Modern radiotherapy using image guidance for unresectable non-small cell lung cancer can improve outcomes in patients treated with chemoradiation therapy
Introduction
Non-small cell lung cancer (NSCLC) continues to be a leading cause of cancer deaths in Americans each year. Approximately 30% of patients initially present with stage III, locally advanced disease (1,2). Radiation therapy (RT) is an effective treatment for solid malignancies, and in lung cancer, the addition of concurrent chemotherapy results in better outcomes than sequential or either treatment alone (3-5). However, the advances in treatment outcomes from concurrent chemoradiation therapy (CRT) come with increases in toxicity rates (3,4,6). Furthermore, avoiding toxicity during RT for thoracic tumors poses a unique set of difficulties due to tumor motion throughout the breathing cycle, the large number of adjacent organs at risk, and possible changes in patient anatomy due to variations in weight during the course of RT (7,8). These changes in tumor position and size during treatment can lead to inadequate tumor coverage (9), as well as larger RT doses to normal tissues, causing higher rates of toxicity (10,11). These problems justify the need for image-guided radiotherapy (IGRT), which may provide greater accuracy.
Technological advances including respiratory gating, four-dimensional computed tomography (4DCT) simulation, motion management techniques (abdominal compression, respiratory gating, and the active breathing coordinator) and IGRT represent techniques developed with the intent of combating the difficulties associated with tumor motion and accurate localization. However, relatively few studies have fully evaluated the efficacy of these treatments. Therefore, we retrospectively reviewed our treatment data to assess the impact of RT-associated technological IGRT advances on overall survival and local progression among patients with NSCLC treated with CRT. We hypothesized that daily orthogonal kilovoltage (kV) image guidance would improve outcomes compared to standard of care weekly megavoltage (MV) image guidance for inoperable NSCLC treated with definitive CRT.
Methods
Patient population
We reviewed a cohort of 159 patients with histologically confirmed NSCLC that were treated between 1/2002 to 9/2012 using an Institutional Review Board (IRB)-approved protocol for this retrospective review. All patients received definitive concurrent CRT. Patients who received cone beam computed tomography (CBCT) for image guidance (n=68) were excluded because the short median follow up time (11.8 months) in this subgroup was less than 12 months (range, 0.23–36.5 months), leaving 91 patient records to evaluate. We subsequently collected pertinent information on patient demographics, tumor characteristics, and treatment plans for patients included in the study. We included patients with stage II node positive (n=4) NSCLC who received definitive CRT. Additionally, we included patients with stage IV oligometastatic NSCLC (n=6), defined as those with a solitary extrathoracic metastasis, who received definitive CRT to the primary tumor and metastatic focus. We included these patients based on the knowledge that they may achieve long-term survival rates comparable to stage III NSCLC (12-14).
Pre-treatment evaluation
Pre-CRT work-up included a complete history and physical examination, complete blood count, serum chemistry profile, chest X-ray, chest computed tomography (CT) scan, positron emission tomography (PET) (82% of patients) scan, and biopsy of mass or nodes. We based our clinical staging on the American Joint Committee on Cancer 6th-edition criteria (15).
Treatment planning
Patients were placed in a supine position with arms up to allow accurate reproducibility of the target lesion among treatment sessions. A large rigid mold was created for each patient. Planning target volumes (PTV) were 0.5 cm beyond the clinical target volume (CTV) for patients treated with 4DCT and IGRT. For patients who did not undergo 4DCT simulation, PTV was 1 cm beyond the CTV. We used three dimensional conformal radiotherapy (3D-CRT) or intensity modulated radiation therapy (IMRT) to deliver the RT through anteroposterior fields first to 40 Gray (Gy) in 1.8 or 2 Gy per fraction per day followed by oblique fields to avoid the spinal cord for an additional 20–26 Gy to a typical total RT dose of 60–66 Gy. If patients presented with bilateral mediastinal lymph node involvement, then we employed IMRT either from the onset of RT or for the boost/off-cord component of the RT. Photon beams of 6- or 15-MV energies were employed to deliver the RT. Tissue inhomogeneity was taken into account with the analytic anisotropic algorithm in the dose calculations of treatment planning. The radiation dose for the spinal cord was <50 Gy. The mean lung dose was <20 Gy, lung V5 <60–70% and lung V20 <37%.
