
Correlation of hemoglobin, albumin, lymphocyte, and platelet score with prognosis in patients with stage III squamous lung cancer
Highlight box
Key findings
• In patients with stage III squamous lung cancer treated with radical radiotherapy, low baseline hemoglobin, albumin, lymphocyte, and platelet (HALP) scores was associated with poorer overall survival and progression-free survival and thus may be a valuable prognostic indicator.
What is known and what is new?
• HALP score has been reported to be correlated with prognosis in a variety of cancers, including gastrointestinal tumors and urological tumors. However, its use in lung cancer is less common, especially in lung squamous cell carcinoma (LUSC), and has not been reported.
• This study was the first to examine HALP scores in patients with stage III LUSC who had undergone radical radiotherapy and was aimed at assessing the prognostic significance of baseline HALP in this population.
What is the implication, and what should change now?
• In recent years, rapid advances have been made in the diagnosis and treatment of lung cancer, but the prognosis of some patients remains poor. Therefore, we should find more suitable biomarkers to guide the diagnose and treatment in lung cancer, which is critical to developing individualized treatment plans.
Introduction
According to the latest International Agency for Research on Cancer report, lung cancer has the second highest incidence rate and the highest mortality rate in the world (1). Non-small cell lung cancer (NSCLC) is one of the major types of lung cancer, accounting for about 85% of all lung cancers, with, lung squamous cell carcinoma (LUSC) is one of the most common histological subtypes of lung cancer, accounting for about 30% of NSCLC cases (2). Due to its insidious onset and lack of specific manifestations, most patients with LUSC are diagnosed at an advanced stage, and radical radiotherapy has become the standard treatment. Despite the recent advances in lung cancer screening and treatment modalities, the overall prognosis for those with LUSC is not satisfactory, with a 5-year overall survival (OS) rate of 10–20% for patients with clinical stage III and IV LUSC (3). Therefore, identifying accurate prognostic biomarkers for tumors is crucial to refining individualized treatment plans and improving the prognosis and survival of patients with LUSC.
A number of studies have shown that the systemic inflammatory status (4) and nutritional status (5,6) are closely related to the survival of patients with cancer, and many common blood indices such as neutrophil:lymphocyte ratio (NLR) (7-9), platelet:lymphocyte ratio (PLR) (10,11), systemic inflammatory response index (SIRI) (12), and prognostic nutritional index (PNI) (13,14) have been used to predict the survival prognosis of patients. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score can reflect the inflammatory and nutritional status of the body in an integrated manner and has been applied to predict patient prognosis in a variety of cancers, including gastrointestinal tumors (15,16) and urological tumors (17,18); however, its utility to prognosticate survival in patients with LUSC treated with radiotherapy has not been extensively reported. In addition, given that clinical stage is an important factor in the prognosis of those with lung cancer, in order to reduce analysis bias, this study retrospectively analyzed the relationship between the pre-radiotherapy HALP score and the prognosis of patients with clinical stage III LUSC who had received radical radiotherapy. The overarching goal of this study was to assess the value of HALP score in predicting survival after radiotherapy for patients with LUSC. We present this article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1513/rc).
Methods
Clinical information
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2020KS024) and informed consent was taken from all the patients. Patients with stage III LUSC who had undergone radical radiotherapy in the Department of Radiotherapy of The Fourth Hospital of Hebei Medical University from January 2011 to December 2020 were enrolled.
Inclusion criteria
The inclusion criteria for patients were the following: (I) histopathologically or cytologically confirmed LUSC; (II) clinical stage III according to the tumor-node-metastasis (TNM) stage of the eighth edition of the American Joint Committee on Cancer (AJCC) lung cancer guidelines, inoperable or refused surgery for personal reasons; (III) distant metastasis excluded by computed tomography (CT), magnetic resonance imaging (MRI), and whole-body positron emission tomography (PET) before treatment; and (IV) no other antitumor treatments such as particle implantation before and after combined radiotherapy.
Exclusion criteria
The exclusion criteria for patients were the following: (I) lack of hematological findings before radiotherapy; (II) accompaniment of serious cardiac, pulmonary, brain, or other medical diseases; (III) accompaniment of malignant tumors in other sites; and (IV) patient failure to complete chest radiotherapy [dose of target (DT) <54 Gy] due to personal reasons or disease progression.
