
Outcomes and risk factors for cardiac surgery during pregnancy: a 13-year, two-centre, retrospective cohort study
Highlight box
Key findings
• Cardiac surgery during pregnancy is associated with high maternal and foetal risks. Combining preoperative left ventricular ejection fraction, pulmonary hy-pertension and intraoperative blood loss can predict postoperative cardiovascu-lar complications and mortality reliably for patients undergoing cardiac surgery during pregnancy.
What is known and what is new?
• During pregnancy, cardiac surgery is associated with high maternal and foetal risks. The risk factors affecting maternal and foetal outcomes are still uncer-tain.
• This study was based on the two largest referral centres for pregnant patients with heart diseases in northern and southern China with a time span of 13 years and it is currently the study with the largest sample size in this field. It provided the largest sample clinical results in China, analysed cardiac surgery outcomes during pregnancy and explored the risk factors in maternal postoperative out-comes.
What is the implication, and what should change now?
• The risk factors discovered in the study are of great significance in helping to assess the condition and establishing a risk assessment system for patients un-dergoing cardiac surgery during pregnancy.
Introduction
Cardiovascular disease is the leading cause of maternal death during pregnancy (1,2). A few patients with urgent and critical heart disease require cardiac surgery during pregnancy to save the mother’s life (3). Due to the physiological changes in pregnancy, pathological and physiological changes of pre-existing or newly developed heart diseases, and surgery-related factors, the mother and infant are exposed to higher complication and mortality risks during the perioperative period (4).
In the past decade, despite increased awareness and clinical care, the clinical outcomes of these patients are still not ideal (5). Due to their rarity, most patients are scattered in various hospitals (6-8). Previous research has mainly focused on case reports, and large-scale clinical research is lacking. Currently, the risk factors affecting maternal and foetal outcomes are uncertain, and commonly used risk prediction systems need to be refined (9).
As the two largest referral centres for pregnant patients with heart diseases in northern and southern China, our team has accumulated ample clinical experience in treating pregnancy-complicated heart diseases, especially aortic dissection and pulmonary hypertension (PH) (10,11). This study aimed to analyse cardiac surgery outcomes during pregnancy and explore the risk factors in maternal postoperative outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-787/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University and the Medical Ethics Committee of Guangdong Provincial People’s Hospital (approval No. KS2023064). The requirement for informed consent was waived due to the retrospective nature of this study. We reviewed the case databases of pregnant women with heart disease at Beijing Anzhen Hospital, Capital Medical University, and Guangdong Provincial People’s Hospital between January 2010 and June 2023. We identified cases based on different cardiac surgeries during pregnancy and excluded catheter-based cardiac interventional procedure (e.g., percutaneous valve and coronary interventions).
The first trimester was defined as a gestational age between 1 and 14 weeks, the second trimester included pregnant patients at 15–27 weeks of gestation, and the third trimester was defined as more than 27 weeks of gestation. Left ventricular ejection fraction (LVEF) was measured using transthoracic echocardiography. PH was defined as right ventricular systolic pressure (RVSP) ≥30 mmHg at rest, on the basis of peak velocity tricuspid regurgitation by echocardiography. Patients with RVSP >70 mmHg were further divided into severe PH.
Maternal death was defined as the death of patients during cardiac surgery, hospitalization after cardiac surgery and within 42 days after the termination of pregnancy. Foetal death is defined as foetal death within the uterus during pregnancy. Neonatal death is defined as the death of a live baby within 28 days after birth. Postoperative new-onset cardiovascular complications were defined as complications not present before surgery but emerged during cardiac surgery or hospitalization after cardiac surgery. Cardiovascular complications included (I) heart failure, (II) venous thromboembolic events, (III) severe arrhythmias, (IV) hypertensive emergencies, (V) PH crisis, and (VI) cerebrovascular events. Venous thromboembolic events included deep vein thrombosis and pulmonary embolism. Severe arrhythmias were considered symptomatic sustained tachyarrhythmia or bradyarrhythmia. Hypertensive emergencies were defined as elevated blood pressure (systolic blood pressure ≥180 mmHg and/or diastolic blood pressure ≥120 mmHg). PH crisis was defined as a condition resulting from pulmonary vasoconstriction, increased pulmonary vascular resistance, and decreased cardiac output, leading to acute right-sided heart failure, hypoxemia, and hypotension.