The typical chemotherapy regimen consisted of intravenous infusional drug delivery consisting of weekly paclitaxel (45 mg/m2) plus carboplatin (AUC =2) or etoposide days 1–5 and 29–33 (50 mg/m2) plus cisplatin day 1, 8, 29, and 36 (50 mg/m2). RT was delivered after the administration of chemotherapy. Both of these chemotherapy regimens were included based on the knowledge that they achieve similar survival results (16).
For RT administration, we imaged patients with either MV films weekly to ascertain portal field shapes as well as patient position setup accuracy, or orthogonal kV films daily as part of IGRT. kV imaging was conducted daily, which was defined as at least four images per week. MV imaging consisted of weekly portal imaging of all the treatment fields. The kV imaging included in this study was only orthogonal kV imaging. No CBCT imaging was included as the median follow up for CBCT was thought to be insufficient (<12 months). Choice of imaging used was based on physician preference as three expert attending physicians were managing patients treated with definitive CRT for inoperable NSCLC.
Statistical analysis
We conducted initial analyses of baseline characteristics (contingency tables) of patients using the Fisher exact test and their corresponding P values are presented. We first analyzed these data for the all patients, followed by comparisons of patients based on IGRT technique. Normality of continuous variables was assessed using the Shapiro-Wilks test. Normally distributed variables were compared using a t-test, while non-normally distributed variables were compared using the Wilcoxon signed rank test. We computed unadjusted Kaplan-Meier curves for product limit survival estimates and locoregional failure, defined from the end of CRT to the event of interest (death or locoregional failure) or last follow up time. These curves were stratified by IGRT technique. Log-rank tests were used to compare strata. We developed Cox Proportional Hazards models for overall survival and locoregional failure. The number of variables in the multi-variable models was determined based on the number of events of interest, which is 1 variable per 10 events. Variables with the lowest P values from univariate models were added to the multi-variable model, however when P values were similar, variables with more clinical significance were included. However, not all significant variables were included in the multivariate analysis. In order for a variable to be a confounding factor, it should affect the association between the outcome and the main predictor (kV/MV status) as well as it should be correlated with outcome and the main predictor. Proportional hazard assumptions were checked by plotting Schoenfeld residuals. All analyses were conducted using SAS v9.3 (SAS Institute, Cary, NC) and the statistical level of significance was <0.05.
Results
Imaging technique frequency
Table 1 shows the frequency by which various imaging techniques were used in this patient population. Fifty-four percent (n=49) of patients received only weekly MV portal imaging during treatment, while 46% (n=42) underwent IGRT using orthogonal kV imaging. Twenty-three percent (n=21) of patients underwent 4DCT simulations, while 77% (n=70) of patients did not. 4DCT was also more common with kV imaging, mostly likely because 4DCT became available in 2011 at our institution. PET scan use was also more common in the kV group (P=0.02).
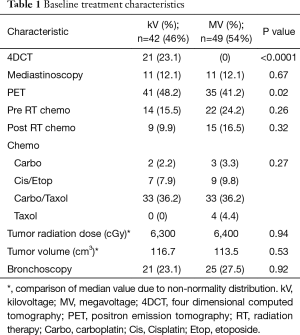
Full table
Baseline characteristics
Median follow up time was 16.3 months (range, 0.3–139.2 months). Baseline characteristics according to IGRT technique are shown in Table 2. Mean age was 65.9 (±10.7) years for the kV group and 63.5 (±13.0) years from the MV group (P=0.34). Median tumor volume was 116.7 cm3 (range 0.73–1,245) in the kV group and 113.5 cm3 (range, 0.86–903 cm3) in the MV group (P=0.53). Median RT dose was 6,300 cGy (range, 5,040–7,020 cGy) in the kV group and 6,400 cGy (range, 5,200–6,840 cGy) in the MV group (P=0.94) (Table 1). Males had a higher distribution of treatment with MV imaging (P=0.05). The distribution of other clinical parameters showed no remarkable differences between the imaging groups (Table 2).