Radiotherapy target area and dose
With consideration to imaging data such as localization CT, ultrasound diagnosis, or PET/CT, the gross tumor volume (GTV) was outlined in the treatment planning system and included the extent of the lung tumor and mediastinal metastatic lymph nodes as determined by imaging. Meanwhile, the clinical target volume (CTV) was a uniform outward expansion of the GTV of 0.6 cm, and the planning target volume (PTV) was a uniform outward expansion of the CTV of 0.5–1.0 cm, with appropriate modifications being made according to the anatomical barrier. Additionally, 95% of the prescribed doses of the PTV was required to be 54–70 Gy, at 1.8–2.2 Gy/dose and a median dose of 60 Gy. All patients were treated with 6 MV X-ray linear accelerator.
Chemotherapy regimens
Chemotherapy was administered using synchronous and sequential chemotherapy. The chemotherapy regimen was platinum-based single-agent or combination chemotherapy; the platinum-based drugs were cisplatin, carboplatin, nedaplatin, and lobaplatin, while the combination drugs were paclitaxel, gemcitabine, docetaxel, and vincristine. According to the general situation of the patient and the patient’s wishes, the treatment regimen was selected. Among the whole group, 155 patients received chemotherapy, 74 of whom were treated with synchronous chemotherapy and 81 with sequential chemotherapy for 1–6 cycles, with a median of 4 cycles.
Observation indicators
Routine blood and liver and kidney function results (including hemoglobin count, albumin count, lymphocyte count, and platelet count) were collected within 1 week before radiotherapy, and the patients’ pre-radiotherapy HALP scores were calculated separately according to the following formula: HALP score = hemoglobin (g/L) × albumin (g/L) × lymphocyte (109/L)/platelet (109/L). The PNI was calculated as follows: PNI = serum albumin value (g/L) + 5 × total peripheral blood lymphocytes (109/L). Moreover, the systemic immune-inflammation index (SII) was calculated as follows: SII = neutrophil count (109/L) × platelet count (109/L)/lymphocyte count (109/L).
Outcomes and follow-up
The outcome evaluation was performed by reviewing chest CT and related imaging within 1 month after radiotherapy. Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, which is divided into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), was applied. All patients were followed up regularly by outpatient or telephone every 3 months for 2 years after radiotherapy and every 6 months thereafter. All cases were followed up until October 31, 2022. OS was defined as the time from the start of radiotherapy to the patient’s death or the last follow-up visit, and progression-free survival (PFS) was defined as the time from the start of radiotherapy to the first disease progression (tumor recurrence, progression, distant metastasis etc.), death from any cause, or the last follow-up visit.
Statistical analysis
The statistical analysis of all data was performed using SPSS 25.0 software (IBM Corporation, Armonk, NY, USA). Comparisons between groups for clinicopathological characteristics were performed using the χ2 test or the Fisher exact test; the optimal truncated values for continuous variables were obtained using X-tile version 3.6.1 software (19) (Yale University, New Haven, CT, USA). The Kaplan-Meier method was applied for survival analysis, and the log-rank method was used to test for survival differences. In univariate and multivariate analyses, the Cox proportional hazards model (with backward selection) was used to assess the independent risk factors for OS and PFS. All statistical tests were two-sided probability tests with a test level of α=0.05, and P<0.05 indicated a statistically significant difference.
Results
Optimal truncation value for continuous variables
The values of HALP score, PNI, and SII were calculated according to their respective formulae. The median value of the HALP score was 29.43, with a range of 3.88–122.38; the median value of PNI was 45.22, with a range of 27.50–65.43; the median value of SII was 805.63, with a range of 140.95–6,081.95. The results showed that the cutoff values for OS and PFS of the HALP score, PNI, and SII were 24.3, 45.2, and 865.4, respectively (Figures 1,2). According to the optimal truncation value, patients were divided into the corresponding high- and low-value groups.
