, interquartile range.
Efficacy
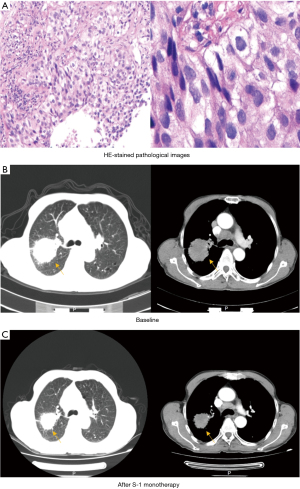
Among all 52 patients, the best response to S-1 treatment was PR in 15 patients (28.8%), SD in 20 patients (38.5%), and progressive disease (PD) in 17 patients (32.7%). The ORR was 28.8%, and the DCR was 67.3% (Table 2). A representative example was presented in Figure 2.
Table 2
| Items | Total (n=52), n (%) |
|---|---|
| PR | 15 (28.8) |
| SD | 20 (38.5) |
| PD | 17 (32.7) |
| ORR | 15 (28.8) |
| DCR | 35 (67.3) |
NSCLC, non-small cell lung cancer; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Survival analysis
The last follow-up date was August 31, 2023. After the follow-up period, 16 patients were still alive, while 36 had passed away. The median follow-up time was 7.4 months (range, 4.8–14.6 months) for all patients and 6.3 months (range, 4.1–13.2 months) for living patients. The median PFS was 2.7 months [95% confidence interval (CI): 2.1–2.8], and the median OS was 9.5 months (95% CI: 6.3–12.6) for the total cohort.
In the subgroup analysis, the median PFS of patients who had received prior radiotherapy (5.5 months; 95% CI: 4.4–6.6) was significantly longer than patients who did not receive prior radiotherapy (2.5 months; 95% CI: 2.1–2.9) (P=0.047); median OS of patients who had received prior radiotherapy (21.0 months; 95% CI: 0–45.9) was significantly longer than patients who did not receive prior radiotherapy (9.5 months; 95% CI: 6.1–12.9) (P=0.01) (Figure 3).

We further analyzed the association between ECOG PS scores and long-term survival in the total cohort. The results showed that patients with ECOG PS score of 1 had better PFS (4.4 months; 95% CI: 2.3–6.6 vs. 1.7 months; 95% CI: 0.9–2.5; P<0.001) and OS (13.5 months; 95% CI: 8.4–18.7 vs. 8.0 months; 95% CI: 2.1–13.8; P=0.01) than patients with ECOG PS score of 2 (Figure 4).

Prognostic factors
A multivariate analysis utilizing the logistic regression model was conducted to identify potential predictive factors of OS, as shown in Table 3. Additionally, univariate logistic regression analysis was performed to assess risk factors, with variables demonstrating a P value of less than 0.2 (ECOG PS scores, brain metastasis, pleural effusion, treatment regimen, number of lines) were included in multivariate regression analysis. S-1 combined therapy was associated with better OS than S-1 monotherapy [odds ratio (OR), 0.03; 95% CI: 0.00–0.64; P=0.03]. While the OS was significantly shorter for those who received S-1 as a later-line treatment than for those who received S-1 as a second-line treatment (OR, 7.61; 95% CI: 1.07–54.02; P=0.042).
Table 3
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (years) | |||||
| <70 | Ref. | ||||
| ≥70 | 0.65 (0.02–20.07) | 0.80 | |||
| Sex | |||||
| Male | Ref. | ||||
| Female | 0.05 (0.00–45.00) | 0.38 | |||
| Smoking status | |||||
| Never | Ref. | ||||
| Current/former | 20.10 (0.13–3,223.61) | 0.25 | |||
| ECOG PS scores | |||||
| 1 | Ref. | ||||
| 2 | 37.05 (0.53–2,581.67) | 0.10 | 3.93 (0.67–22.97) | 0.13 | |
| Histologic features | |||||
| Squamous cell carcinoma | Ref. | ||||
| Adenocarcinoma | 3.13 (0.14–71.10) | 0.47 | |||
| Liver metastasis | |||||
| No | Ref. | ||||
| Yes | 3.369 (0.09–132.77) | 0.52 | |||
| Brain metastasis | |||||
| No | Ref. | ||||
| Yes | 0.00 (0.00–0.84) | 0.045 | 0.09 (0.00–2.24) | 0.14 | |
| Pleural effusion | |||||
| No | Ref. | ||||
| Yes | 22.57 (0.51–1,002.68) | 0.11 | 3.33 (0.42–26.59) | 0.26 | |
| PD-L1 | |||||
| Negative/undone | Ref. | ||||
| Positive | 2.49 (0.10–60.04) | 0.58 | |||
| Previous surgical resection | |||||
| No | Ref. | ||||
| Yes | 2.46 (0.02–322.99) | 0.72 | |||
| Previous radiotherapy | |||||
| No | Ref. | ||||
| Yes | 0.12 (0.00–19.99) | 0.42 | |||
| Treatment regimen | |||||
| S-1 monotherapy | Ref. | ||||
| S-1 combined therapy | 0.01 (0.00–1.284) | 0.06 | 0.03 (0.00–0.64) | 0.03* | |
| Number of lines | |||||
| Second-line therapy | Ref. | ||||
| ≥ Third-line treatment | 186.77 (1.41–24,779.78) | 0.04 | 7.61 (1.07–54.02) | 0.042* | |
*, P<0.05. NSCLC, non-small cell lung cancer; OR, odds ratio; CI, confidence interval; ref., reference; ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death-ligand 1.