Statistical analysis
Continuous variables with normal distribution were expressed as means ± standard deviations (SDs), and differences in means were compared using an independent sample Student test (two groups) or analysis of variance (multiple groups) after performing the Shapiro-Wilk test. Non-normally distributed variables were reported as medians and interquartile ranges (IQRs), and non-parametric Mann-Whitney U tests were used for between-group comparisons. Categorical data were summarized as ratios and percentages, and differences in proportions were tested with the χ2 or Fisher exact test. Univariate and multivariate binary stepwise logistic regression analyses were performed to identify the risk factors associated with endpoint events. To avoid missing meaningful variables, the significance threshold (P) for univariate analysis was relaxed to 0.2 for the univariate analysis. The Box-Tidwell method was used to test the linear relationship between continuous independent and dependent variables. The tolerance or variance inflation factor was used to diagnose multicollinearity between independent variables. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were analysed to evaluate and compare the prediction effects of different models. Differences were considered statistically significant at a two-tailed P value of 0.05. All statistical tests were performed using SPSS software (version 26.0; SPSS Inc., Chicago, IL, USA). The cut-off value for outcome was identified using the R statistical software (version 4.2.1) and the following software package: “proc”.
Results
From January 2010 to June 2023, a total of 7,492 pregnant women with heart disease were identified in the patient databases of Beijing Anzhen Hospital, Capital Medical University, and Guangdong Provincial People’s Hospital. After applying the exclusion criteria, 140 patients underwent cardiac surgery during pregnancy, with 74 patients and 66 patients in each centre respectively (Figure 1). The number of annual cardiac surgeries performed during pregnancy showed an increasing trend over the years (Figure 2), of which six patients died.
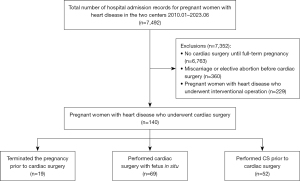

There were three methods for managing the foetus during cardiac surgery: (I) terminating the pregnancy prior to cardiac surgery; (II) performing cardiac surgery with the foetus in situ; and (III) performing a caesarean section (CS) prior to cardiac surgery. Preoperative maternal baseline characteristics, surgery information, cardiopulmonary bypass (CPB) information, and postoperative information are shown in Tables 1-4.
Table 1
| Variables | Value (n=140) |
|---|---|
| Age, years | 29 (26.1–33.0) |
| Body mass index, kg/m2 | 23.3 (20.8–25.6) |
| Gestation when cardiac surgery, weeks | 26 (20.9–32.2) |
| Gravida | |
| 1 | 15 (10.7) |
| 2 | 70 (50.0) |
| 3 or >3 | 55 (39.3) |
| Parity | |
| 0 | 38 (27.1) |
| 1 | 67 (47.9) |
| 2 or >2 | 35 (25.0) |
| Trimester | |
| First | 15 (10.7) |
| Second | 70 (50.0) |
| Third | 55 (39.3) |
| Cardiac diagnosis | |
| Aortic disease | 51 (36.4) |
| Valvular heart disease | 42 (30.0) |
| Cardiac tumour | 11 (7.9) |
| Endocarditis | 17 (12.1) |
| Others | 19 (13.6) |
| Previous cardiac surgery | 23 (16.4) |
| Comorbidities | |
| Hypertension | 15 (10.7) |
| Diabetes mellitus | 4 (2.9) |
| Renal insufficiency | 2 (1.4) |
| Cerebrovascular disease | 5 (3.6) |
| Hepatic insufficiency | 3 (2.1) |
| Marfan | 29 (20.7) |
| Arrhythmia | 17 (12.1) |
| NYHA classification | |
| I | 2 (1.4) |
| II | 85 (60.7) |
| III | 34 (24.3) |
| IV | 19 (13.6) |
| PH | 35 (25.0) |
| Severe PH | 14 (10.0) |
| LVEF, % | 62 (58.2–67.5) |
Values are presented as numbers (%) or median (IQR). NYHA, New York Heart Association; PH, pulmonary hypertension; LVEF, left ventricular ejection fraction; IQR, interquartile range.
Table 2
| Variables | Value (n=140) |
|---|---|
| Emergency surgery | 59 (42.1) |
| Type of surgery | |
| Isolated valve surgery | 57 (40.7) |
| Isolated aortic surgery | 10 (7.1) |
| Combined aortic + valve surgery | 35 (25.0) |
| Resection cardiac tumour | 12 (8.6) |
| Other | 26 (18.6) |
| Oxytocin use during surgery | 28 (20.0) |
| Vaginal balloon | 34 (24.3) |
| Allogeneic blood transfusion | 80 (57.1) |
| Intraoperative blood loss, mL | 600 (200–1,000) |
| Operation time, min | 285 (195–399) |
Values are presented as numbers (%) or median (IQR). IQR, interquartile range.