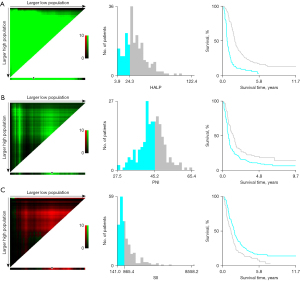
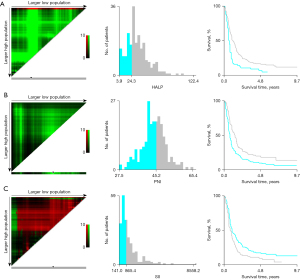
Baseline clinical characteristics of the whole group of patients
A total of 206 patients with stage III LUSC treated with radical radiotherapy were included in this study, including 193 males and 13 females. There were 79 patients in the low-HALP-score group, accounting for 38.3% of all patients, and 127 patients in the high-HALP-score group, accounting for 61.7% of all patients (Figure 3). The mean age at presentation for the whole group was 61 years, and the age range at presentation was 28–83 years. The majority (80.6%) of patients had a history of smoking, and 36.4% of the patients had a history of alcohol consumption. About half (n=96) of the patients in the study population had stage IIIB disease, with a fewer number of patients with stage IIIC and IIIA disease, at 64 and 46 patients, respectively. After radiotherapy, the distribution of patients in terms of response status was as follows: CR, 6 cases; PR, 147 cases; SD, 50 cases; and PD, 3 cases. The objective remission rate of the entire cohort was 74.3%. The baseline clinical characteristics of the patients are shown in Table 1.
Table 1
| Variables | Total number (%) | HALP group | P | |
|---|---|---|---|---|
| Low-HALP score group (%) | High-HALP score group (%) | |||
| Gender | 0.63 | |||
| Female | 13 (6.3) | 6 (7.6) | 7 (5.5) | |
| Male | 193 (93.7) | 73 (92.4) | 120 (94.5) | |
| Age | 0.06 | |||
| ≤60 years | 100 (48.5) | 26 (32.9) | 74 (58.3) | |
| >60 years | 106 (51.5) | 53 (67.1) | 53 (41.7) | |
| Smoking history | 0.08 | |||
| No | 40 (19.4) | 18 (22.8) | 22 (17.3) | |
| Yes | 166 (80.6) | 61 (77.2) | 105 (82.7) | |
| Alcohol consumption history | 0.03 | |||
| No | 131 (63.6) | 54 (68.4) | 77 (60.6) | |
| Yes | 75 (36.4) | 25 (31.6) | 50 (39.4) | |
| T stage | 0.11 | |||
| T1 | 6 (2.9) | 3 (3.8) | 3 (2.4) | |
| T2 | 30 (14.6) | 6 (7.6) | 24 (18.9) | |
| T3 | 71 (34.5) | 30 (38.0) | 41 (32.3) | |
| T4 | 99 (48.1) | 40 (50.6) | 59 (46.5) | |
| N stage | 0.08 | |||
| N0–1 | 27 (13.1) | 9 (11.4) | 18 (14.2) | |
| N2 | 102 (49.5) | 29 (36.7) | 73 (57.5) | |
| N3 | 77 (37.4) | 41 (51.9) | 36 (28.3) | |
| TNM stage | 0.02 | |||
| IIIA | 46 (22.3) | 16 (20.3) | 30 (23.6) | |
| IIIB | 96 (46.6) | 26 (32.9) | 70 (55.1) | |
| IIIC | 64 (31.1) | 37 (46.8) | 27 (21.3) | |
| Prescription dose | 0.58 | |||
| ≤60 Gy | 135 (65.5) | 45 (57.0) | 90 (70.9) | |
| >60 Gy | 71 (34.5) | 34 (43.0) | 37 (29.1) | |
| Concurrent chemotherapy | 0.051 | |||
| No | 50 (24.3) | 27 (34.2) | 23 (18.1) | |
| Yes | 156 (75.7) | 52 (65.8) | 104 (81.9) | |
| Radiotherapy efficacy | 0.43 | |||
| CR + PR | 153 (74.3) | 58 (73.4) | 95 (74.8) | |
| SD | 50 (24.3) | 18 (22.8) | 32 (25.2) | |
| PD | 3 (1.4) | 3 (3.8) | 0 | |
| PNI score | 0.001 | |||
| <45.2 | 120 (58.2) | 55 (69.6) | 65 (51.2) | |
| ≥45.2 | 86 (41.7) | 24 (30.4) | 62 (48.8) | |
| SII score | <0.001 | |||
| ≤865.4 | 111 (53.9) | 22 (27.8) | 99 (78.0) | |
| >865.4 | 95 (46.1) | 57 (72.2) | 28 (22.0) | |
LUSC, lung squamous cell carcinoma; HALP, hemoglobin, albumin, lymphocyte, and platelet score; TNM, tumor-node-metastasis; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PNI, prognostic nutritional index; SII, systemic immune-inflammation index.
