
Outcomes of lung volume reduction surgery for emphysema: unilateral and bilateral
Highlight box
Key findings
• For patients undergoing lung volume reduction surgery (LVRS) for severe emphysema, bilateral LVRS is associated with greater functional improvement at the expense of longer air leak, chest tube, and length of stay days, compared to unilateral LVRS.
What is known and what is new?
• LVRS involves the removal of the most affected emphysematous lobe, to reduce hyperinflation and allow the remaining healthier lung to function more effectively.
• LVRS has historically been performed bilaterally, but can also be performed unilaterally in selected patients, with similar comparable functional improvement.
What is the implication, and what should change now?
• The long- and short-term functional and procedural outcomes should be considered when selecting patients for surgical intervention.
• One should consider unilateral LVRS for high-risk patients.
Introduction
In the 1990s, before the National Emphysema Treatment Trial (NETT), surgeons experimented with different techniques to perform lung volume reduction surgery (LVRS). These techniques included sternotomy versus thoracoscopy and unilateral versus bilateral. Unilateral proponents argued that a staged procedure provided less morbidity and a longer duration of palliation than bilateral LVRS (1-3). Other case series showed that the length of stay, morbidity, and mortality with bilateral LVRS were similar to unilateral LVRS, with greater improvement in functional outcomes (4).
The NETT, which mandated a bilateral approach, reported a 30-day mortality of 2.2% and a 90-day mortality of 7.9% (5). However, these rates included “high-risk” emphysema patients. Since then, excluding this subgroup of patients, other bilateral case series have shown improved outcomes with 0–1.5% operative mortality and 0–2.2% 90-day mortality (6,7).
This multicenter retrospective study compares functional outcomes, morbidity, and mortality of unilateral versus bilateral LVRS according to final approach and surgeon preference. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1655/rc).
Methods
Ethical statement
This study complied with the standards of the Declaration of Helsinki (as revised in 2013) and current ethical guidelines. The Institutional Review Board at Mayo Clinic Florida (#21-006676) approved this study on July 27th, 2021. The other institution was informed and agreed with the study. Individual consent for this retrospective analysis was waived.
Study design
We queried our electronic medical records service for patients who underwent LVRS from January 1st, 2011, to December 31st, 2021, at Mayo Clinic Florida and Mayo Clinic Rochester. A total of thirteen surgeons performed all cases. Following selection criteria from the NETT (5), many surgeons performed bilateral LVRS unless there was a strong or moderate contraindication. One surgeon in this study had an established preference for the unilateral technique. For these patients, laterality was decided upon the location of the optimal emphysema target. Patients who had a wedge, segmental, or lobar resection for nodules suspected of cancer and benefitted from a lung volume reduction effect were excluded. Cases were analyzed by final approach, not intention-to-treat.
Functional outcomes were recorded pre-operatively and post-operatively at 3, 6, and 12 months, when available. If more than one test was performed within the post-operative period, we used the best result for analysis. We included residual volume (RV%), forced expiratory volume in the first second (FEV1%), diffusing capacity for carbon monoxide (DLCO%), 6-minute walk test (6MWT), and use of supplemental oxygen at rest and exercise or nights. We evaluated the whole cohort and then divided patients into two groups according to laterality, for comparison. Additionally, we assessed post-procedural outcomes following the Minimal Clinically Important Change Score (MCID) for the entire cohort and subgroups, based on RV decrease equal to or more than 8% or 430 mL, FEV1% increase of equal to or more than 10%, and 6MWT increase of equal to or more than 26 meters. Missing data, either pre- or post-procedural, was excluded from analysis for each functional outcome. Therefore, the number of patients in each group for every variable is listed under the column or row “N” in tables.
For the demographic and clinical baseline, current smoking was defined as habitual smoking less than a year before surgery. The surgical details and post-operative outcomes were recorded from the electronic medical record notes. Prolonged air leak was defined as >7 days. Subjective symptoms evaluation and oxygen requirements were based on patients’ descriptions, as documented in the outpatient clinic follow-up notes.
From the unilateral group, 19 patients underwent subsequent contralateral, staged LVRS. Their pre- and post-operative outcomes analyzed in this manuscript refer to the initial procedure. A separate, follow-up study will evaluate these subgroups of patients after their second intervention.