Table 3
| Variables | Value (n=139) |
|---|---|
| CPB time, min | 111 (75.3–170.5) |
| Clamping time, min | 70 (45.1–95.7) |
| Minimum nasopharyngeal temperature, ℃ | 32 (27.3–35.3) |
| Minimum rectal temperature, ℃ | 33 (28.2–35.7) |
| Deep hypothermic circulatory arrest | 29 (20.8) |
Values are presented as numbers (%) or median (IQR). CPB, cardiopulmonary bypass; IQR, interquartile range.
Table 4
| Variables | Value (n=140) |
|---|---|
| ICU stay time, days | 2 (1.5–4.4) |
| Postoperative complications | |
| Heart failure | 11 (7.9) |
| Arrhythmia | 17 (12.1) |
| Renal injury | 13 (9.3) |
| Hepatic injury | 2 (1.4) |
| Dialysis | 2 (1.4) |
| Cerebrovascular events | 4 (2.9) |
| Pulmonary infection | 6 (4.3) |
| Reoperation | 12 (8.6) |
Values are presented as numbers (%) or median (IQR). ICU, intensive care unit; IQR, interquartile range.
The maternal and foetal mortality rates are shown in Table 5. The maternal mortality rate was 4.3%. There was no significant difference in maternal mortality rates among trimesters and foetal management groups. The foetal mortality rate was 35.7%. There were significant differences in the foetal mortality rates among the trimesters. The foetal mortality rate was significantly lower in the third trimester (12.7%) than in the first (53.3%) or second (50.0%) trimesters. Foetal mortality rates were also significantly different among the different foetal management groups. The foetal mortality rate was significantly lower in cases that received CS prior to cardiac surgery than received cardiac surgery with fetus in situ.
Table 5
| Variables | Overall (n=140) | Trimester of cardiac surgery | |||
|---|---|---|---|---|---|
| First (n=15) | Second (n=70) | Third (n=55) | P value | ||
| Maternal mortality | |||||
| Overall (n=140) | 6/140 (4.3) | 0/15 | 3/70 (4.3) | 3/55 (5.5) | >0.99 |
| Terminating the pregnancy prior to cardiac surgery (n=19) | 2/19 (10.5)** | 0/4 | 2/15 (13.3) | – | >0.99 |
| Performing cardiac surgery with foetus in situ (n=69) | 1/69 (1.4)** | 0/11 | 0/52 | 1/6 (16.7) | 0.09 |
| Performing CS prior to cardiac surgery (n=52) | 3/52 (5.8)** | – | 1/3 (33.3) | 2/49 (4.1) | 0.17 |
| Foetal mortality | |||||
| Overall (n=140) | 50/140 (35.7) | 8/15 (53.3) | 35/70 (50.0) | 7/55 (12.7) | <0.001 |
| Terminating the pregnancy prior to cardiac surgery (n=19) | 19/19 (100.0)* | 4/4 (100.0) | 15/15 (100.0) | – | – |
| Performing cardiac surgery with foetus in situ (n=69) | 23/69 (33.3)* | 4/11 (36.4) | 17/52 (32.7) | 2/6 (33.3) | >0.99 |
| Performing CS prior to cardiac surgery (n=52) | 8/52 (15.4)* | – | 3/3 (100.0) | 5/49 (10.2) | 0.003 |
Values are presented as numbers (%). Maternal mortality: performing cardiac surgery with the foetus in situ: Second vs. Third, P=0.047. Performing CS prior to cardiac surgery: Second vs. Third, P=0.03. Foetal mortality: total population: First vs. Third, P=0.02. Second vs. Third, P=0.04. Performing CS prior to cardiac surgery: Second vs. Third, P=0.04. *, P<0.05; **, P=0.14. CS, caesarean section.
There were six deaths, including five from heart failure and one from cerebral infarction. The maternal deaths are shown in Table 6. In this study, 22 patients experienced postoperative new-onset cardiovascular complications or death, including six cases of heart failure, five cases of arrhythmias, three cases of heart failure combined with arrhythmias, two cases of cerebrovascular events and six deaths. There were significant differences between the group that experienced postoperative new-onset cardiovascular complications or death and the group that did not in terms of New York Heart Association (NYHA) classification, presence of PH, preoperative LVEF, CPB time, CPB clamping time, and intensive care unit (ICU) stay time (Table 7). Considering that due to the small sample size, some clinically significant and previous literature significant factors may be omitted, we adjusted the significance threshold to 0.2 to include age, operation time, intraoperative blood loss, and whether allogeneic blood was infused in the univariate and multivariate analyses. Univariate analysis showed significant differences between the two groups in presence of PH, preoperative LVEF, intraoperative blood loss, operation time, CPB time, and ICU stay time (Table 8).
