
Comparison of three methods in the surgical treatment of mediastinal roof tumors
Highlight box
Key findings
• We found that both robotic-assisted thoracoscopic surgery (RATS) and video-assisted thoracoscopic surgery (VATS) resections of tumors at the top of the mediastinum are safe and feasible, with advantages over traditional surgery. Subgroup analysis suggested that VATS may be more suitable for tumors in the anterior region, whereas RATS may be more suitable for tumors in the posterior region.
What is known and what is new?
• Previous studies comparing surgical techniques for mediastinal tumors have predominantly focused on anterior mediastinal or thymic tumors, with conclusions indicating that VATS and RATS resections of anterior mediastinal tumors are safe and feasible. However, there is a lack of research focusing on the mediastinal roof. This study aimed to evaluate the effectiveness and safety of different surgical approaches for resecting tumors at the top of the mediastinum by collecting and analyzing clinical data from patients undergoing surgery for mediastinal top tumors.
What is the implication, and what should change now?
• The findings of this study suggest that appropriate surgical approaches should be chosen based on the anatomical features of specific body regions.
Introduction
The mediastinum has a narrow space and complicated anatomical structure. Even benign tumors can cause serious compression symptoms, so the principle of mediastinal tumor treatment is based on surgical resection (1). The mediastinum is roughly conical in shape, wider at the bottom, and the space at the top of the mediastinum is the narrowest, which exacerbates the risk of compression symptoms and complicates surgical intervention due to the dense vasculature and innervation in this area.
Conventional open surgeries (COSs) for mediastinal tumors include median sternal split, partial sternal split, anterolateral incision, transcervical approach, and paraspinal approach, which are chosen based on the tumor location (2-4). In line with their development, minimally invasive technology, video-assisted thoracoscopic surgery (VATS), and robotic-assisted thoracoscopic surgery (RATS) have been successively applied to mediastinal surgery (5,6). The emergence of the da Vinci robot-assisted system expanded the possibilities of minimally invasive techniques with its enhanced screen resolution, three-dimensional vision, and superior stability and flexibility of the operating arm (7,8). Following the proliferation of surgical modalities, attention has shifted to comparing the efficacy and safety of RATS, VATS, and COS. To date, relevant studies have mainly focused on anterior mediastinal thymoma surgery, and little attention has been paid to the top of the mediastinum; the efficacy and safety differences among the three surgical modalities for surgical treatment of tumors on the top of the mediastinum are not yet clear (9-11).
This study aimed to evaluate the surgical outcomes, postoperative recurrence, and survival rates of patients who underwent resection of tumors at the top of the mediastinum using RATS, VATS, and COS. Subgroup analyses were conducted to minimize bias and provide a comprehensive comparison of the efficacy and safety of these surgical modalities. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-946/rc).
Methods
Patients
This retrospective cohort study was conducted to analyze the clinical data of patients who underwent resection of mediastinal roof tumors in the First Medical Center of the PLA General Hospital from January 2013 to January 2023. The cases were divided into three groups according to the surgical modality: the RATS, the VATS, and the COS groups.
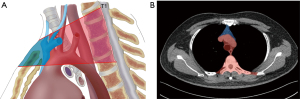
The inclusion criteria were as follows: (I) computed tomography (CT) or magnetic resonance imaging (MRI) examination suggested that the mediastinal tumor is located in the top of the mediastinum (as shown in Figure 1A); the largest diameter of the tumor was located above the level of the aortic arch and the lowest edge of the tumor was located above the bifurcation trachea), and the patient underwent surgical treatment. (II) Cardiac, pulmonary, hepatic, and renal functions were basically normal in preoperative examination, with no serious comorbidities. (III) No clear history of tuberculosis, pyothorax, traumatic hemopneumothorax, and other diseases.
The exclusion criteria were as follows: (I) simultaneous management of lung lesions unrelated to the mediastinal tumor during surgery. (II) Patients who had undergone radiotherapy and chemotherapy before surgery. (III) Patients with preoperative malnutrition or hypoproteinemia. (IV) Patients with other preoperative medical history that may have affected the operation.
Surgical approach
RATS
Single-lumen endotracheal intubation after general anesthesia was routinely used, assisted by artificial pneumothorax, and the artificial pneumothorax pressure was generally maintained at about 8–10 mmHg, so that the lung on the operative side was slightly atrophied and the mediastinal lesion was fully revealed. The surgical approach, position, and incision location were selected and adjusted appropriately according to the tumor location. The lateral thoracic approach was the most common: the patient assumed a 45° lateral position, an incision was made in the mid-axillary line of the 5th–6th intercostal into the cavity lens, the two main operating holes were established on the mid-axillary line of the 2nd intercostal and axillary anterior line of the 5th intercostal, an additional auxiliary hole could be added as needed for the procedure. The main principles of incision selection included incision toward the tumor, the triangular distribution of perforation, the right and left operating arm holes, left and right operating arm holes, and cavity lens holes were more than 10 cm apart, and appropriate relaxation of robot arm spacing was ensured to avoid mutual interference with the mechanical arm. During installation of robotic arm, generally the left arm was installed with pericardial grasping forceps, and the right arm was selected according to the needs of unipolar electrocoagulation hook, bipolar electrocoagulation hook (Maryland forceps), ultrasonic knife, and so on. After installation of the robotic auxiliary system, the mediastinal pleura around the tumor was opened, and careful anatomical differentiation was performed to bluntly and sharply free the mass, identify and protect the surrounding important blood vessels, nerves, and other tissues, and the mass was exposed and completely resected.