The low-HALP-score group (<24.3) and the high-HALP-score group (≥24.3) were further compared in terms of gender, age, smoking history, alcohol consumption history, and TNM stage. The results suggested that patients in the high-HALP-score group were more likely to have a high PNI (P=0.001) and a low SII (P<0.001), while HALP score was significantly associated with patients’ alcohol consumption history (P=0.03) and TNM stage (P=0.02); however, there were no significant correlations with gender, age, smoking history, T stage, N stage, concurrent chemotherapy, prescription dose or radiotherapy efficacy (Table 1).
Survival analysis
All patients were followed up until October 31, 2022; two cases were lost to follow-up, The median follow-up time was 18.5 months, ranging from 2 months to 138 month, and 176 patients died. The median OS for the whole group was 19.0 months [95% confidence interval (CI): 16.16–21.83 months] with a range of 2–138 months, and the OS rates at 1, 2, and 3 years were 65.3%, 36.1%, and 20.2%, respectively. The median PFS for the whole group was 11.0 months (95% CI: 9.26–12.73 months), and the PFS rates at 1, 2, and 3 years were 42.6%, 22.0%, and 17.7%, respectively.
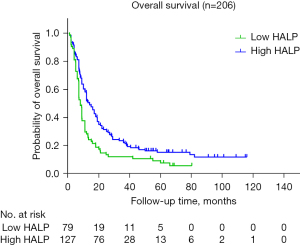
Further analysis revealed that the median OS was 11.0 months (95% CI: 6.89–15.11 months) and 22.0 months (95% CI: 20.36–23.63 months) in the low- and high-HALP-score groups, respectively. The 1-, 2-, and 3-year OS rates were 48.6%, 21.6%, and 16.2% in the low-HALP-score group, respectively, while those in the high-HALP-score group were 72.6%, 43.7%, and 25.3%, respectively. Kaplan-Meier curves were constructed, and log-rank tests showed significant differences between groups with low and high HALP scores (P<0.001) (Figure 4).
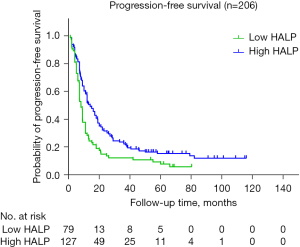
The median PFS was 8.0 months (95% CI: 7.07–8.92 months) and 13.0 months (95% CI: 10.15–15.81 months) in the low-HALP-score and high-HALP-score groups, respectively. In the low-HALP-score group, 1-, 2-, and 3-year PFS rates were 28.3%, 13.5%, and 9.5%, respectively, while those in the high-HALP-score group were 50.3%, 30.1%, and 20.5%, respectively. Kaplan-Meier curves were constructed, and log-rank tests showed significant differences between the low- and high-HALP-score groups (P=0.002) (Figure 5).
In the univariate Cox proportional hazards model for OS, a low HALP score was found to be significantly associated with a poor prognosis [hazard ratio (HR) =1.698, 95% CI: 1.261–2.286; P<0.001]. Meanwhile, the following variables were associated with OS: later N stage, TNM stage, no concurrent chemotherapy, poor radiotherapy efficacy, lower PNI score, and higher SII score (Table 2).