Statistical analysis
Categorical variables were summarized as counts and percentages; continuous nonparametric variables were reported as median and interquartile range (IQR). Pre- and post-operative continuous values were compared using Wilcoxon Signed Rank tests. Categorical variables were analyzed using Fisher’s exact test. Kaplan-Meier method was performed to estimate survival, with a log-rank test to compare survival between the unilateral and bilateral groups. At-risk patients were considered from time of procedure until date of death, end of follow-up period, date of sequential LVRS for unilateral patients, or proceeding to lung transplant. All tests were two-sided, with a P value <0.05 considered statistically significant, using IBM SPSS Statistics (Version 28.0).
Results
A total of 119 consecutive patients were included in this study. Demographic and clinical characteristics are described in Table 1 and perioperative outcomes are described in Table 2. For the combined cohort, the median length of stay and follow-up time were 6 days (IQR, 4, 11) and 2.6 years (IQR, 0.7, 4.9), respectively. The most common complication was prolonged air leak (37%, N=44). For the entire cohort, RV%, FEV1%, DLCO%, and 6MWT improved significantly post-LVRS (Figure 1A,1B). There was a 25% (N=24) reduction in the number of patients dependent on supplemental oxygen, with a median decrease of 1.5 L in oxygen requirements (Table 3). In the 3–6-month follow-up visit, 77% (N=92) patients reported subjective improvement of symptoms.
Table 1
| Characteristics | Unilateral (N=64) | Bilateral (N=55) | P value |
|---|---|---|---|
| Age at surgery, years | 66 [61, 72] | 65 [60, 68] | 0.09 |
| Sex | >0.99 | ||
| Male | 35 (54.7) | 30 (54.5) | |
| Female | 29 (45.3) | 25 (45.5) | |
| Race | 0.60 | ||
| White | 61 (95.3) | 51 (92.7) | |
| Black or African American | 1 (1.6) | 1 (1.8) | |
| Other | 2 (3.1) | 3 (5.5) | |
| BMI, kg/m2 | 24.9 [22.1, 28.1] | 25.2 [23.1, 29] | 0.24 |
| Smoking history | 0.98 | ||
| Former | 59 (92.2) | 51 (92.7) | |
| Current | 4 (6.3) | 3 (5.5) | |
| Never | 1 (1.6) | 1 (1.8) | |
| Supplemental oxygen | |||
| Yes | 52 (81.3) | 44 (80.0) | 0.86 |
| Liters at rest | 2 [0, 3] | 2 [0, 2] | 0.83 |
| Liters on exertion/night | 2 [1.5, 4] | 3 [2, 4] | 0.88 |
| Comorbidities | |||
| Hypertension | 32 (50.0) | 22 (40.0) | 0.28 |
| Gastroesophageal reflux disease | 26 (40.6) | 11 (20.0) | 0.02 |
| Coronary artery disease | 10 (15.6) | 2 (3.6) | 0.03 |
| Chronic kidney disease | 6 (9.4) | 5 (9.1) | 0.96 |
| Diabetes mellitus | 5 (7.8) | 3 (5.5) | 0.61 |
| Congestive heart failure | 5 (7.8) | 0 | 0.03 |
| Previous lung intervention | 0.03 | ||
| Endoscopic lung volume reduction | 5 (7.8) | 1 (1.8) | |
| Previous lung surgery | 3 (4.7) | 0 | |
| Thoracoscopic diaphragm plication | 1 (1.6) | 0 | |
| Lung decortication | 1 (1.6) | 0 | |
| Pulmonary function tests | |||
| RV% | 218.0 [192.0, 241.0] | 227.0 [203.0, 257.0] | 0.22 |
| FEV1% | 29.5 [22.5, 36.5] | 29.0 [23.0, 37.0] | 0.70 |
| DLCO% | 31.5 [22.0, 39.0] | 33.0 [27.0, 42.0] | 0.20 |
| 6MWT (m) | 311.0 [284.6, 345.3] | 301.0 [256.0, 347.7] | 0.33 |
Data are presented as median [interquartile range] or n (%). BMI, body mass index; RV, residual volume; FEV1, forced expiration in 1 second; DLCO, diffusing capacity for carbon monoxide; 6MWT, 6-minute walk test.