VATS
VATS is similar to RATS, and the surgical approach, position, and incision position were selected and adjusted appropriately according to the tumor location. The subxiphoid approach was often used for anterior region tumors. The patient was placed in a supine position, and a longitudinal incision of about 3 cm was made under the xiphoid process. The rectus abdominis muscle was incised and bluntly separated from the anterior mediastinum along the posterior wall of the sternum above the diaphragm, and 5 mm Trocars were inserted into the intersections of the midclavicular line and the lower edge of the costal arches bilaterally to place laparoscopic grasping forceps and an ultrasonic knife, and 10 mm Trocars were inserted into the subxiphoid incisions to place a laparoscopic source of light. The mediastinal pleura and posterior sternal space were opened to expose the mass and carefully distinguish the anatomy, the mass was then bluntly and sharply separated, with care taken to protect the peripheral blood vessels and nerves, and completely resect the mass. The posterior region tumors were mostly taken via the lateral thoracic approach, which is similar to the robotic-assisted surgical approach.
COS
After successful general anesthesia, an appropriate surgical access was selected according to the location of the tumor, and the position and incision could be adjusted according to the location and size of the mediastinal tumor. For anterior tumors, the longitudinal sternal approach was often used: with the patient in a supine position, an incision was made in the middle of the anterior chest wall about 15 cm long; the incision was made layer by layer to reach the sternum, the sternum was split longitudinally along the middle, and a sternal spreader was used to open it out, and then the mass was separated bluntly and sharply. Attention was paid to the protection of peripheral blood vessels and nerves, and the complete resection of mass was completed; posterior tumors could be treated with transcervical or posterior approach, and the transcervical approach was performed by making a curved incision in the neck of the chest, with an arc length of 10 cm above the sternum. In the posterior approach, the patient was placed in a prone position, an incision about 15 cm long was made in the middle of the spine, with the skin, subcutaneous tissue, and supraspinatus ligament being incised in turn. The 2nd, 3rd, and 4th vertebral spinous processes and the joint protrusions and transverse processes on the side of the vertebral body tumor were revealed, and then the 2nd, 3rd, and 4th vertebral spinous processes and the transverse processes were cut and resected; the 2nd, 3rd, and 4th ribs were resected, the mass was exposed, and then the mass was bluntly and sharply separated. With care taken to protect the surrounding blood vessels and nerves, the mass was completely resected.
Postoperative management and follow-up
Unless there were comorbidities requiring intervention, discharge was routinely performed 1–2 days after drain removal. The follow-up program after discharge of patients mainly comprised chest CT with contrast; the initial follow-up time was 6–12 months, and then follow-up was conducted once a year. Patients who did not regularly visit the outpatient clinic were followed up by telephone until September 2023. Patients who were lost to follow-up were evaluated based on the most recent electronic medical record.
Study design
Since the location of the tumor has a large impact on the choice of surgical modality and access, in this study, the top of the mediastinum was divided into two regions, the anterior and posterior regions, based on different anatomical features (Figure 1B). Due to the narrow space at the top of the mediastinum, which may affect the surgical execution when the tumor size is large, in this study, the cases with the largest tumor diameter ≥30 mm were analyzed individually to compare the efficacy of the three surgical approaches in resecting larger tumors in the narrow space.
Data collection
The primary outcome was the postoperative composite adverse outcomes (complications ≥1). These complications were further analyzed by organ system. Secondary outcomes included surgical cost (¥), inpatient cost (¥), operation time (min), intraoperative bleeding (mL), drainage within three days (mL), total postoperative drainage (mL), thoracic drainage time (h), peak postoperative axillary temperature (℃), postoperative hospital stays (day), and so on. If the missing data conformed to the normal distribution, the mean of the same group of data was substituted. Preoperative demographic and clinical characteristics were assessed to identify potential confounding variables. If the normal distribution was not met, the median of the same group of data was used instead.
Statistical analysis
To explore the effect of each factor on the occurrence of composite adverse outcomes, SHAP values (SHapley Additive exPlanations) were used to interpret the role and characteristic importance of each factor (12,13). For categorical variable factors, we used numerical definitions for each variable (Table 1).
Table 1
| Variable factor | Option | Number |
|---|---|---|
| Surgical modality | COS | 1 |
| VATS | 2 | |
| RATS | 3 | |
| Gender | Male | 1 |
| Female | 2 | |
| Location of the mass | Anterior region | 1 |
| Posterior region | 2 | |
| Pathological type | Cystic lesion | 1 |
| Neurogenic tumor | 2 | |
| Thyroid and parathyroid tumor | 3 | |
| Thymic tumor | 4 | |
| Other types | 5 | |
| History of smoking | Yes | 1 |
| No | 0 | |
| Hypertension | Yes | 1 |
| No | 0 | |
| Diabetes | Yes | 1 |
| No | 0 | |
| Coronary artery disease | Yes | 1 |
| No | 0 | |
| History of mediastinal surgery | Yes | 1 |
| No | 0 |
COS, conventional open surgery; VATS, video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery.