Toxicities
The incidence of AEs is shown in Table 4. Among the total cohort of patients, both the S-1 groups were well tolerated. The most common adverse reactions were anemia (12, 23.1%), platelet count decreased (5, 9.6%), elevated creatinine (5, 9.6%), pneumonitis (5, 9.6%), fatigue (4, 7.7%), elevated AST/ALT (4, 7.7%), white blood cell count decreased (2, 3.8%), neutrophil count decreased (2, 3.8%), dizziness (2, 3.8%), hypothyroidism (2, 3.8%), hand-foot syndrome (1, 1.9%), abdominal distension (1, 1.9%), elevated serum amylase (1, 1.9%). Most of the AEs were grade 1–2, only 3 (5.7%) patients showed grade 3–4 AEs (Table 4).
Table 4
| Treatment-related AEs | Any grade | Grade ≥3 |
|---|---|---|
| Anemia | 12 (23.1) | 0 (0.0) |
| White blood cell count decreased | 2 (3.8) | 0 (0.0) |
| Neutrophil count decreased | 2 (3.8) | 2 (3.8) |
| Platelet count decreased | 5 (9.6) | 1 (1.9) |
| Pneumonitis | 5 (9.6) | 0 (0.0) |
| Nausea and/or vomiting | 0 (0.0) | 0 (0.0) |
| Hand-foot syndrome | 1 (1.9) | 0 (0.0) |
| Dizziness | 2 (3.8) | 0 (0.0) |
| Abdominal distension | 1 (1.9) | 0 (0.0) |
| Fatigue | 4 (7.7) | 0 (0.0) |
| Hypertension | 0 (0.0) | 0 (0.0) |
| Hypothyroidism | 2 (3.8) | 0 (0.0) |
| Elevated creatinine | 5 (9.6) | 0 (0.0) |
| Elevated AST/ALT | 4 (7.7) | 0 (0.0) |
| Elevated serum amylase | 1 (1.9) | 0 (0.0) |
Data are presented as n (%). AE, adverse event; ALT, alanine aminotransferase; AST, aspartate transaminase.
Discussion
In the realm of advanced NSCLC patients who have experienced progression following platinum-based chemotherapy and ICI therapy, docetaxel monotherapy has emerged as the preferred treatment option. However, the effectiveness of docetaxel as second-line chemotherapy is not satisfactory. In a study conducted by Hanna et al., patients with advanced NSCLC who had previously received chemotherapy were given docetaxel monotherapy (16). The ORR was found to be less than 10%, the median PFS was less than 3 months, and the median OS ranged from 6 to 8.3 months. Moreover, the toxicities associated with docetaxel monotherapy were notably severe. Additionally, not all patients were able to tolerate docetaxel, particularly those with ECOG PS scores ≥2. ICIs have emerged as the preferred treatment modality for disease progression following initial platinum-based chemotherapy. Various studies, including CheckMate-078 (17), KEYNOTE-010 (18), and OAK (19) have illustrated that single-agent immunotherapy can result in extended survival rates in the second-line setting when compared to docetaxel. Nevertheless, the utilization of immunotherapy monotherapy in second-line treatment has been constrained by the elevated expenses associated with immunotherapy agents and the escalating utilization of programmed death 1 (PD1)/PD-L1 inhibitors in first-line therapy. Anlotinib was approved by the National Medical Products Administration (NMPA) in China in 2008 as a third-line treatment for NSCLC (20). However, drug resistance eventually emerged, and the improvement in OS was finite.