Table 2
| Characteristics | Unilateral (N=64) | Bilateral (N=55) | P value |
|---|---|---|---|
| Surgical approach | <0.001 | ||
| VATS | 61 (95.3) | 25 (45.5) | |
| Sternotomy | 0 | 28 (50.9) | |
| Thoracotomy | 3 (4.7) | 2 (3.6) | |
| Target lobe | – | ||
| RUL/LUL | – | 52 (94.5) | |
| RUL + RML/LUL | – | 1 (1.8) | |
| RUL + RLL/LUL | – | 1 (1.8) | |
| RLL/LLL | – | 1 (1.8) | |
| RUL | 42 (65.6) | – | |
| LUL | 15 (23.4) | – | |
| RUL + RLL | 5 (7.8) | – | |
| RUL + RML | 1 (1.6) | – | |
| RML + RLL | 1 (1.6) | – | |
| Duration of surgery (min) | 65.5 [50, 99] | 132.5 [117, 187] | <0.001 |
| Air leak (days) | 2.5 [0, 9] | 5 [1, 14] | 0.03 |
| Chest tube (days) | 4 [3, 10.5] | 7 [4, 15] | 0.01 |
| ICU stay (days) | 0 [0, 1] | 0 [0, 2] | 0.07 |
| Length of stay (days) | 5 [4, 9] | 8 [6, 14] | <0.001 |
| Discharged with chest tube | 9 (14.1) | 11 (20.0) | 0.39 |
| Complications | |||
| Prolonged air leak (>7 days) | 20 (31.3) | 24 (43.6) | 0.16 |
| Subcutaneous emphysema | 11 (17.2) | 6 (10.9) | 0.33 |
| Pneumonia | 4 (6.3) | 8 (14.5) | 0.13 |
| Bleeding requiring transfusion | 3 (4.7) | 8 (14.5) | 0.12 |
| Hemothorax | 1 (1.6) | 1 (1.8) | 0.91 |
Data are presented as median [interquartile range] or n (%). VATS, video-assisted thoracoscopic surgery; RUL, right upper lobe; LUL, left upper lobe; RML, right middle lobe; RLL, right lower lobe; LLL, left lower lobe; ICU, intensive care unit.

Table 3
| Functional outcomes | N† | Pre-LVRS | Post-LVRS | Delta | Percent change | P value |
|---|---|---|---|---|---|---|
| RV% | 81 | 226 [199.5, 251.5] | 157 [129.5, 198.5] | −68 [−86, −31] | −30.1% | <0.001 |
| FEV1% | 102 | 29 [21.7, 36.3] | 40.5 [29.3, 53] | +10.5 [3, 20] | +36.2% | <0.001 |
| DLCO% | 80 | 31.5 [25, 40.8] | 39 [29.3, 48.8] | +5 [−1, 14] | +15.9% | <0.001 |
| 6MWT (m) | 70 | 310.2 [263.5, 341.3] | 335.7 [294.7, 380.5] | +24.2 [−21.3, 80] | +7.8% | 0.02 |
| Supplemental oxygen | 119 | |||||
| Yes | 96 (80.7) | 72 (60.5) | −24 | −25% | <0.001 | |
| Liters at rest | 2 [0, 3] | 0 [0, 0.3] | −2 | −100% | <0.001 | |
| Liters at exertion | 2 [2, 4] | 1.5 [0, 2] | −0.5 | −25% | <0.001 |
Data are presented as median [interquartile range] or n (%). †, patients with pre- and post-functional test results were included in the analysis. LVRS, lung volume reduction surgery; RV, residual volume; FEV1, forced expiration in 1 second; DLCO, diffusing capacity for carbon monoxide; 6MWT, 6-minute walk test.
Patients were grouped into unilateral (N=64) and bilateral (N=55) LVRS. Two patients had strong contraindications to bilateral LVRS, one for prior lung decortication and the other for prior diaphragm plication. Another two patients were intended to be bilateral, but the contralateral side was aborted due to extensive air leaks from the initial side, so a unilateral approach was decided. One surgeon preferred unilateral LVRS and performed 86% of these cases. Gastroesophageal reflux disease, coronary artery disease, congestive heart failure, and a prior lung intervention were significantly more prevalent in the unilateral group, including 5 previous bronchoscopic lung volume reduction (BLVR) interventions with unidirectional valves (Table 1). No other demographic or clinical differences were found between the two groups. Bilateral LVRS resulted in significantly longer duration of surgery, air leak and chest tube days, and length of hospital stay, than unilateral LVRS. Post-procedural complications were similar between the two groups (Table 2).