All data were analyzed using SPSS 26.0 software (IBM Corp., Armonk, NY, USA). If the measurement information conformed to normal distribution, it was expressed as (mean ± standard deviation), and t-test or analysis of variance (ANOVA) was used for comparison between groups; if the measurement information did not conform to normal distribution, it was expressed as median {M [P25, P75]}, and Kruskal-Wallis H test was used for comparison between groups. Count data were expressed as the number of cases and percentages, and comparisons between groups were made using the Pearson χ2 test or the Fisher exact probability test.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital (protocol #S2024-039-02) and the requirement for individual consent for this retrospective analysis was waived.
Results
A total of 213 cases of resection of mediastinal roof tumors were enrolled: 41 cases of COS (19.2%), 115 cases of VATS (54.0%), and 57 cases of RATS (26.8%). The preoperative characteristics of the cases in the three groups are shown in Table 2. Nine patients (4.2%) were missing from the data of surgical cost and inpatient cost, and the median data of the same group were used instead of the missing values because the two groups of data did not conform to the normal distribution. The resection of the lesions was accomplished in the three groups of patients, with four cases in the VATS group converted to open thoracotomy because of surgical difficulties: two cases were converted because of the high location of the mass, one case was converted because of severe thoracic adhesion, and one case was converted because of the high risk of hemorrhage; there were no converted open thoracotomy cases in the RATS group. During postoperative follow-up, a total of eight patients (3.8%) were lost to follow-up, and the remaining 205 patients (96.2%) obtained follow-up results. One patient in each of the VATS and RATS groups experienced postoperative recurrence, one patient with thymoma in the VATS group had a recurrence of severe-rate myasthenia gravis at one year postoperatively, and one patient with mediastinal lipoblastoma in the RATS group had a recurrence at two years postoperatively. Two deaths occurred in the VATS group, one patient died of renal clear cell carcinoma metastasis three years after surgery, and one patient died of pancreatic cancer five years after surgery; there were no deaths in the RATS and COS groups during follow-up.
Table 2
| Characteristics | COS cases (n=41) | VATS cases (n=115) | RATS cases (n=57) | P value |
|---|---|---|---|---|
| Age, years | 49±10 | 48±13 | 43±14 | 0.02 |
| Height, cm | 165.8±6.5 | 165.2±7.6 | 168.4±7.4 | 0.03 |
| Weight, kg | 68 [59.5, 74.5] | 68 [59, 81] | 69 [60.5, 82] | 0.82 |
| Tumor location | <0.001 | |||
| Anterior region | 30 (30.6) | 57 (58.2) | 11 (11.2) | |
| Posterior region | 11 (9.6) | 58 (50.4) | 46 (40.0) | |
| BMI, kg/m2 | 24.3 [22.2, 26.3] | 25.3 [22.4, 28.3] | 25 [21,4, 28.3] | 0.56 |
| Smoking history, yes | 10 (24.4) | 41 (35.7) | 24 (42.1) | 0.19 |
| Maximum tumor diameter, mm | 32.5 [23.8, 50.2] | 32.0 [24.9, 44.5] | 38.8 [30.4, 54.0] | 0.03 |
| Gender, female | 23 (56.1) | 52 (45.2) | 23 (40.4) | 0.30 |
| Pathological type | <0.001 | |||
| Cystic lesion | 8 (13.1) | 45 (73.8) | 8 (13.1) | |
| Neurogenic tumor | 5 (7.7) | 27 (41.5) | 33 (50.8) | |
| Thymic tumor | 18 (40.9) | 23 (52.3) | 3 (6.8) | |
| Thyroid and parathyroid tumor | 6 (66.7) | 2 (22.2) | 1 (11.1) | |
| Other | 4 (11.8) | 18 (52.9) | 12 (35.3) |
Data are expressed as mean ± SD, median [IQR] and n (%). COS, conventional open surgery; VATS, video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery; BMI, body mass index; SD, standard deviation; IQR, interquartile range.
Ranking of the importance of the features revealed that surgical approach was the most important factor influencing the occurrence of composite adverse outcome, and maximum tumor diameter was the second most important factor (Figure 2). The SHAP summary plot showed that surgical approach of RATS and tumor location in anterior region were associated with a decreased risk of composite adverse outcomes; the surgical approach of COS, increased maximum tumor diameter, and increased patient weight were associated with increased risk of composite adverse outcomes (Figure 3).