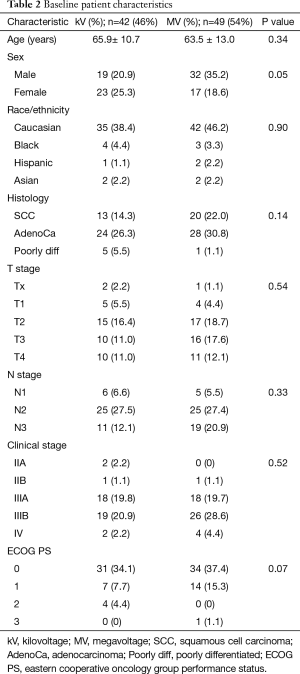
Full table
Impact of imaging technique on survival
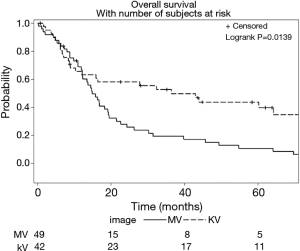
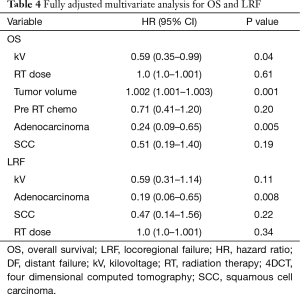
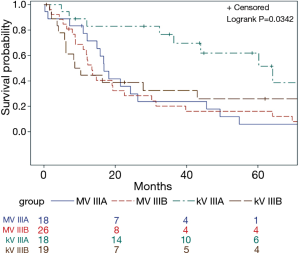
Imaging with daily orthogonal kV’s was associated with a decreased risk of mortality on univariate analysis [hazard ratio (HR) 0.55; 95% CI (0.34–0.89); P=0.02] (Table 3). Median survival for patients who underwent daily orthogonal kV imaging was 36.4 months (95% CI (10.3–78.2)] compared to 14.9 months [95% CI (12.1–19.3); P=0.01] for those who underwent weekly MV imaging (Figure 1). On multivariate analysis (Table 4), when adjusting for RT dose, tumor volume, Pre RT chemotherapy, and histology, kV imaging demonstrated an association with decreased mortality [HR 0.59; 95% CI (0.35–0.99); P=0.04]. In this model, increasing tumor volume [HR 1.002; 95% CI (1.001–1.003); P=0.001] was associated with increased mortality, while adenocarcinoma histology [HR 0.24; 95% CI (0.006–0.65); P=0.005] was associated with lower mortality. Stage IIIA disease imaged with kV (Figure S1), was associated with the longest survival [64.1 months; 95% CI (32.4–78.2 months)] compared to kV stage IIIB [9.4 months; 95% CI (5.8–43.0 months)], MV stage IIIA [16.8 months, 95 CI (12.1–26.3 months)], and MV stage IIIB [13.4 months, 95% CI (8.8–22.6 months); P=0.03].
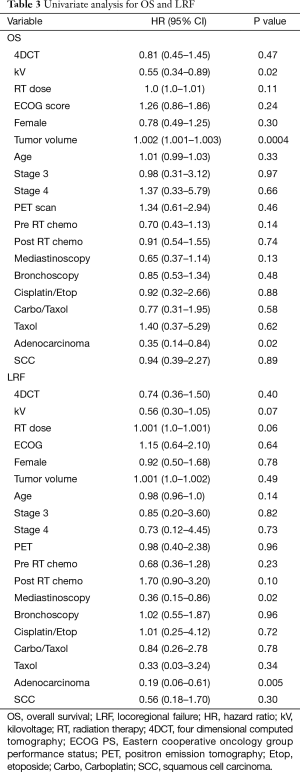
Full table
Full table
Impact of imaging technique on progression
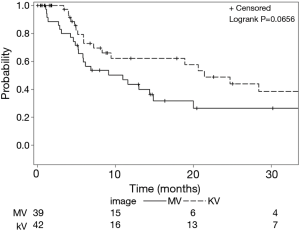
Use of kV imaging also showed a marginal association with lower risk of locoregional failure. Median time to locoregional progression (Figure 2) in patients imaged with kV was 21.4 months [95% CI (8.3–71.2 months)] compared to 10.9 months [95% CI (5.3–14.9 months)] in patients imaged with MV (P=0.065). On multivariate analysis (Table 4), use of kV imaging was marginally associated with a lower risk of locoregional failure [HR 0.59; 95% CI (0.31–1.14); P=0.11].