Post-LVRS, RV%, FEV1%, DLCO%, and 6MWT improved twice as much in the bilateral group compared to the unilateral group, however, only DLCO% and 6MWT were significant (Table 4, Table S1, Figure 2A-2D). Use of supplemental oxygen was reduced by approximately 25% for both. Regarding MCID, 73 patients exceeded the RV% reduction, 52 patients the FEV1% increase, and 35 patients the 6MWT increase, with bilateral patients fulfilling these criteria more frequently in across the three parameters (Table 4, Table S2). Nineteen patients exceeded all three criteria, 6 from the unilateral group and 13 from the bilateral group (P=0.03).
Table 4
| Functional outcomes | Unilateral LVRS delta | Bilateral LVRS delta | P value |
|---|---|---|---|
| RV% | |||
| N | 39 | 42 | – |
| Median [IQR] | −45 [−82, −31] | −77.5 [−94.8, −37] | 0.07 |
| FEV1% | |||
| N | 52 | 50 | – |
| Median [IQR] | 6.3 [3, 15.8] | 13 [4, 23] | 0.07 |
| DLCO% | |||
| N | 36 | 44 | – |
| Median [IQR] | 2 [−3.8, 11] | 9 [2.1, 15.5] | 0.02 |
| 6MWT (m) | |||
| N | 33 | 37 | – |
| Median [IQR] | −4 [−41.9, 58.7] | 42.8 [1.56, 110.2] | 0.007 |
| Supplemental O2 | |||
| Patients requiring oxygen pre-LVRS, n | 52 | 45 | – |
| Patients not requiring oxygen post-LVRS†, n (%) | 13 (25.0) | 11 (24.4) | 0.95 |
| Liters at rest, median [IQR] | 0 [−2, 0] | −2 [−2, 0] | 0.17 |
| Liters on exertion, median [IQR] | −1 [−2, 0] | −1.5 [−2.1, 0] | 0.59 |
| MCID criteria | |||
| N | 64 | 55 | – |
| RV% (−8%), n (%) | 35 (54.7) | 38 (69.1) | 0.12 |
| FEV1% (+10%), n (%) | 21 (32.8) | 31 (56.4) | 0.01 |
| 6MWT (+26 m), n (%) | 13 (20.3) | 22 (40.0) | 0.02 |
| All criteria, n (%) | 6 (9.4) | 13 (23.6) | 0.04 |
| Mortality | |||
| N | 64 | 55 | – |
| 30-day, n (%) | 1 (1.6) | 2 (3.6) | 0.6 |
| 90-day, n (%) | 1 (1.6) | 3 (5.5) | 0.33 |
| 1-year, n (%) | 1 (1.6) | 5 (9.1) | 0.06 |
Patients with pre- and post-functional test results were included in the analysis. †, number of patients who were on supplemental oxygen prior to LVRS and no longer required it after surgery. LVRS, lung volume reduction surgery; RV, residual volume; IQR, interquartile range; FEV1, forced expiration in 1 second; DLCO, diffusing capacity for carbon monoxide; 6MWT, 6-minute walk test; MCID, Minimal Clinically Important Change Score.

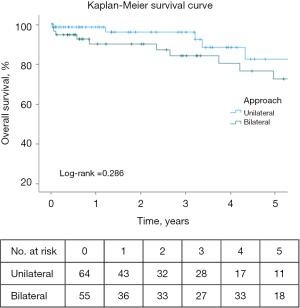
For the entire cohort, 30-day and 90-day mortality were 2.5% (N=3) and 3.4% (N=4), respectively. At 30 days, there were 3 deaths: 1 (1.6%) from the unilateral group and 2 (3.6%) from the bilateral group (P=0.60). At 90 days, there was 1 more death from the bilateral patients (1 vs. 3, P=0.33) (Table 4, Table S2). Of the 3 deaths in the bilateral group, two were sternotomy patients. Kaplan-Meier survival curve censored at one year, estimated survival was 98.4% for unilateral and 90.9% for bilateral LVRS (P=0.09) (Figure 3).