The overall composite adverse outcome rate was 29.6%, and there was no statistically significant difference in the comparison among the three groups (26.4% vs. 30.6% vs. 36.6%, P=0.44, for RATS, VATS, and COS, respectively). The highest frequency of postoperative complications was neurological complications (23.9%), followed by postoperative pleural infection or effusion (7.3%). The percentage of postoperative development of pleural infection or effusion was higher in the COS group than that in the RATS group and the VATS group (14.6% vs. 1.8% vs. 7.0%, respectively, P=0.04), and the difference was statistically significant. Univariate analysis showed that the VATS group had the shortest operation time [median, 95 vs. 115 (RATS) vs. 131 min (COS), P<0.001]; intraoperative bleeding was lower in the RATS and VATS groups than in the COS group (median, 20 vs. 100 mL, P <0.001; 20 vs. 100 mL, P<0.001); the RATS group had the shortest postoperative hospital stay [median, 4 vs. 6 (COS) vs. 5 d (VATS), P<0.001]; thoracic drainage time in the RATS group was shorter than that of the COS group (median, 48 vs. 72 h, P=0.001); and postoperative hospital stay in the VATS group was shorter than that of the COS group (median, 5 vs. 6 d, P<0.001); surgical cost was lowest in the COS group [median, 5,968 vs. 7,092 (VATS) vs. 47,829 (RATS), P<0.001]; inpatient cost were lowest in the VATS group (median, 26,483 vs. 29,817 vs. 67,624, P <0.001) (Table 3).
Table 3
| Characteristics | COS cases (n=41) | VATS cases (n=115) | RATS cases (n=57) | P value |
|---|---|---|---|---|
| Composite adverse outcome | 15 (36.6) | 34 (30.6) | 14 (26.4) | 0.44 |
| Neurological complications | ||||
| Horner syndrome | 6 (14.6) | 12 (10.4) | 10 (17.5) | 0.41 |
| Numbness in the operated limb | 2 (4.9) | 5 (4.3) | 4 (7.0) | 0.78 |
| Hoarseness of voice | 2 (4.9) | 5 (4.3) | 1 (1.8) | 0.70 |
| Delayed response | 0 | 1 (0.9) | 0 | >0.99 |
| Dizziness and nausea | 1 (2.4) | 0 | 0 | 0.19 |
| Respiratory complications | ||||
| Pleural infection/effusion | 6 (14.6)2,3 | 8 (7.0)1 | 1 (1.8)1 | 0.04 |
| Pulmonary injury | 1 (2.4) | 3 (2.6) | 2 (3.5) | >0.99 |
| Cardiovascular complications | ||||
| Atrial fibrillation | 0 | 1 (0.9) | 0 | >0.99 |
| Cerebral infarction | 0 | 1 (0.9) | 0 | >0.99 |
| Other | ||||
| Chylothorax | 0 | 3 (2.6) | 1 (1.8) | 0.82 |
| Subcutaneous emphysema | 0 | 2 (1.7) | 0 | >0.99 |
| Poor wound healing | 1 (2.4) | 1 (0.9) | 0 | 0.42 |
| Incomplete resection | 0 | 2 (1.7) | 0 | >0.99 |
| Conversion to thoracotomy | 0 | 4 (3.5) | 0 | 0.31 |
| Recurrence | 0 | 1 (0.9) | 1 (1.9) | 0.71 |
| Death | 0 | 2 | 0 | >0.99 |
| Surgical cost, CNY | 5,968 [3,639, 7,850]2,3 | 7,092 [5,643, 9,745]1,3 | 47,829 [31,775, 49,031]1,2 | <0.001 |
| Inpatient cost, CNY | 29,817 [27,215, 37,722]2,3 | 26,483 [22,736, 35,024]1,3 | 67,624 [61,361, 77,475]1,2 | <0.001 |
| Operation time, min | 131 [100, 184]2 | 95 [72, 120]1,3 | 115 [88, 148]2 | <0.001 |
| Intraoperative bleeding, mL | 100 [50, 125]2, 3 | 20 [10, 50]1 | 20 [10, 50]1 | <0.001 |
| Drainage within 3 days, mL | 280 [175, 450] | 250 [120, 420] | 220 [100, 408] | 0.38 |
| Total postoperative drainage, mL | 340 [195, 510] | 275 [120, 520] | 245 [100, 450] | 0.20 |
| Thoracic drainage time, h | 72 [72, 108]2,3 | 72 [48, 94]1 | 48 [24, 84]1 | 0.002 |
| Peak postoperative axillary temperature, ℃ | 37.2 [37.0, 37.5] | 37.1 [36.9, 37.4] | 37 [36.8, 37.4] | 0.052 |
| Postoperative hospital-stay, days | 6 [5, 7]2,3 | 5 [3, 6]1,3 | 4 [2, 5]1,2 | <0.001 |
Data are expressed as median [IQR] and n (%). Data presented in the table are accompanied by superscript numerals in the top right corner to represent the results of pairwise comparison between the three groups, 1 for COS group, 2 for VATS group, 3 for RATS group. When a number appears in the upper right corner of each data, it means that there is a statistically significant difference between the data in this group and the corresponding group data in the upper right corner. COS, conventional open surgery; VATS, video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery; IQR, interquartile range; CNY, Chinese yuan.
Results of subgroup analysis
Comparison with cases with tumors located in the anterior or posterior region and tumor maximum diameter ≥30 mm individually showed a better balance of baseline demographic and clinical characteristics among the three groups.