Table 2
| Variables | OS | PFS | |||
|---|---|---|---|---|---|
| aHR (95% CI) | P | aHR (95% CI) | P | ||
| Gender | |||||
| Female | 1 (Reference) | NA | 1 (Reference) | NA | |
| Male | 1.423 (0.786–2.624) | 0.23 | 1.413 (0.763–2.615) | 0.27 | |
| Age | |||||
| ≤60 years | 1 (Reference) | NA | 1 (Reference) | NA | |
| >60 years | 1.151 (0.866–1.529) | 0.33 | 1.058 (0.796–1.405) | 0.67 | |
| Smoking history | |||||
| No | 1 (Reference) | NA | 1 (Reference) | NA | |
| Yes | 1.123 (0.799–1.578) | 0.51 | 1.096 (0.793–1.569) | 0.53 | |
| Alcohol consumption history | |||||
| No | 1 (Reference) | NA | 1 (Reference) | NA | |
| Yes | 1.264 (0.938–1.704) | 0.12 | 1.325 (0.983–1.785) | 0.07 | |
| T stage | |||||
| T1 | 1 (Reference) | NA | 1 (Reference) | NA | |
| T2 | 1.158 (0.443–3.027) | 0.76 | 1.052 (0.402–2.749) | 0.92 | |
| T3 | 2.052 (0.826–5.094) | 0.12 | 1.818 (0.732–4.516) | 0.20 | |
| T4 | 2.112 (0.858–5.199) | 0.10 | 1.886 (0.765–4.647) | 0.17 | |
| N stage | |||||
| N0–1 | 1 (Reference) | NA | 1 (Reference) | NA | |
| N2 | 1.165 (0.742–1.827) | 0.51 | 1.174 (0.748–1.842) | 0.49 | |
| N3 | 1.929 (1.217–1.827) | 0.005 | 1.822 (1.151–2.885) | 0.01 | |
| TNM stage | |||||
| IIIA | 1 (Reference) | NA | 1 (Reference) | NA | |
| IIIB | 1.220 (0.842–1.767) | 0.29 | 1.206 (0.833–1.746) | 0.32 | |
| IIIC | 2.250 (1.526–3.317) | <0.001 | 2.014 (1.368–2.964) | <0.001 | |
| Prescription dose | |||||
| ≤60 Gy | 1 (Reference) | NA | 1 (Reference) | NA | |
| >60 Gy | 0.791 (0.591–1.059) | 0.12 | 0.816 (0.610–1.093) | 0.17 | |
| Concurrent chemotherapy | |||||
| No | 1 (Reference) | NA | 1 (Reference) | NA | |
| Yes | 0.589 (0.428–0.810) | 0.001 | 0.680 (0.495–0.934) | 0.02 | |
| Radiotherapy efficacy | |||||
| CR + PR | 1 (Reference) | NA | 1 (Reference) | NA | |
| SD | 1.224 (0.883–1.698) | 0.23 | 1.219 (0.879–1.691) | 0.24 | |
| PD | 2.865 (1.157–7.090) | 0.02 | 3.858 (1.565–9.509) | 0.003 | |
| PNI score | |||||
| <45.2 | 1 (Reference) | NA | 1 (Reference) | NA | |
| ≥45.2 | 2.304 (1.710–3.105) | <0.001 | 2.214 (1.643–2.982) | <0.001 | |
| SII score | |||||
| ≤865.4 | 1 (Reference) | NA | 1 (Reference) | NA | |
| >865.4 | 1.458 (1.098–1.936) | 0.009 | 1.371 (1.032–1.822) | 0.03 | |
| HALP score | |||||
| ≥24.3 | 1 (Reference) | NA | 1 (Reference) | NA | |
| <24.3 | 1.698 (1.261–2.286) | <0.001 | 1.584 (1.176–2.132) | 0.002 | |
OS, overall survival; PFS, progression-free survival; LUSC, lung squamous cell carcinoma; aHR, adjusted hazard ratio; CI, confidence interval; NA, not available; TNM, tumor-node-metastasis; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; HALP, hemoglobin, albumin, lymphocyte, and platelet.
In the univariate Cox proportional hazards model of PFS, a low HALP score was found to be significantly associated with poor prognosis (HR =1.584, 95% CI: 1.176–2.132; P=0.002). Meanwhile, the following variables were associated with PFS: later N stage, TNM stage, no concurrent chemotherapy, poor radiotherapy efficacy, lower PNI score, and higher SII score (Table 2).
Factors identified as significant in the univariate analysis were further analyzed, and incorporated into the Cox multivariate regression model. The results indicated that a low HALP score was an independent risk factor for OS (HR =1.538, 95% CI: 1.137–2.079; P=0.005) and PFS (HR =1.399, 95% CI: 1.033–1.895; P=0.03). In addition, the independent risk factors for OS were low PNI score (HR =2.010, 95% CI: 1.479–2.732; P<0.001), advanced TNM stage (HR =1.921, 95% CI: 1.292–2.875; P=0.001), and no concurrent chemotherapy (HR =0.705, 95% CI: 0.511–0.973; P=0.03) (Table 3). Meanwhile, the independent risk factors for PFS were a low PNI score (HR =1.966, 95% CI: 1.446–2.673; P<0.001) and advanced TNM stage (HR =1.709, 95% CI: 1.152–2.543; P=0.008) (Table 4).