Discussion
As with the NETT and other cohort studies (5-9), we found that LVRS significantly improves patients’ pulmonary functions tests (PFTs) and exercise capacity and decreases their requirements of supplemental oxygen (Table 4). Our estimated survival after LVRS was higher than the reported in the same large-scale trial, and similar to other recent single-center studies which have also described improved survival (6-12) (Table 5). Following the lessons learned from the NETT, we generally exclude high-risk patients (FEV1% and DLCO% less than 20%) and those with homogeneous emphysema (5,9). The results of this study solidify the efficacy and safety of LVRS for advanced emphysema in high-volume LVRS centers.
Table 5
| Series | Years | N | Mean age, years | Pre-FEV1% | Pre-6MWT, m | Laterality | Approach | Mortality, % | Survival, % | Δ RV% | Δ FEV1% | Δ DLCO% | Δ 6MWT, m | LOS, days | Prolonged air leak | Chest tube days | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30-day | 90-day | 1-year | 3-year | 5-year | ||||||||||||||||
| Ciccone 2003 | 1993–2000 | 250 | 62 | 25 | 348 | Bilateral | Sternotomy | NA | 4 | 93.6 | 84.4 | 67.7 | −93 | +14 | +5 | +129.8 | 9 | 45.2 | NA | |
| NETT 2011 | 1998–2002 | 580 | 66.5 | 26.8 | 371 | Bilateral | 70% sternotomy | 2.2 | 7.9 | 89 | 75 | 60 | NA | NA | +8 | +47.3 | 10 | NA | 7 | |
| Ginsburg 2016 | 2004–2014 | 91 | 62.5 | 25.8 | 380 | 88% bilateral | 12% sternotomy | 0 | 0 | 99 | NA | 78 | −64 | +11.1 | 5.2 | +39.2 | 8 | 57 | NA | |
| Horwood 2019 | 2006–2017 | 135 | 63.2 | 29.1 | 379 | 95% bilateral | 97.8% VATS | 1.5 | 2.2 | 94 | 91 | 71 | −42 | +5.3 | +5.8 | +19.5 | 8 | 23.2 | NA | |
| Buttery 2023 | 2016–2019 | 34 | 65 | 32 | NA | Unilateral | VATS | 0 | 0 | 97.7 | NA | NA | −36.1 | +24 | NA | NA | 9 | NA | 8 | |
| Bascarevic 2024 | 2007–2017 | 39 | NA | 52.7 | 367 | 79.5% unilateral | Thoracotomy | 0 | 0 | 100 | NA | NA | −0.49 L | +0.32 L | NA | +60.4 | 13 | 67.7 | 12 | |
| Buttery 2024 | 2017–2022 | 244 | 63.4 | 32.2 | NA | NA | VATS | 1.6 | 2.9 | NA | NA | 75 | NA | NA | NA | NA | 12 | NA | 11 | |
| Ours | 2011–2021 | 119 | 65 | 29 | 306.4 | 46% bilateral | 24% sternotomy | 2.5 | 3.4 | 95 | 93.3 | 87 | −68 | +10.5 | +5 | +24.2 | 6 | 37 | 6 | |
FEV1, forced expiration in 1 second; 6MWT, 6-minute walk test; RV, residual volume; DLCO, diffusing capacity for carbon monoxide; LOS, length of stay; NA, not available; NETT, National Emphysema Treatment Trial; VATS, video-assisted thoracoscopic surgery.
Multiple studies have compared post-operative functional outcomes between unilateral and bilateral LVRS, showing greater improvement in the latter (2,13,14). McKenna et al. described an FEV1% improvement of 31% in unilateral vs. 57% in bilateral patients (13), while Kotloff et al. reported an absolute FEV1 increase of 0.16±0.22 L for unilateral versus 0.25±0.31 L for bilateral LVRS (P<0.001) (15). In our study, FEV1% improvement was proportionally greater for the bilateral group, but not statistically significant (P=0.07). There was no statistical difference in RV% decrease between groups, despite the larger reduction in lung volume for the bilateral patients and, in fact, use of supplemental oxygen decreased more in the unilateral group. Significant changes were reported in DLCO% and 6MWT, favoring the bilateral approach (Table 4). The bilateral group also exceeded in the number of patients who achieved MCID criteria compared to the unilateral
Perioperative outcomes comparing bilateral to unilateral are conflicting. Oey et al. showed that the unilateral (N=39) compared to the bilateral (N=26) LVRS resulted in a shorter ICU stay (2 vs. 7 days; P=0.04) and shorter hospital stay (16 vs. 28 days; P=0.004) (2). Another larger study found no statistical difference in the mean length of stay (11.4±1 vs. 10.9±1 days) between the two approaches. When compared to our findings, we reported a shorter median total length of stay for the unilateral procedures (P<0.001) as well as ICU days, though not significant.