Anterior region
The overall rate of composite adverse outcomes was 20.4%, and there was no statistically significant difference in the comparison among the three groups (30.0% vs. 14.0% vs. 27.3%, COS, VATS, RATS, respectively, P=0.18). Univariate analysis showed that intraoperative bleeding in the RATS and VATS groups was less than that in the COS group (median, 20 vs. 100 mL, P<0.001; 20 vs. 100 mL, P<0.001); postoperative hospital stay in the RATS and VATS groups was shorter than that in the COS group (median, 4 vs. 6 d, P=0.008; 3 vs. 6 d, P<0.001); operation time and thoracic drainage time were less in the VATS group than in the COS group (median, 100 vs. 133 min, P<0.001); (median, 48 vs. 72 h, P<0.001); surgical cost were lowest in the COS group (median, 5,937 vs. 7,854 vs. 47,613, P<0.001); the VATS group had the lowest inpatient cost (median, 26,317 vs. 29,414 vs. 66.758, P<0.001) (Table 4).
Table 4
| Characteristics | COS cases (n=30) | VATS cases (n=57) | RATS cases (n=11) | P value |
|---|---|---|---|---|
| Age, years | 49±12 | 51±12 | 51±16 | 0.70 |
| Height, cm | 166.3±6.8 | 165.1±7.8 | 168.0±8.0 | 0.45 |
| Weight, kg | 68 [62, 75.3] | 67 [58, 81.3] | 69 [64, 78] | 0.93 |
| BMI, kg/m2 | 24.4 [22.4, 26.3] | 25.3 [22,5, 27.9] | 24.5 [23.4, 29.1] | 0.72 |
| Maximum tumor diameter, mm | 30.1 [23.7, 38.4] | 26.0 [20.6, 37.2] | 26.8 [18.6, 36.9] | 0.26 |
| Gender, female | 16 (53.3) | 24 (42.1) | 5 (45.5) | 0.61 |
| Smoking history, yes | 6 (20.0) | 23 (40.4) | 4 (36.4) | 0.16 |
| Composite adverse outcomes | 9 (30.0) | 8 (14.0) | 3 (27.3) | 0.18 |
| Surgical cost, CNY | 5,937 [3,892, 7,825]2,3 | 7,854 [6,607, 14,323]1,3 | 47,613 [41,920, 48,169]1,2 | <0.001 |
| Inpatient cost, CNY | 29,414 [26,843, 37,545]2,3 | 26,317 [23,550, 32,670]1,3 | 66,758 [60,379, 73,271]1,2 | <0.001 |
| Operation time, min | 133 [105, 191]2 | 100 [83, 120]1 | 110 [95, 176] | 0.003 |
| Intraoperative bleeding, mL | 100 [50, 113]2,3 | 20 [10, 50]1 | 20 [10, 50]1 | <0.001 |
| Drainage within 3 days, mL | 335 [210, 455] | 250 [90, 4420] | 405 [110, 500] | 0.15 |
| Total postoperative drainage, mL | 435 [217, 520] | 250 [90, 526] | 405 [110, 500] | 0.14 |
| Thoracic drainage time, h | 72 [72, 102]2,3 | 48 [48, 72]1 | 72 [24, 96]1 | <0.001 |
| Peak postoperative axillary temperature, ℃ | 37.2 [37, 37.6] | 37.0 [36.8, 37.4] | 36.9 [36.8, 37.5] | 0.13 |
| Postoperative hospital-stay, days | 6 [5, 7]2,3 | 3 [3, 6]1 | 4 [3, 5]1 | <0.001 |
Data are expressed as mean ± SD, median [IQR] and n (%). Data presented in the table are accompanied by superscript numerals in the top right corner to represent the results of pairwise comparison between the three groups, 1 for COS group, 2 for VATS group, 3 for RATS group. When a number appears in the upper right corner of each data, it means that there is a statistically significant difference between the data in this group and the corresponding group data in the upper right corner. COS, conventional open surgery; VATS, video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery; BMI, body mass index; IQR, interquartile range; SD, standard deviation; CNY, Chinese yuan.
Posterior region
The overall rate of composite adverse outcomes was 37.4%, and the rate of composite adverse outcomes was lower in the RATS group than in the COS and VATS groups (23.9% vs. 44.8% vs. 54.5%, respectively, P=0.04), and the difference was statistically significant. Univariate analysis showed that the postoperative hospital stay in the RATS group was shorter than that in the COS and VATS groups (median, 3 vs. 5.5 vs. 5 d, P<0.001). Surgical and inpatient cost in the COS and VATS groups were lower than that in the RATS group (median, 6,107 vs. 47,977, P<0.001; 6,146 vs. 47,977, P<0.001); (median, 33,477 vs. 67,624, P<0.001; 26,963 vs. 67,624, P<0.001) (Table 5).