Table 3
| Variables | aHR (95%CI) | P |
|---|---|---|
| HALP score | ||
| ≥24.3 | 1 (Reference) | NA |
| <24.3 | 1.538 (1.137–2.079) | 0.005 |
| PNI score | ||
| ≥45.2 | 1 (Reference) | NA |
| <45.2 | 2.010 (1.479–2.732) | <0.001 |
| TNM stage | ||
| IIIA | 1 (Reference) | NA |
| IIIB | 1.140 (0.785–1.655) | 0.49 |
| IIIC | 1.921 (1.292–2.875) | 0.001 |
| Concurrent chemotherapy | ||
| No | 1 (Reference) | NA |
| Yes | 0.705 (0.511–0.973) | 0.03 |
OS, overall survival; LUSC, lung squamous cell carcinoma; aHR, adjusted hazard ratio; CI, confidence interval; NA, not available; HALP, hemoglobin, albumin, lymphocyte, and platelet; PNI, prognostic nutritional index; TNM, tumor-node-metastasis.
Table 4
| Variables | aHR (95% CI) | P |
|---|---|---|
| HALP score | ||
| ≥24.3 | 1 (Reference) | NA |
| <24.3 | 1.399 (1.033–1.895) | 0.03 |
| PNI score | ||
| ≥45.2 | 1 (Reference) | NA |
| <45.2 | 1.966 (1.446–2.673) | <0.001 |
| TNM stage | ||
| IIIA | 1 (Reference) | NA |
| IIIB | 1.180 (0.814–1.710) | 0.38 |
| IIIC | 1.709 (1.152–2.543) | 0.008 |
PFS, progression-free survival; LUSC, lung squamous cell carcinoma; aHR, adjusted hazard ratio; CI, confidence interval; NA, not available; HALP, hemoglobin, albumin, lymphocyte, and platelet; PNI, prognostic nutritional index; TNM, tumor-node-metastasis.
Discussion
In recent years, rapid advances have been made in the treatment of lung cancer, but the prognosis of some patients remains poor. Therefore, developing suitable biomarkers to assist in prognostic prediction for the patients is critical to developing individualized treatment plans. Numerous studies have shown that the degree of systemic inflammation can reflect the dynamic balance between antitumor and protumor functions of the body and is inseparable from tumor development (4,20). Meanwhile, tumor-related malnutrition and underlying metabolic alterations in tumors likewise influence tumor invasion, metastasis, and treatment (5,6). HALP score, which combines measures of hemoglobin, lymphocyte, platelets, and albumin, can reflect the inflammatory status and nutritional status of the body in a comprehensive manner and has been shown to correlate with patient prognosis in a variety of cancers, including gastrointestinal tumors (16,21) and urological tumors (17,18). However, its use in lung cancer is less common, especially for LUSC squamous lung cancer, and has not been extensively reported. This study was the first of its kind to examine HALP scores in patients with stage III LUSC treated with radical radiotherapy and was aimed at assessing the prognostic significance of baseline HALP scores in this population. The cutoff HALP values were determined in the study using X-Tile software, and the study population was divided into a high-HALP-score group and a low-HALP-score group. Survival analysis showed that patients with low HALP scores had a relatively poor OS and PFS, and the differences were statistically significant. Cox multifactorial analysis showed that the baseline HALP score could be used as a predictor of survival in patients with stage III LUSC treated with radical radiotherapy.
Xu et al. (21) retrospectively analyzed the clinical data of 582 patients with pancreatic cancer who had undergone radical resection; the cutoff value of HALP in this study was 44.56, and the results showed that low HALP score was associated with early recurrence and short survival after pancreatic cancer surgery and was independent risk factor for early recurrence and short-term survival. In another study on the prognosis of patients with prostate cancer, Bendari et al. (18) used a cutoff value of 22.2 for the HALP score. Their results indicated that a low HALP score is an independent prognostic factor for both patients with multiple metastases and limited metastases of prostate cancer. Similar results were obtained in another study on gastric mesenchymal cell tumors (16), which included a total of 591 patients with gastrointestinal mesenchymal tumors who underwent radical resection. The cutoff value for HALP score was 31.5, and a low HALP score was demonstrated to be an independent risk factor for poor recurrence-free survival. In another study on the prognosis of patients with esophageal squamous carcinoma, the cutoff value for the HALP score was 31.8. The results of the study suggested that preoperative HALP score is an independent prognostic indicator for patients with esophageal squamous carcinoma undergoing radical resection (15).
Our study was a retrospective analysis of 206 patients with stage III LUSC who received radical radiotherapy, and a HALP score cutoff value of 23.2 was used. It was found that the OS and PFS of patients in the group with high HALP score before radiotherapy were significantly higher than those in the group with a low score; moreover, HALP score before radiotherapy was an independent risk factor for OS and PFS, which was consistent with the results of the studies mentioned above.