Mortality has been the main concern when considering surgical over medical treatment, as evidenced in the NETT (5,9,16). However, subsequent long-term analysis of the trial and further studies demonstrating low mortality rates have supported the surgical treatment of advanced emphysema (6,7,10-12,16). For 30-day mortality, rates have improved from the NETT and have been subsequently reported at 0% (17) and in our study, 2.5% (N=3). For 90-day mortality, we reported 3.4% (N=4) from the entire cohort. Comparing unilateral to bilateral LVRS for this time frame, Oey et al. found a non-significant lower mortality rate in the unilateral group (3% vs. 8%; P=0.34) (2). On the contrary, McKenna et al. described a slightly higher post-operative mortality for unilateral than bilateral LVRS (3.5% vs. 2.5%) (13). They surmised that the smaller increase in functional outcomes with unilateral LVRS contributed to increased respiratory failure in the post-operative period (13,14). In contrast, our 4 operative mortalities were attributable to prolonged air leaks. These patients had prolonged air leaks complicated by pneumonia and respiratory failure. This is not a new finding. From propensity-matched patients in the NETT, air leaks led to greater overall complications, were more likely to develop pneumonia, as well as to be readmitted to the ICU, which we also evidenced in our cohort (4). Even with buttressed staple lines, there is no consistent way to eliminate air leaks (4); therefore, the rationale for unilateral LVRS is to minimize the severity of air leaks and the associated complications.
Other less invasive techniques have been developed for achieving lung volume reduction through BLVR. Recently, large multicenter study compared unilateral LVRS to BLVR, including operative and functional outcomes, and reported similar findings between the groups (11). Median length of stay was significantly shorter for BLVR (3 vs. 9 days; P=0.006). In functional outcomes, BLVR showed greater improvement in FEV1%, while unilateral LVRS showed greater reduction in RV%, though neither was statistically significant. Compared to our unilateral LVRS group results, our patients achieved a greater increase in FEV1% (6.3% vs. 1.1%), as well reduction in RV% (−77.5% vs. −36.1%) (11). At 12-month follow-up, Buttery et al. and our study reported one death in the unilateral LVRS group, 2.1% vs. 1.6%, respectively (11).
Unilateral LVRS can be followed with a subsequent contralateral or staged LVRS procedure. Several studies have compared functional outcomes and complications of staged versus bilateral LVRS, achieving comparable perioperative and functional outcomes (3,18,19). A separate, second study will assess this subgroup of patients from our population.
This multicenter study contributes to the debate between unilateral versus bilateral LVRS, with a sample size comparable to other large LVRS case series as seen in Table 5. We acknowledge the limitations of our study. The retrospective nature is subject to selection bias, however, the selection of patients was mostly decided by surgeon preference and not due to contra-indications. Moreover, we performed the analysis by final approach, not-intention to treat, which mitigates the influence of intra-operative change of plans. We consider this a strength since as it presents as a natural experiment since no surgeon changed their preferred approach unless there was a specific contraindication. Another limitation is that, nearly half of the bilateral group was performed via a sternotomy. Although that approach is not currently the surgical technique of choice, it was standard practice during the earlier years of our cohort.
Conclusions
Bilateral LVRS is associated with greater long-term functional improvement at the expense of longer air leak, chest tube, and length of stay days, compared to unilateral LVRS.