Table 5
| Characteristics | COS cases (n=11) | VATS cases (n=58) | RATS cases (n=46) | P value |
|---|---|---|---|---|
| Age, years | 49±8 | 46±13 | 41±13 | 0.10 |
| Height, cm | 164.5±5.8 | 165.4±7.5 | 168.5±7.3 | 0.07 |
| Weight, kg | 66.3±10.0 | 70.3±13.5 | 71.4±15.7 | 0.56 |
| BMI, kg/m2 | 24.3±2.6 | 25.6±4.6 | 25±4.5 | 0.61 |
| Maximum tumor diameter, mm | 38.3 [30.5, 74.1] | 37.4 [31.0, 46.0] | 42.3 [32.9, 57.2] | 0.33 |
| Gender, female | 7 (63.6) | 28 (48.3) | 18 (39.1) | 0.31 |
| Smoking history, yes | 4 (36.4) | 18 (31.0) | 20 (43.5) | 0.43 |
| Composite adverse outcomes | 6 (54.5) | 26 (44.8) | 11 (23.9) | 0.04 |
| Surgical cost, CNY | 6,107 [2,912, 7,207]3 | 6,146 [5,215, 7,786]3 | 47,977 [30,680, 49,302]1, 2 | <0.001 |
| Inpatient cost, CNY | 33,477 [28,391, 40,826]3 | 26,963 [22,141, 36,444]3 | 67624 [61,471, 78,458]1, 2 | <0.001 |
| Operation time, min | 132 [96, 199]2 | 85 [70, 120]1, 3 | 115 [85, 136]2 | 0.01 |
| Intraoperative bleeding, mL | 100 [50, 350] | 20 [10, 50] | 25 [10, 50] | 0.051 |
| Drainage within 3 days, mL | 198 [100, 325] | 225 [140, 415] | 210 [100, 341] | 0.20 |
| Total postoperative drainage, mL | 253 [100, 639] | 275 [140, 500] | 237 [92.5, 360] | 0.37 |
| Thoracic drainage time, h | 72 [30, 114] | 72 [48, 96] | 48 [24, 78] | 0.33 |
| Peak postoperative axillary temperature, ℃ | 37.1 [36.9, 37.4] | 37.2 [36.9, 37.4] | 37 [36.7, 37.3] | 0.23 |
| Postoperative hospital-stay, days | 5.5 [4, 8.75]3 | 5 [4, 7]3 | 3 [2, 5]1,2 | <0.001 |
Data are expressed as mean ± SD, median [IQR] and n (%). Data presented in the table are accompanied by superscript numerals in the top right corner to represent the results of pairwise comparison between the three groups, 1 for COS group, 2 for VATS group, 3 for RATS group. When a number appears in the upper right corner of each data, it means that there is a statistically significant difference between the data in this group and the corresponding group data in the upper right corner. COS, conventional open surgery; VATS, video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery; BMI, body mass index; IQR, interquartile range; SD, standard deviation; CNY, Chinese yuan.
Maximum tumor diameter ≥30 mm
The overall incidence of composite adverse outcomes was 34.8%, with no statistically significant difference between the three groups (37.5% vs. 37.7% vs. 28.9%, P=0.60). Univariate analysis showed that intraoperative bleeding was less in the RATS and VATS groups than in the COS group (30 vs. 100 mL, P=0.001; 20 vs. 100 mL, P<0.001); postoperative hospital stay was shortest in the RATS group (3 vs. 6 vs. 5 d, P<0.001). Surgical and inpatient cost were higher in the RATS group than in the COS and VATS groups (median, 47,914 vs. 4,965 vs. 6,923, P<0.001) (median, 67,935 vs. 30,027 vs. 27,319, P<0.001) (Table 6).
Table 6
| Characteristics | COS cases (n=24) | VATS cases (n=69) | RATS cases (n=45) | P value |
|---|---|---|---|---|
| Age, years | 47±11 | 47±14 | 41±14 | 0.08 |
| Height, cm | 164.3±5.8 | 166.3±7.6 | 169.3±7.0 | 0.02 |
| Weight, kg | 67.5 [57.3, 72.8] | 69 [60, 80] | 70.5 [60, 84.5] | 0.50 |
| BMI, kg/m2 | 24 [21.9, 26.1] | 25.3 [22,4, 28.0] | 24.5 [20.8, 28.5] | 0.71 |
| Gender, female | 15 (62.5) | 30 (43.5) | 16 (35.6) | 0.10 |
| Smoking history, yes | 6 (25.0) | 25 (36.2) | 21 (46.7) | 0.20 |
| Composite adverse outcomes | 9 (37.5) | 26 (37.7) | 13 (28.9) | 0.60 |
| Surgical cost, CNY | 4,965 [3,420, 7,558]3 | 6,923 [5,505, 9,070]3 | 47,914 [33,177, 49,266]1,2 | <0.001 |
| Inpatient cost, CNY | 30,027 [26,843, 39,616]3 | 27,319 [22,929, 38,653]3 | 67,935 [61,619, 78,380]1,2 | <0.001 |
| Operation time, min | 123 [102, 177]2 | 95 [72, 139]1 | 115 [91, 148] | 0.02 |
| Intraoperative bleeding, mL | 100 [50, 137.5]2,3 | 20 [10, 50]1 | 30 [10, 50]1 | <0.001 |
| Drainage within 3 days, mL | 208 [101, 430] | 310 [140, 510] | 220 [100, 408] | 0.45 |
| Total postoperative drainage, mL | 298 [108, 510] | 310 [140, 573] | 245 [103, 450] | 0.50 |
| Thoracic drainage time, h | 72 [72, 96] | 72 [48, 96] | 48 [36, 84] | 0.12 |
| Peak postoperative axillary temperature, ℃ | 37.2 [36.9, 37.4] | 37.1 [36.9, 37.4] | 36.9 [36.7, 37.4] | 0.01 |
| Postoperative hospital-stay, days | 6 [5, 7.75]3 | 5 [3, 7]3 | 3 [2.5, 5]1,2 | <0.001 |
Data are expressed as mean ± SD, median [IQR] and n (%). Data presented in the table are accompanied by superscript numerals in the top right corner to represent the results of pairwise comparison between the three groups, 1 for COS group, 2 for VATS group, 3 for RATS group. When a number appears in the upper right corner of each data, it means that there is a statistically significant difference between the data in this group and the corresponding group data in the upper right corner. COS, conventional open surgery; VATS, video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery; BMI, body mass index; IQR, interquartile range; SD, standard deviation; CNY, Chinese yuan.