In this study, we first assessed the efficacy and safety of combining S-1 with other therapies in advanced NSCLC patients. 25% of patients were in the monotherapy group, and 75.0% of patients were in the combination group. The ORR was 28.8% of the total population. Median PFS and OS were 2.7 and 9.5 months for the total cohort, respectively. The fluorouracil-based oral prodrug S-1 was approved in Japan in 2004 for the treatment of NSCLC. A meta-analysis conducted by Abdel-Rahman et al. demonstrated that S-1-based regimens are associated with favorable efficacy outcomes in NSCLC (21).
Of particular note, compared with patients who had not received prior radiotherapy, patients who had received prior radiotherapy exhibited superior OS and PFS curves. Analysis of the PACIFIC trial demonstrated that the concurrent administration of chemoradiotherapy and immunotherapy led to a significant improvement in OS, with a median OS of 47.5 months compared to 29.1 months [hazard ratio (HR), 0.72; 95% CI: 0.59 to 0.89] (22) and a median PFS of 16.9 months compared to 5.6 months (HR, 0.55; 95% CI: 0.45 to 0.68) between the immunotherapy group and placebo group. It may be that immunotherapy was used during radiotherapy for all patients who had received prior radiotherapy, of which the synergy between immunotherapy and radiotherapy enhanced therapeutic responses. Moreover, it was observed that patients with an ECOG PS of 1 exhibited enhanced PFS and OS in comparison to those with a PS of 2. The ECOG PS score serves as a comprehensive indicator of a patient’s general health condition and is commonly utilized in guiding recommendations for antitumor therapies (23). A recent multicenter study conducted in Japan revealed a significant correlation between an ECOG PS score of ≥2 and a diminished OS outcome (24).
Additionally, results from multivariate logistic regression analysis indicated that the use of combination therapy involving S-1 independently predicted OS. This discovery aligned with the findings of the clinical trial (11,25,26), which demonstrated that combination therapy with S-1 significantly enhanced antitumor efficacy. However, the small sample size may limit the accuracy of the conclusions. A large multicenter sample are needed in future studies due to the possibility of bias in small sample sizes.
Utilizing multivariate logistic regression analysis, our research identified the treatment line as an independent adverse prognostic factor for OS. Specifically, individuals who received S-1 as a later-line therapy experienced a significantly decreased OS compared to those who received it as a second-line treatment. The later the treatment lines, and the worse the physical function, the poorer the prognosis, the initiation of the S-1 treatment regimen promptly is recommended to optimize outcomes.
In our cohort, the most common AE was anemia, which occurred in 23.1% of patients. Followed by platelet count decreased, elevated creatinine and pneumonitis, which occurred in 9.6% of patients. Most of the AEs were grade 1–2, only 5.7% of patients showed grade 3–4 AEs. In a clinical trial by Nishijima-Futami et al. (26), the addition of S-1 to bevacizumab did not increase toxicity. A meta-analysis also demonstrated that the S-1-based regimens showed milder AEs in high-grade nausea/vomiting, anorexia, leukopenia, neutropenia, and febrile neutropenia (all P<0.05) (27).
Of note, there are certain limitations in this study. First, the limited sample size in this study had implications for statistical power, potentially introducing selection and measurement biases. Due to the small sample size, conclusions may not be generalized to the entire population. Further multicenter large sample studies are needed in the future. Furthermore, the retrospective design of this study introduced inherent limitations associated with retrospective data collection, potentially impacting survival time and treatment response.
Conclusions
In conclusion, the study findings suggest that S-1 exhibits potential efficacy and tolerability with minimal toxicity as a second-line or subsequent treatment for advanced NSCLC, in comparison to standard treatment options such as docetaxel monotherapy or docetaxel plus ramucirumab, regardless of whether it is administered as monotherapy or in combination. Moreover, combined therapy with S-1 and treatment line emerged as two significant independent predictors of OS. Given the constraints of the small sample size, additional prospective investigations are warranted to explore the potential efficacy of this therapeutic approach.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-940/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-940/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-940/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-940/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Zhejiang Cancer Hospital, Zhejiang, China (No. IRB-2023-615) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Wood SL, Pernemalm M, Crosbie PA, et al. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev 2014;40:558-66. [Crossref] [PubMed]
- Matsuzawa R, Morise M, Ito K, et al. Efficacy and safety of second-line therapy of docetaxel plus ramucirumab after first-line platinum-based chemotherapy plus immune checkpoint inhibitors in non-small cell lung cancer (SCORPION): a multicenter, open-label, single-arm, phase 2 trial. EClinicalMedicine 2023;66:102303. [Crossref] [PubMed]
- Chen B, Wang J, Pu X, et al. The efficacy and safety of immune checkpoint inhibitors combined with chemotherapy or anti-angiogenic therapy as a second-line or later treatment option for advanced non-small cell lung cancer: a retrospective comparative cohort study. Transl Lung Cancer Res 2022;11:2111-24.