Acknowledgments
Part of this study was presented in oral form at the European Society of Thoracic Surgeons Annual Conference in June 2023 in Milan, Italy.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1655/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1655/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1655/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1655/coif). S.F.B. serves as an unpaid editorial board member of Journal of Thoracic Disease from September 2024 to August 2026. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study complied with the standards of the Declaration of Helsinki (as revised in 2013) and current ethical guidelines. The Institutional Review Board at Mayo Clinic Florida (#21-006676) approved this study on July 27th, 2021. The other institution was informed and agreed with the study. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hazelrigg SR, Boley TM, Grasch A, et al. Surgical strategy for lung volume reduction surgery. Eur J Cardiothorac Surg 1999;16:S57-60. [Crossref] [PubMed]
- Oey IF, Waller DA, Bal S, et al. Lung volume reduction surgery--a comparison of the long term outcome of unilateral vs. bilateral approaches. Eur J Cardiothorac Surg 2002;22:610-4. [Crossref] [PubMed]
- Pompeo E, Mineo TC. Long-term outcome of staged versus one-stage bilateral thoracoscopic reduction pneumoplasty. Eur J Cardiothorac Surg 2002;21:627-33; discussion 633. [Crossref] [PubMed]
- DeCamp MM Jr, McKenna RJ Jr, Deschamps CC, et al. Lung volume reduction surgery: technique, operative mortality, and morbidity. Proc Am Thorac Soc 2008;5:442-6. [Crossref] [PubMed]
- Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881-93. [Crossref] [PubMed]
- Ginsburg ME, Thomashow BM, Bulman WA, et al. The safety, efficacy, and durability of lung-volume reduction surgery: A 10-year experience. J Thorac Cardiovasc Surg 2016;151:717-724.e1. [Crossref] [PubMed]
- Horwood CR, Mansour D, Abdel-Rasoul M, et al. Long-Term Results After Lung Volume Reduction Surgery: A Single Institution's Experience. Ann Thorac Surg 2019;107:1068-73. [Crossref] [PubMed]
- Ciccone AM, Meyers BF, Guthrie TJ, et al. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg 2003;125:513-25. [Crossref] [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Bascarevic S, Ercegovac M, Hoda MA, et al. Twenty four-month follow-up after bullectomy, unilateral and bilateral lung volume reduction surgery: a single-center retrospective analysis of consecutive cases. Eur J Med Res 2024;29:279. [Crossref] [PubMed]
- Buttery SC, Banya W, Bilancia R, et al. Lung volume reduction surgery versus endobronchial valves: a randomised controlled trial. Eur Respir J 2023;61:2202063. [Crossref] [PubMed]
- Buttery SC, Lewis A, Alzetani A, et al. Survival following lung volume reduction procedures: results from the UK Lung Volume Reduction (UKLVR) registry. BMJ Open Respir Res 2024;11:e002092. [Crossref] [PubMed]
- McKenna RJ Jr, Brenner M, Fischel RJ, et al. Should lung volume reduction for emphysema be unilateral or bilateral? J Thorac Cardiovasc Surg 1996;112:1331-8; discussion 1338-9. [Crossref] [PubMed]
- Serna DL, Brenner M, Osann KE, et al. Survival after unilateral versus bilateral lung volume reduction surgery for emphysema. J Thorac Cardiovasc Surg 1999;118:1101-9. [Crossref] [PubMed]
- Kotloff RM, Tino G, Palevsky HI, et al. Comparison of short-term functional outcomes following unilateral and bilateral lung volume reduction surgery. Chest 1998;113:890-5. [Crossref] [PubMed]
- McCarthy DP, Taylor LJ, DeCamp MM. Analysis of Recent Literature on Lung Volume Reduction Surgery. Thorac Surg Clin 2021;31:119-28. [Crossref] [PubMed]
- Agzarian J, Miller JD, Kosa SD, et al. Long-term survival analysis of the Canadian Lung Volume Reduction Surgery trial. Ann Thorac Surg 2013;96:1217-22. [Crossref] [PubMed]
- Hazelrigg SR, Boley TM, Magee MJ, et al. Comparison of staged thoracoscopy and median sternotomy for lung volume reduction. Ann Thorac Surg 1998;66:1134-9. [Crossref] [PubMed]
- Oey IF, Morgan MD, Spyt TJ, et al. Staged bilateral lung volume reduction surgery - the benefits of a patient-led strategy. Eur J Cardiothorac Surg 2010;37:846-52. [Crossref] [PubMed]