Discussion
This study suggests that the use of kV image guidance associates with an improved OS and lower rates of locoregional failure. Given the similar RT techniques used between patients imaged with MV portal films and kV IGRT, we examined the differences between these imaging modalities that might explain the disparity in OS and disease control. The OS survival benefit of kV imaging probably stems from improvements in locoregional control. While distant metastasis probably plays a more important role in mortality in NSCLC, local failure is also a cause of increased mortality (17). One potential confounder in these results is that kV imaging was used more frequently in more recent years when technological advances were available. Despite adjusting for these variables, such as PET scan and 4DCT, they may still have provided survival benefits to those patients for whom these technologies were employed.
There are several potential advantages to kV IGRT. kV imaging is associated with lower radiation exposure compared to MV portal film verification because the radiation dose per kV image (0.01–0.1 cGy) is smaller than that of an MV image (1–5 cGy) (18). This allows IGRT with orthogonal kV imaging to be done daily as opposed to MV imaging, which is typically done weekly. Studies have demonstrated that the use of daily imaging results in optimal setup margins compared to weekly imaging (19,20). In lung cancer patients, less than daily image guidance results in 20–43% of fractions incurring setup errors of 5 mm or greater (20). However, when daily imaging was used, errors were reduced to 6% (20).
Barney et al. compared daily shifts in prostate cancer patients treated with CBCT to kV imaging and found that daily shifts differ enough between the two modalities to effect target coverage in 25% of cases (21). Given that CBCT and kV imaging were done at similar time intervals in this study, there are probably other factors in addition to frequency at play causing daily kV imaging to result in improved outcomes compared to weekly MV imaging. One of these factors is that orthogonal kV imaging generally has better contrast and image quality than MV imaging. For example, Pisani et al found that interobserver alignment was more variable with MV imaging than kV imaging (22), which can lead to increased set up uncertainties (23). Song et al. also found that use of MV imaging associated with larger segmented volumes and increased variability in local delineation in prostate cancer patients (24). Enhanced image contrast for soft tissues with low to moderate imaging doses also allows for improved patient set up accuracy and alignment of the target volume within the reference frame of the treatment beam (25), which argues for the benefit of daily kV image guidance. Imaging with kV films also allows for better representation of bony structures and identification of objects such as fiducial markers (23), which can result in better patient outcomes. For example, kV imaging with fiducial markers translates to improvements in biochemical tumor control in high-risk prostate cancer patients (26).
Finally, from a technical aspect, oblique angled MV portal imaging and orthogonal kV imaging should theoretically lead to the same level of setup accuracy. However, in reality the levels of accuracy can be different (worse for oblique angled portal imaging setup). These differences in imaging may contribute to a larger setup uncertainty with MV imaging in addition to the differences caused by image quality between kV and MV images. Therefore, IGRT using kV orthogonal imaging can provide benefits of lower exposure, better visualization of anatomy, and may allow the clinician to reduce the PTV. Thus, the improved OS and disease control seen in this study with kV imaging is most likely due to improved treatment set up accuracy over MV portal imaging.
Conclusions
In this study, the use of daily orthogonal kV imaging during CRT for inoperable NSCLC associated with better survival and disease control when compared to weekly standard of care MV imaging. The strength of the findings implies the need for larger confirmatory studies. Further study of these techniques remains necessary to better comprehend the impact of RT associated technological advances in patients with NSCLC treated with CRT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective review was approved by the Institutional Review Board (IRB) (CR00003827).