Discussion
With reference to several reports, the prevalence of mediastinal tumors is 0.73–0.9% (14-16). Currently, the treatment strategy for mediastinal tumors is based on surgery, but surgical resection is often difficult due to the complex anatomy of the mediastinum (17,18). After years of development, RATS and VATS have emerged as new modalities for the treatment of mediastinal tumors. Current studies comparing open and minimally invasive mediastinal tumors surgery have focused only on anterior mediastinal thymomas, and few studies have paid attention to the mediastinal roof tumors (2,19,20). According to a survey based on 9,263 individuals, the prevalence of upper mediastinal tumors is about 0.27%, accounting for about 35% of mediastinal tumors as a whole, and about 80% of intrathoracic goiters and about 20% of neurogenic tumors are located in the upper mediastinum (14,21). Due to the many anatomical structures, it is more difficult to expose the tumor field of the top mediastinum under the traditional surgical method, and the limited bony space often causes difficulties in intraoperative manipulation; these reasons lead to the extreme difficulty of surgery in the upper mediastinum and the higher probability of postoperative complications. Due to the different anatomical characteristics, it is not known whether the conclusions of the studies related to anterior mediastinal tumors can be applied to mediastinal roof tumors, and for this reason, we conducted a comparative study of different surgical approaches for mediastinal roof tumors.
Due to the overall high percentage of composite adverse outcomes (29.6%), we explored the SHAP value distribution of the overall sample for each characteristic; we found that RATS was associated with a decreased risk of occurrence of composite adverse outcomes. This suggests that RATS may be advantageous in narrow-area surgery. The SHAP value distribution suggests that tumors in the anterior region have a lower risk of composite adverse outcomes compared with those in the posterior region. This result aligns with our understanding that the anatomy of the anterior region is simpler compared to that of the posterior region, and the likelihood of unnecessary injuries during the operation is smaller. In a comparison of different pathology types, patients with neurogenic tumors had the highest rate of composite adverse outcomes after surgery. This is similar to other reports of higher rates of postoperative complications in neurogenic tumors, because most of the neurogenic tumors are closely related to the nerves, which are more prone to cause injuries during surgery (22).
After subdividing the anatomical levels of the top of the mediastinum, in the anterior region, there was no statistically significant difference in the rate of postoperative composite adverse outcomes among the three surgical modalities. This is different from the report by O’Sullivan et al. (23), RATS and VATS did not have a significant advantage in reducing the rate of postoperative complications in anterior region tumors. Subgroup analysis of the anterior region showed that the VATS group was better than the COS group in three indicators, namely intraoperative bleeding, thoracic drainage time, and postoperative hospital stay. Taken together, VATS, with its good postoperative recovery indicators and lower treatment costs, seems to be more appropriate for mediastinal roof tumors in the anterior region.
Subgroup analysis of the posterior region showed that the RATS group was superior to the VATS and COS groups in terms of two aspects: composite adverse outcomes and postoperative hospital stays, a result that suggests the advantages of robotic-assisted systems in performing surgery in areas of confined space and anatomical complexity, which can improve the prognosis of patients. However, the cost of surgery and inpatient care in the RATS group was still significantly higher than that in the VATS and COS groups, and it is believed that RATS will be a reasonable surgical choice for posterior region tumor as the cost of treatment borne by patients decreases with the improvement of health insurance policies. The subgroup analysis of the maximum tumor diameter ≥30 mm showed that the postoperative hospital stay in the RATS group was less than that in the VATS and COS groups, and the operation time and intraoperative bleeding in the RATS were less than that in the COS group, a result that suggests the potential of robotic-assisted surgery in coping with mediastinal tumors of larger sizes.
We delineated the levels of the mediastinal apex and compared the perioperative outcomes of resecting mediastinal roof tumors with different surgical approaches, but there are limitations to the applicability of this result. This study was a single-center retrospective study, which may have incurred bias. The cases included in this study were not randomized for surgical access, and there is an inherent bias due to the lack of randomization of surgical approaches. Due to the limitation of the number of cases, this study did not analyze subgroups of pathological types and did not explore the differences in surgical approaches among different types of tumors.