- Mao S, Zhou F, Liu Y, et al. ICI plus chemotherapy prolonged survival over ICI alone in patients with previously treated advanced NSCLC. Cancer Immunol Immunother 2022;71:219-28. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. [Crossref] [PubMed]
- Miura K, Shirasaka T, Yamaue H, et al. S-1 as a core anticancer fluoropyrimidine agent. Expert Opin Drug Deliv 2012;9:273-86. [Crossref] [PubMed]
- Nokihara H, Lu S, Mok TSK, et al. Randomized controlled trial of S-1 versus docetaxel in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy (East Asia S-1 Trial in Lung Cancer). Ann Oncol 2017;28:2698-706. [Crossref] [PubMed]
- Sugawara S, Nakagawa K, Yamamoto N, et al. Japanese subgroup analysis of a phase III study of S-1 versus docetaxel in non-small cell lung cancer patients after platinum-based treatment: EAST-LC. Int J Clin Oncol 2019;24:485-93. [Crossref] [PubMed]
- Nishino K, Imamura F, Kumagai T, et al. A randomized phase II study of bevacizumab in combination with docetaxel or S-1 in patients with non-squamous non-small-cell lung cancer previously treated with platinum based chemotherapy (HANSHIN Oncology Group 0110). Lung Cancer 2015;89:146-53. [Crossref] [PubMed]
- Xie XH, Wang F, Lin XQ, et al. Anlotinib Plus S-1 for Patients with EGFR Mutation-Negative Advanced Squamous Cell Lung Cancer with PS Scores of 2-3 After Progression of Second-Line or Later-Line Treatment. Cancer Manag Res 2020;12:12709-14. [Crossref] [PubMed]
- Chihara Y, Yoshimura A, Date K, et al. Phase II Study of S-1 and Paclitaxel Combination Therapy in Patients with Previously Treated Non-Small Cell Lung Cancer. Oncologist 2019;24:1033-e617. [Crossref] [PubMed]
- Gridelli C, Shepherd FA. Chemotherapy for elderly patients with non-small cell lung cancer: a review of the evidence. Chest 2005;128:947-57. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized Phase III Trial of Pemetrexed Versus Docetaxel in Patients With Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy. J Clin Oncol 2023;41:2682-90. [Crossref] [PubMed]
- Wu YL, Lu S, Cheng Y, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol 2019;14:867-75. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Zhou M, Chen X, Zhang H, et al. China National Medical Products Administration approval summary: anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy. Cancer Commun (Lond) 2019;39:36. [Crossref] [PubMed]
- Abdel-Rahman O, ElHalawani H. S-1-based regimens for locally advanced/metastatic non-small-cell lung cancer: a meta-analysis. Future Oncol 2016;12:701-13. [Crossref] [PubMed]
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:1301-11. [Crossref] [PubMed]
- Schiller JH, Cleary J, Johnson D. Lung cancer: review of the ECOG experience. Eastern Cooperative Oncology Group. Oncology 1997;54:353-62. [Crossref] [PubMed]
- Hanaoka M, Hino H, Shiomi A, et al. The Eastern Cooperative Oncology Group Performance Status as a prognostic factor of stage I-III colorectal cancer surgery for elderly patients: a multi-institutional retrospective analysis. Surg Today 2022;52:1081-9. [Crossref] [PubMed]
- Takiguchi Y, Seto T, Ichinose Y, et al. Long-term administration of second-line chemotherapy with S-1 and gemcitabine for platinum-resistant non-small cell lung cancer: a phase II study. J Thorac Oncol 2011;6:156-60. [Crossref] [PubMed]
- Nishijima-Futami Y, Minami S, Futami S, et al. Phase II study of S-1 plus bevacizumab combination therapy for patients previously treated for non-squamous non-small cell lung cancer. Cancer Chemother Pharmacol 2017;79:1215-20. [Crossref] [PubMed]
- Chen J, Wang J, Wu X, et al. Meta-analysis for the efficacy of S-1-based regimens as the first-line treatment in Asian chemotherapy-naive patients with advanced non-small-cell lung cancer. Future Oncol 2017;13:2195-207. [Crossref] [PubMed]