References
- Provencio M, Isla D, Sánchez A, et al. Inoperable stage III non-small cell lung cancer: Current treatment and role of vinorelbine. J Thorac Dis 2011;3:197-204. [PubMed]
- Chen VW, Ruiz BA, Hsieh MC, et al. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer 2014;120 Suppl 23:3781-92. [Crossref] [PubMed]
- Sause W, Kolesar P, Taylor S IV, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000;117:358-64. [Crossref] [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692-9. [PubMed]
- Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol 2005;23:5910-7. [Crossref] [PubMed]
- Ross CS, Hussey DH, Pennington EC, et al. Analysis of movement of intrathoracic neoplasms using ultrafast computerized tomography. Int J Radiat Oncol Biol Phys 1990;18:671-7. [Crossref] [PubMed]
- Stevens CW, Munden RF, Forster KM, et al. Respiratory-driven lung tumor motion is independent of tumor size, tumor location, and pulmonary function. Int J Radiat Oncol Biol Phys 2001;51:62-8. [Crossref] [PubMed]
- Zhang P, Yorke E, Hu YC, et al. Predictive treatment management: incorporating a predictive tumor response model into robust prospective treatment planning for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014;88:446-52. [Crossref] [PubMed]
- Gunter T, Ali I, Matthiesen C, et al. Gross tumour volume variations in primary non-small-cell lung cancer during the course of treatment with stereotactic body radiation therapy. J Med Imaging Radiat Oncol 2014;58:384-91. [Crossref] [PubMed]
- Schaake EE, Rossi MM, Buikhuisen WA, et al. Differential motion between mediastinal lymph nodes and primary tumor in radically irradiated lung cancer patients. Int J Radiat Oncol Biol Phys 2014;90:959-66. [Crossref] [PubMed]
- Jabbour SK, Daroui P, Moore D, et al. A novel paradigm in the treatment of oligometastatic non-small cell lung cancer. J Thorac Dis 2011;3:4-9. [PubMed]
- Albain KS, Crowley JJ, LeBlanc M, et al. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 1991;9:1618-26. [PubMed]
- O'Connell JP, Kris MG, Gralla RJ, et al. Frequency and prognostic importance of pretreatment clinical characteristics in patients with advanced non-small-cell lung cancer treated with combination chemotherapy. J Clin Oncol 1986;4:1604-14. [PubMed]
- Lababede O, Meziane MA, Rice TW. TNM staging of lung cancer: a quick reference chart. Chest 1999;115:233-5. [Crossref] [PubMed]
- Santana-Davila R, Devisetty K, Szabo A, et al. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small-cell lung cancer: an analysis of Veterans Health Administration data. J Clin Oncol 2015;33:567-74. [Crossref] [PubMed]
- Consonni D, Pierobon M, Gail MH, et al. Lung cancer prognosis before and after recurrence in a population-based setting. J Natl Cancer Inst 2015;107:djv059. [Crossref] [PubMed]
- Walter C, Boda-Heggemann J, Wertz H, et al. Phantom and in-vivo measurements of dose exposure by image-guided radiotherapy (IGRT): MV portal images vs. kV portal images vs. cone-beam CT. Radiother Oncol 2007;85:418-23. [Crossref] [PubMed]
- Yeung AR, Li J, Shi W, et al. Optimal image-guidance scenario with cone-beam computed tomography in conventionally fractionated radiotherapy for lung tumors. Am J Clin Oncol 2010;33:276-80. [PubMed]
- Higgins J, Bezjak A, Hope A, et al. Effect of image-guidance frequency on geometric accuracy and setup margins in radiotherapy for locally advanced lung cancer. Int J Radiat Oncol Biol Phys 2011;80:1330-7. [Crossref] [PubMed]
- Barney BM, Lee RJ, Handrahan D, et al. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT). Int J Radiat Oncol Biol Phys 2011;80:301-5. [Crossref] [PubMed]
- Pisani L, Lockman D, Jaffray D, et al. Setup error in radiotherapy: on-line correction using electronic kilovoltage and megavoltage radiographs. Int J Radiat Oncol Biol Phys 2000;47:825-39. [Crossref] [PubMed]
- Gill S, Thomas J, Fox C, et al. Electronic portal imaging vs kilovoltage imaging in fiducial marker image-guided radiotherapy for prostate cancer: an analysis of set-up uncertainties. Br J Radiol 2012;85:176-82. [Crossref] [PubMed]
- Song WY, Chiu B, Bauman GS, et al. Prostate contouring uncertainty in megavoltage computed tomography images acquired with a helical tomotherapy unit during image-guided radiation therapy. Int J Radiat Oncol Biol Phys 2006;65:595-607. [Crossref] [PubMed]
- Korreman S, Rasch C, McNair H, et al. The European Society of Therapeutic Radiology and Oncology-European Institute of Radiotherapy (ESTRO-EIR) report on 3D CT-based in-room image guidance systems: a practical and technical review and guide. Radiother Oncol 2010;94:129-44. [Crossref] [PubMed]
- Zelefsky MJ, Kollmeier M, Cox B, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2012;84:125-9. [Crossref] [PubMed]