Table 6
| Case | Surgery date | Age, years | Gestation at cardiac surgery, weeks | NYHA classification | LVEF, % | PH | Cardiac diagnosis | Cardiac surgery |
Cause of death |
Time of death |
Foetal management |
Foetus outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2012 | 25 | 28 | IV | 62 | Severe | MI | MVR | Heart failure | 30 days after surgery | Performing CS prior to cardiac surgery | Died |
| 2 | 2012 | 28 | 22 | IV | 54 | Moderate | AS, MI | AVR, MVP, CABG | Heart failure | 10 days after surgery | Performing CS prior to cardiac surgery | Died |
| 3 | 2012 | 30 | 32 | IV | 76 | Mild | Aortic dissection | Bentall-Sun’s surgery | Heart failure | 28 days after surgery | Performing CS prior to cardiac surgery | Alive |
| 4 | 2013 | 31 | 22 | IV | 34 | Moderate | Aortic dissection | Bentall-Sun’s surgery, CABG | Heart failure | 3 days after surgery | Terminating pregnancy prior to cardiac surgery | Died |
| 5 | 2016 | 22 | 33 | IV | 33 | Severe | Aortic dissection | Bentall-Sun’s surgery, CABG, delayed sternal closure | Cerebral infarction | 15 days after surgery | Performing cardiac surgery with foetus in situ |
Died |
| 6 | 2022 | 30 | 24 | III | 60 | Moderate | Ascending aortic aneurysm, PDA | Bentall-Sun’s surgery, ligation of PDA, MVP | Heart failure | 9 days after surgery | Terminating pregnancy prior to cardiac surgery | Died |
NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; PH, pulmonary hypertension; MI, mitral insufficiency; AS, aortic stenosis; PDA, patent ductus arteriosus; MVR, mitral valve replacement; AVR, aortic valve replacement; MVP, mitral valvuloplasty; CABG, coronary artery bypass grafting; CS, caesarean section.
Table 7
| Variables | Postoperative new-onset cardiovascular complications or death |
t/Z/χ2 | P value | |
|---|---|---|---|---|
| No (n=118) | Yes (n=22) | |||
| Age, years | 29.0 (26.8–33.0) | 27.5 (25.0–31.3) | −1.802 | 0.07 |
| Body mass index, kg/m2 | 23.7 (19.5–28.8) | 22.6 (19.0–26.8) | 1.095 | 0.27 |
| Gestation at cardiac surgery, weeks | 25.1 (16.2–33.5) | 26.9 (20.2–31.7) | −1.326 | 0.19 |
| Gravida | 2.582 | 0.29 | ||
| 1 | 13 (11.1) | 2 (9.1) | ||
| 2 | 62 (52.5) | 8 (36.4) | ||
| 3 or >3 | 43 (36.4) | 12 (54.5) | ||
| Parity | 1.077 | 0.67 | ||
| 0 | 30 (25.4) | 8 (36.4) | ||
| 1 | 58 (49.2) | 9 (40.9) | ||
| 2 or >2 | 30 (25.4) | 5 (22.7) | ||
| Trimester | 2.582 | 0.29 | ||
| First | 13 (11.0) | 2 (9.1) | ||
| Second | 62 (52.5) | 8 (36.4) | ||
| Third | 43 (36.4) | 12 (54.5) | ||
| Cardiac diagnosis | 4.402 | 0.34 | ||
| Aneurysm or dissection | 42 (35.6) | 9 (40.9) | ||
| Regurgitation/stenosis | 33 (28.0) | 9 (40.9) | ||
| Cardiac tumour | 11 (9.3) | 0 | ||
| Endocarditis | 14 (11.9) | 3 (13.6) | ||
| Others | 18 (15.3) | 1 (4.5) | ||
| Previous cardiac surgery | 20 (16.9) | 3 (13.6) | 0.005 | 0.94 |
| Comorbidities | 0.021 | >0.99 | ||
| Hypertension | 12 (10.2) | 3 (13.6) | 0.012 | 0.91 |
| Diabetes mellitus | 4 (3.4) | 0 | 1.389 | >0.99 |
| Renal insufficiency | 2 (1.7) | 0 | 0.000 | >0.99 |
| Cerebrovascular disease | 5 (4.2) | 0 | 0.128 | >0.99 |
| Hepatic insufficiency | 3 (2.5) | 0 | 0.000 | >0.99 |
| Marfan | 19 (16.1) | 10 (45.5) | 0.000 | >0.99 |