We also analyzed the association of PNI and SII with patient prognosis. PNI score, which includes measures of both serum albumin (22,23) and peripheral blood lymphocytes, was initially used to predict the risk of complications after gastrointestinal surgery (24) and has been confirmed in recent years by several studies to be significantly associated with prognosis in various solid tumors, including esophageal (25), colorectal (26), and gastric cancers (14). Our study also confirmed PNI to be significantly associated with survival prognosis, and we found that it was an independent risk factor for OS and PFS in patients with stage III LUSC treated with radical radiotherapy.
SII is a combination of peripheral blood lymphocyte, neutrophil, and platelets, and it was found that compared with traditional predictors such as PLR and NLR, SII can more objectively reflect the balance between the patient’s inflammatory response and immune response; moreover, it has been shown to be associated with poor prognosis in various solid tumors, including lung cancer, gastric cancer, cervical cancer, bladder cancer, and colorectal cancer. Our findings also indicated that pre-radiotherapy SII was an independent influencing factor for OS in patients with stage III LUSC receiving radical radiotherapy, which is consistent with the results of previous studies (27-31).
This study involved certain limitations which should be addressed. First, the cutoff values of HALP scores were calculated by X-tile software according to the baseline blood parameters of 206 patients included in this study, but there is no uniform standard at this stage. Second, our study did not explore the trend of HALP score during radiotherapy and its relationship with survival prognosis or the relationship between adverse effects of radiotherapy and HALP score. Third, there is a potential bias in the low-HALP group, where more patients received sequential radiation compared to the high-HALP group. Finally, the patients in this study were from a single center and the sample size was relatively small, which might have led to selection bias.
Conclusions
This study was the first to investigate the utility of HALP score in predicting the prognosis of patients with stage III LUSC. The results showed that HALP score was significantly correlated with patients’ OS and PFS and was an independent factor for OS. Therefore, HALP score can be used as a simple, feasible, and effective prognostic indicator in clinical practice for the treatment of stage III LUSC. Future multicenter, large-sample, and prospective studies are needed to validate the prognostic value of HALP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1513/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1513/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1513/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1513/coif). A.O. received honoraria from AstraZeneca, Chugai Pharmaceutical, MSD, Ono Pharmaceutical, and Bristl Meiers Squibb; also received research grants from Chugai Pharmaceutical, Taiho Pharma, Kyowa Kirin, Boehringer Ingelheim, Daiichi Sankyo, Nippon Kayaku, and Lilly. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2020KS024) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res 2019;11:943-53. [Crossref] [PubMed]
- Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin 2022;72:409-36. [Crossref] [PubMed]
- Dolan RD, McSorley ST, Horgan PG, et al. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;116:134-46. [Crossref] [PubMed]
- Hao S, Ge P, Su W, et al. Steady-State Delivery and Chemical Modification of Food Nutrients to Improve Cancer Intervention Ability. Foods 2024;13:1363. [Crossref] [PubMed]
- Rinninella E, Cintoni M, Raoul P, et al. Effects of nutritional interventions on nutritional status in patients with gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN 2020;38:28-42. [Crossref] [PubMed]
- Song H, Jeong MJ, Cha J, et al. Preoperative neutrophil-to-lymphocyte, platelet-to-lymphocyte and monocyte-to-lymphocyte ratio as a prognostic factor in non-endometrioid endometrial cancer. Int J Med Sci 2021;18:3712-7. [Crossref] [PubMed]
- Yang L, Huang Y, Zhou L, et al. High pretreatment neutrophil-to-lymphocyte ratio as a predictor of poor survival prognosis in head and neck squamous cell carcinoma: Systematic review and meta-analysis. Head Neck 2019;41:1525-35. [Crossref] [PubMed]