Conclusions
In conclusion, robotic-assisted as well as video-assisted treatment of mediastinal roof tumors is safe and feasible, and RATS as well as VATS improves short-term prognostic outcomes, including intraoperative bleeding, thoracic drainage time, and postoperative hospital stay, and the probability of developing a postoperative pleural infection or effusion is lower when compared with COS. The results of the subgroup analyses suggest that VATS may be more appropriate for anterior region mediastinal tumors, whereas RATS is advantageous for posterior region tumors and bulky mediastinal tumors, but the financial burden of RATS for patients needs to be weighed.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-946/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-946/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-946/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-946/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Ethics Committee of the Chinese People’s Liberation Army General Hospital (protocol #S2024-039-02) and the requirement for individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoffman OA, Gillespie DJ, Aughenbaugh GL, et al. Primary mediastinal neoplasms (other than thymoma). Mayo Clin Proc 1993;68:880-91. [Crossref] [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [Crossref] [PubMed]
- Friedant AJ, Handorf EA, Su S, et al. Minimally Invasive versus Open Thymectomy for Thymic Malignancies: Systematic Review and Meta-Analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Mehta C, Raparia K, Bharat A. Anterior Mediastinal Myelolipoma. Ann Thorac Surg 2017;103:e81. [Crossref] [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE. Imaged thoracoscopic surgery: a new thoracic technique for resection of mediastinal cysts. Ann Thorac Surg 1992;53:318-20. [Crossref] [PubMed]
- Yoshino I, Hashizume M, Shimada M, et al. Video-assisted thoracoscopic extirpation of a posterior mediastinal mass using the da Vinci computer enhanced surgical system. Ann Thorac Surg 2002;74:1235-7. [Crossref] [PubMed]
- DeRose JJ Jr, Swistel DG, Safavi A, et al. Mediastinal mass evaluation using advanced robotic techniques. Ann Thorac Surg 2003;75:571-3. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65; discussion 265-6. [Crossref] [PubMed]
- Tianci C, Shen Z, Chen S, et al. Median sternotomy versus minimally invasive thymectomy for early-stage thymoma: A systematic review and meta-analysis protocol. Medicine (Baltimore) 2019;98:e18359. [Crossref] [PubMed]
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. [Crossref] [PubMed]
- Holleran TJ, Napolitano MA, Crowder HR, et al. Clinical Outcomes and Technical Approach of Thymectomy in the Veterans Health Administration. Ann Thorac Surg 2022;113:1648-55. [Crossref] [PubMed]
- Lundberg S, Lee S. A Unified Approach to Interpreting Model Predictions; Proceedings of the 31st International Conference On Neural Information Processing Systems; Long Beach, CA, USA. 4–9 December 2017; pp. 4768-77.
- Chen H, Lundberg S, Lee SI. Explaining models by propagating Shapley values of local components. In: Shaban-Nejad A, Michalowski M, Buckeridge DL (eds). Explainable AI in Healthcare and Medicine. Springer; Berlin, Germany: 2021.; pp. 261-70.
- Henschke CI, Lee IJ, Wu N, et al. CT screening for lung cancer: prevalence and incidence of mediastinal masses. Radiology 2006;239:586-90. [Crossref] [PubMed]
- Araki T, Nishino M, Gao W, et al. Anterior Mediastinal Masses in the Framingham Heart Study: Prevalence and CT Image Characteristics. Eur J Radiol Open 2015;2:26-31. [Crossref] [PubMed]
- Yoon SH, Choi SH, Kang CH, et al. Incidental Anterior Mediastinal Nodular Lesions on Chest CT in Asymptomatic Subjects. J Thorac Oncol 2018;13:359-66. [Crossref] [PubMed]
- Alvarado CE, Worrell SG, Bachman KC, et al. Robotic Approach Has Improved Outcomes for Minimally Invasive Resection of Mediastinal Tumors. Ann Thorac Surg 2022;113:1853-8. [Crossref] [PubMed]
- Radkani P, Joshi D, Barot T, et al. Robotic video-assisted thoracoscopy: minimally invasive approach for management of mediastinal tumors. J Robot Surg 2018;12:75-9. [Crossref] [PubMed]
- Orsini B, Santelmo N, Pages PB, et al. Comparative study for surgical management of thymectomy for non-thymomatous myasthenia gravis from the French national database EPITHOR. Eur J Cardiothorac Surg 2016;50:418-22. [Crossref] [PubMed]
- Youssef SJ, Louie BE, Farivar AS, et al. Comparison of open and minimally invasive thymectomies at a single institution. Am J Surg 2010;199:589-93. [Crossref] [PubMed]
- Fujimoto K, Hara M, Tomiyama N, et al. Proposal for a new mediastinal compartment classification of transverse plane images according to the Japanese Association for Research on the Thymus (JART) General Rules for the Study of Mediastinal Tumors. Oncol Rep 2014;31:565-72. [Crossref] [PubMed]
- Chen X, Ma Q, Wang S, et al. Surgical treatment of thoracic dumbbell tumors. Eur J Surg Oncol 2019;45:851-6. [Crossref] [PubMed]
- O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review of robotic versus open and video assisted thoracoscopic surgery (VATS) approaches for thymectomy. Ann Cardiothorac Surg 2019;8:174-93. [Crossref] [PubMed]