| Arrhythmia | 15 (12.7) | 2 (9.1) | 0.015 | 0.90 |
| NYHA classification | 16.500 | <0.001 | ||
| I | 2 (1.7) | 0 | ||
| II | 77 (67.0) | 6 (28.6) | ||
| III | 26 (22.6) | 6 (28.6) | ||
| IV | 10 (8.7) | 9 (42.9) | ||
| PH | 25 (21.2) | 10 (45.5) | 5.824 | 0.02 |
| Severe PH | 10 (8.5) | 4 (18.2) | 1.013 | 0.31 |
| LVEF, % | 63.0 (59.8–68.0) | 58.0 (38.5–62.0) | −3.387 | <0.001 |
| Emergency surgery | 49 (41.5) | 10 (45.5) | 0.117 | 0.82 |
| Type of surgery | 2.938 | 0.58 | ||
| Isolated valve surgery | 46 (39.0) | 11 (50.0) | ||
| Isolated aortic surgery | 8 (6.8) | 2 (9.1) | ||
| Combined aortic + valve surgery | 30 (25.4) | 5 (22.7) | ||
| Resection cardiac tumour | 12 (10.2) | 0 | ||
| Other | 22 (18.6) | 4 (18.2) | ||
| Oxytocin use during surgery | 25 (21.2) | 3 (13.6) | 0.273 | 0.60 |
| Vaginal balloon use | 29 (24.6) | 5 (22.7) | 0.034 | >0.99 |
| Allogeneic blood transfusion | 64 (54.2) | 16 (72.7) | 2.589 | 0.16 |
| Intraoperative blood loss, mL | 600.0 (200.0–1,000.0) | 800.0 (300.0–1,350.0) | −1.572 | 0.12 |
| Operation time, min | 284.5 (192.5–381.3) | 330.0 (205.0–547.5) | −1.798 | 0.07 |
| CPB | 118 (100.0) | 21 (95.5) | −1.495 | 0.08 |
| CPB time, min | 108.5 (74.3–158.8) | 135.0 (98.8–199.0) | −2.001 | 0.048 |
| Clamping time, min | 66.5 (44.0–91.8) | 86.5 (52.8–116.3) | −1.990 | 0.046 |
| Minimum nasopharyngeal temperature, ℃ | 33.0 (29.2–35.3) | 31.0 (25.5–34.9) | −1.041 | 0.30 |
| Minimum rectal temperature, ℃ | 34.0 (31.0–35.7) | 32.0 (25.9–35.3) | −1.376 | 0.17 |
| Deep hypothermic circulatory arrest | 23 (19.5) | 6 (27.3) | 0.292 | 0.59 |
| ICU stay time, days | 2.0 (1.0–3.7) | 3.0 (2.0–8.8) | −2.694 | 0.007 |
| Foetal management | 0.880 | 0.66 | ||
| Terminated pregnancy prior to cardiac surgery | 15 (12.7) | 4 (18.2) | ||
| Performed cardiac surgery with foetus in situ | 60 (50.8) | 9 (40.9) | ||
| Performed CS prior to cardiac surgery | 43 (36.4) | 9 (40.9) | ||
Values are presented as numbers (%) or median (interquartile range). NYHA, New York Heart Association; PH, pulmonary hypertension; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; ICU, intensive care unit; CS, caesarean section.
Table 8
| Variables | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age, years | 0.901 | 0.809–1.004 | 0.06 | ||||
| NYHA classification, n (%) | 125,877,941.649 | 0.000– | 0.99 | 1.270 | 0.266–6.077 | 0.76 | |
| PH | 3.100 | 1.201–8.002 | 0.02 | 57.427 | 5.553–593.932 | 0.001 | |
| LVEF, % | 0.914 | 0.872–0.957 | <0.001 | 0.825 | 0.748–0.911 | <0.001 | |
| Allogeneic blood transfusion | 2.250 | 0.823–6.151 | 0.11 | ||||
| Intraoperative blood loss, mL | 1.001 | 1.000–1.002 | 0.04 | 1.005 | 1.002–1.008 | 0.001 | |
| Operation time, min | 1.004 | 1.001–1.007 | 0.02 | 0.997 | 0.989–1.002 | 0.50 | |
| CPB time, min | 1.009 | 1.002–1.015 | 0.007 | 0.992 | 0.973–1.011 | 0.38 | |
| Clamping time, min | 1.011 | 1.000–1.022 | 0.06 | ||||
| ICU stay time, days | 1.077 | 1.018–1.139 | 0.01 | 1.149 | 1.017–1.298 | 0.03 | |
NYHA, New York Heart Association; PH, pulmonary hypertension; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; ICU, intensive care unit; OR, odds ratio; CI, confidence interval.