- Ma SJ, Yu H, Khan M, et al. Evaluation of Optimal Threshold of Neutrophil-Lymphocyte Ratio and Its Association With Survival Outcomes Among Patients With Head and Neck Cancer. JAMA Netw Open 2022;5:e227567. [Crossref] [PubMed]
- Yamamoto T, Kawada K, Obama K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int J Mol Sci 2021;22:8002. [Crossref] [PubMed]
- Zhou K, Cao J, Lin H, et al. Prognostic role of the platelet to lymphocyte ratio (PLR) in the clinical outcomes of patients with advanced lung cancer receiving immunotherapy: A systematic review and meta-analysis. Front Oncol 2022;12:962173. [Crossref] [PubMed]
- Hu M, Xu Q, Yang S, et al. Pretreatment systemic inflammation response index (SIRI) is an independent predictor of survival in unresectable stage III non-small cell lung cancer treated with chemoradiotherapy: a two-center retrospective study. Ann Transl Med 2020;8:1310. [Crossref] [PubMed]
- Zhang L, Ma W, Qiu Z, et al. Prognostic nutritional index as a prognostic biomarker for gastrointestinal cancer patients treated with immune checkpoint inhibitors. Front Immunol 2023;14:1219929. [Crossref] [PubMed]
- Zheng Z, Zhu H, Cai H. Preoperative Prognostic Nutritional Index Predict Survival in Patients With Resectable Esophageal Squamous Cell Carcinoma. Front Nutr 2022;9:824839. [Crossref] [PubMed]
- Feng JF, Wang L, Yang X. The preoperative hemoglobin, albumin, lymphocyte and platelet (HALP) score is a useful predictor in patients with resectable esophageal squamous cell carcinoma. Bosn J Basic Med Sci 2021;21:773-81. [Crossref] [PubMed]
- Zhao Z, Yin XN, Wang J, et al. Prognostic significance of hemoglobin, albumin, lymphocyte, platelet in gastrointestinal stromal tumors: A propensity matched retrospective cohort study. World J Gastroenterol 2022;28:3476-87. [Crossref] [PubMed]
- Acar O, Ayhan M, Demir B, et al. HALP Score as a New Prognostic Factor for Patients with Metastatic Bladder Cancer. J Coll Physicians Surg Pak 2023;33:1405-9. [Crossref] [PubMed]
- Bendari A, Bendari A, Zhong X, et al. Hemoglobin-Albumin-Lymphocyte-Platelet (HALP) Score as a Predictive Value of Incidental Prostate Cancer for Patients Going for Transurethral Resection of the Prostate (TURP): A Single-Center Study. Cureus 2024;16:e57736. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Kou J, Huang J, Li J, et al. Systemic immune-inflammation index predicts prognosis and responsiveness to immunotherapy in cancer patients: a systematic review and meta-analysis. Clin Exp Med 2023;23:3895-905. [Crossref] [PubMed]
- Xu SS, Li S, Xu HX, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol 2020;26:828-38. [Crossref] [PubMed]
- Domblides C, Lartigue L, Faustin B. Control of the Antitumor Immune Response by Cancer Metabolism. Cells 2019;8:104. [Crossref] [PubMed]
- Zhang TN, Yin RH, Wang LW. The prognostic and predictive value of the albumin-bilirubin score in advanced pancreatic cancer. Medicine (Baltimore) 2020;99:e20654. [Crossref] [PubMed]
- Yang X, Song X, Zhang L, et al. Prognostic role of the pretreatment C-reactive protein/albumin ratio in gastric cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e19362. [Crossref] [PubMed]
- Oyama K, Oba M, Oshima Y, et al. Predicting short-term life expectancy of patients with end-stage gastric cancer using Onodera's prognostic nutritional index. Int J Clin Oncol 2021;26:364-9. [Crossref] [PubMed]
- Li J, Zhu N, Wang C, et al. Preoperative albumin-to-globulin ratio and prognostic nutritional index predict the prognosis of colorectal cancer: a retrospective study. Sci Rep 2023;13:17272. [Crossref] [PubMed]
- Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal 2019;33:e22964. [Crossref] [PubMed]
- Qiu Y, Zhang Z, Chen Y. Prognostic Value of Pretreatment Systemic Immune-Inflammation Index in Gastric Cancer: A Meta-Analysis. Front Oncol 2021;11:537140. [Crossref] [PubMed]
- Huang H, Liu Q, Zhu L, et al. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer. Sci Rep 2019;9:3284. [Crossref] [PubMed]
- Huang Y, Gao Y, Wu Y, et al. Prognostic value of systemic immune-inflammation index in patients with urologic cancers: a meta-analysis. Cancer Cell Int 2020;20:499. [Crossref] [PubMed]
- Yordanagil M, Bakir H, Güler Avci G, et al. Do haematological parameters such as HALP and Lymphocyte to C-reactive protein ratio predict tumor response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer? Pol Przegl Chir 2022;95:1-5. [Crossref] [PubMed]