Table 8 presents the best-fitting binary logistic regression model for the factors associated with postoperative new-onset cardiovascular complications or death. Outcomes of postoperative new-onset cardiovascular complications or death were associated with preoperative LVEF, the presence of PH, and intraoperative blood loss.
The predictive performance of the different models was assessed using ROC curves. Figure 3 shows the predictive performance of individual risk factors or different combinations. The ROC curve suggested that the combined prediction of these three factors yielded the best results, with the AUC of 0.803. Analysis using the ‘proc’ package in R revealed that patients with LVEF ≤61.5% had a risk of adverse outcomes 4.205 times higher than that of patients with LVEF >61.5%. Similarly, patients with an intraoperative blood loss >1,150 mL had a risk of adverse outcomes 4.226 times higher than that of patients with intraoperative blood loss ≤1,150 mL.

Discussion
During pregnancy, cardiac surgery is associated with high maternal and foetal risks. In this study, the maternal and foetal mortality rates were 4.3% and 35.7%, respectively. Combining the presence of PH, preoperative LVEF, and intraoperative blood loss volume helps predict the overall incidence of postoperative new-onset cardiovascular complications or death.
This retrospective study was based on data from two of the largest Obstetrics and Gynecology Medical Centres for Severe Cardiovascular Diseases in northern and southern China. These two centres have annual delivery volumes of 2,600 and 2,300 cases, respectively, and annual cardiac surgery volumes of 15,000 and 5,500 cases, respectively, making them two of the three largest cardiac centres in China. We identified 7,492 cases of pregnant women with heart disease complications over the past 13 years, with 140 patients undergoing cardiac surgery during pregnancy.
Among the patients who underwent cardiac surgery during pregnancy included in this study, the proportion of patients with aortic dissection was the highest, and 70% of patients had PH. Aortic dissection and PH were the two most critical conditions, and patients had a high risk of cardiac complications (2,10). With the continuous optimization and improvement of comprehensive clinical management of these patients over the years, maternal and foetal outcomes have continuously improved. This study showed that the maternal mortality rate was lower than the 7.3% reported in a meta-analysis (12). We believe that this could be due to (I) increased multidisciplinary understanding of the pathophysiology of pregnant women with heart diseases at both centres; (II) optimization of clinical management processes; (III) focused and advanced multidisciplinary teams; (IV) a high level of cardiac surgery expertise; and (V) improved postoperative monitoring and treatment (1,11).
Our data indicated that the timing of cardiac surgery and whether to terminate pregnancy during surgery did not significantly impact maternal mortality (13). This finding contradicts the recommendation in the 2018 European Society of Cardiology guidelines that suggests cardiac surgery should ideally be performed ‘before 28 weeks’ of pregnancy due to high risks (2). While cardiac surgery should be avoided as much as possible during pregnancy because of the high maternal-foetal risks, it can be conducted at any stage of pregnancy if there is a need to save the mother’s life. Similarly, there is no need to proactively perform cardiac surgery in the early or mid-pregnancy stages because of concerns regarding maternal deterioration and increased surgical risks.
Over the past 13 years, we have been committed to continuously optimizing the clinical management of such patients. At the beginning we focused on only saving the mothers’ lives, while abandoning the foetuses. Then we could successfully treat pregnant women and foetuses, giving priority to delivery, ensuring maternal safety and protecting the foetuses. The overall foetal mortality rate was 35.7%, which was higher than the reported 10–30% in the literature (14,15). This may be related to the fact that these two centres focused mainly on treating patients with severe conditions, such as PH and aortic dissection. Foetal outcomes were significantly influenced by maternal functional status. Foetal management approaches, surgical factors, maternal postoperative status and social factors can also have a significant impact on the foetus in those undergoing cardiac surgery during pregnancy. Improving foetal outcomes while ensuring maternal safety has become an increasingly important topic for patients of this kind. Different foetal management strategies during surgery significantly impact foetal outcomes, necessitating an evaluation of foetal development, cardiac surgery factors, and the risk of preterm birth to make comprehensive decisions (16,17). Owing to perioperative factors, the foetal mortality rate of patients undergoing cardiac surgery with a foetus in situ is significantly higher than that of patients who undergo CS prior to cardiac surgery. We initiated further clinical research to analyse the causes of adverse foetal outcomes in patients who underwent cardiac surgery in situ.
Postoperative new-onset cardiovascular complications affect the postoperative quality of life of mothers and increase healthcare costs. For example, they can affect whether a pregnant woman can conceive again and tolerate the pregnancy until delivery. The maternal cardiac status after the surgery for patients performing cardiac surgery with foetus in situ also has a significant impact on the long-term foetal outcome (11). Maternal heart failure and severe arrhythmias can potentially result in intrauterine foetal demise, miscarriage, preterm birth, and foetal growth restriction (15). In this study, in addition to six maternal deaths, 16 patients had new-onset heart failure and arrhythmia after surgery. Multivariate regression analysis revealed that PH, preoperative LVEF, and intraoperative blood loss volume were independent risk factors predicting postoperative new-onset cardiovascular complications or death.
LVEF assessment of left ventricular function is of great significance. Patients with decreased preoperative LVEF and poor cardiac function have significantly increased difficulty recovering after surgery, CPB, pregnancy, and delivery (16,18). Preoperative medical treatment to maintain cardiac function as much as possible is important for reducing the overall perioperative risk. Left ventricular dysfunction is usually diagnosed if the LVEF of a woman is under 50%, which should be adjusted based on the specific pathological and physiological changes in heart disease and pregnancy. We found that LVEF less than 61.5% was an important risk factor for maternal outcomes after cardiac surgery. This also indicates from another perspective that for the high hemodynamic status of pregnant women, the previous criteria for determining the normal LVEF value may need revaluation (19). It may be necessary to use a different LVEF value in pregnancy to assess the risks of cardiac surgery (20,21).
PH is an independent risk factor for perinatal heart failure. The results of individual studies on PH in the Registry on Pregnancy and Cardiac Disease (ROPAC) showed that the incidence of heart failure in patients with PH is 30%, and the incidence of arrhythmia is also high (13). PH in patients undergoing cardiac surgery during pregnancy is mainly secondary to primary left heart system diseases, such as mitral stenosis and congenital heart disease (22). The persistence of primary diseases without correction often leads to unsatisfactory therapeutic effects of specific drugs for reducing pulmonary arterial pressure. Patients with this type of PH generally have a longer course of disease and a more severe condition (23). Due to factors such as pulmonary endothelial remodelling and impaired right heart function caused by a longer course of disease, PH and right heart function may not necessarily be alleviated after cardiac surgery, and the adverse effects of PH may persist for a long time. In addition, surgery, CPB, changes in maternal hormonal levels, and other factors can negatively impact the pulmonary circulation, significantly increasing the probability of postoperative adverse events in these patients (24-26). The 2018 European Society of Cardiology (ESC) guidelines recommend that patients with concomitant PH avoid or terminate pregnancy early (2). This study found an impact of PH on postoperative new-onset cardiovascular complications or death; however, it cannot confirm whether there are differences in the effects of different severities of PH, which may be related to the small sample size.
The intraoperative blood loss volume is a risk factor for predicting the overall incidence of postoperative cardiovascular and cerebrovascular complications and death. If the overall intraoperative blood loss exceeds 1,150 mL, it is necessary to be vigilant because the probability of postoperative complications in patients will greatly increase (27). The increase in intraoperative bleeding could be from cardiac surgery and obstetric reasons (28). Unfortunately, the data from this retrospective study cannot distinguish the specific source of bleeding. The increased transfusion volume and the use of blood products caused by increased bleeding may lead to increased right heart failure due to fluid overload (29). Judicious volume therapy should be administered under comprehensive monitoring of right heart function, especially for patients in the middle and late stages of pregnancy.
While preparing for this study, we found that there is currently no maternal and foetal risk prediction system specifically designed for pregnant women undergoing cardiac surgery, which may be mainly related to the lack of large-scale case studies (2,5,9). The currently recommended risk prediction systems are primarily based on the preoperative clinical characteristics of the mother. However, surgery-related factors can notably impact the mother and foetus. The current risk prediction factors do not include intraoperative or postoperative factors. Therefore, establishing a risk prediction system that includes preoperative clinical features and intraoperative and postoperative factors has become an urgent need. In addition, it is necessary to dynamically predict maternal and foetal risks based on changes in conditions during the perioperative and perinatal periods.
Limitations
Although this study has the largest sample size of patients undergoing cardiac surgery during pregnancy, the sample size might still be insufficient and large multicentre studies are needed. Further, there was a lack of predictive factors for foetal outcomes in the data analysis. This was mainly because various factors influence the choice of foetal management during cardiac surgery, and the outcome of the foetus is closely related to the management method. The factors affecting foetal outcomes are significantly different among different treatment methods and need to be demonstrated separately.
Conclusions
Cardiac surgery during pregnancy is associated with high maternal and foetal risks. Combining preoperative LVEF, PH, and intraoperative blood loss can predict postoperative cardiovascular complications and mortality reliably.
Acknowledgments
The authors would like to thank Professor Sheng Wang for excellent technical support and Professor Jiakai Lu for critically reviewing the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-787/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-787/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-787/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-787/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University and the Medical Ethics Committee of Guangdong Provincial People’s Hospital (approval No. KS2023064). The requirement for informed consent was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roos-Hesselink J, Baris L, Johnson M, et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J 2019;40:3848-55. [Crossref] [PubMed]
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165-241. [Crossref] [PubMed]
- Shook LL, Barth WH Jr. Cardiac Surgery During Pregnancy. Clin Obstet Gynecol 2020;63:429-46. [Crossref] [PubMed]
- Morton A. Physiological Changes and Cardiovascular Investigations in Pregnancy. Heart Lung Circ 2021;30:e6-e15. [Crossref] [PubMed]
- Silversides CK, Grewal J, Mason J, et al. Pregnancy Outcomes in Women With Heart Disease: The CARPREG II Study. J Am Coll Cardiol 2018;71:2419-30. [Crossref] [PubMed]
- Lu A, Ye Y, Hu J, et al. Case Series: Video-Assisted Minimally Invasive Cardiac Surgery During Pregnancy. Front Med (Lausanne) 2021;8:781690. [Crossref] [PubMed]
- Hu J, Ye Y, Lu A, et al. Pregnancy outcomes in patients with heart disease in China. Am J Cardiol 2020;125:1718-24. [Crossref] [PubMed]
- Wang Z, Sun H, Zhang C, et al. Outcomes of acute type A aortic dissection repair during pregnancy. Int J Gynaecol Obstet 2023;161:927-33. [Crossref] [PubMed]
- Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J 2010;31:2124-32. [Crossref] [PubMed]
- Ma WG, Zhu JM, Chen Y, et al. Aortic dissection during pregnancy and postpartum in patients with Marfan syndrome: a 21-year clinical experience in 30 patients. Eur J Cardiothorac Surg 2020;58:294-301. [Crossref] [PubMed]
- Wang J, Lu J. Anesthesia for Pregnant Women with Pulmonary Hypertension. J Cardiothorac Vasc Anesth 2021;35:2201-11. [Crossref] [PubMed]
- van Steenbergen GJ, Tsang QHY, van der Heijden OWH, et al. Timing of cardiac surgery during pregnancy: a patient-level meta-analysis. Eur Heart J 2022;43:2801-11. [Crossref] [PubMed]
- Sliwa K, van Hagen IM, Budts W, et al. Pulmonary hypertension and pregnancy outcomes: data from the Registry Of Pregnancy and Cardiac Disease (ROPAC) of the European Society of Cardiology. Eur J Heart Fail 2016;18:1119-28. [Crossref] [PubMed]
- Liu Y, Han F, Zhuang J, et al. Cardiac operation under cardiopulmonary bypass during pregnancy. J Cardiothorac Surg 2020;15:92. [Crossref] [PubMed]
- Pfaller B, Sathananthan G, Grewal J, et al. Preventing Complications in Pregnant Women With Cardiac Disease. J Am Coll Cardiol 2020;75:1443-52. [Crossref] [PubMed]
- Jha N, Jha AK, Chand Chauhan R, et al. Maternal and Fetal Outcome After Cardiac Operations During Pregnancy: A Meta-Analysis. Ann Thorac Surg 2018;106:618-26. [Crossref] [PubMed]
- Siu SC, Evans KL, Foley MR. Risk Assessment of the Cardiac Pregnant Patient. Clin Obstet Gynecol 2020;63:815-27. [Crossref] [PubMed]
- Meng X, Han J, Wang L, et al. Aortic dissection during pregnancy and postpartum. J Card Surg 2021;36:2510-7. [Crossref] [PubMed]
- Campens L, Baris L, Scott NS, et al. Pregnancy outcome in thoracic aortic disease data from the Registry Of Pregnancy And Cardiac disease. Heart 2021;107:1704-9. [Crossref] [PubMed]
- Liu YY, Li HY, Jiang WJ, et al. Treatment of patients with aortic disease during pregnancy and after delivery. J Int Med Res 2017;45:1359-68. [Crossref] [PubMed]
- Braverman AC, Mittauer E, Harris KM, et al. Clinical Features and Outcomes of Pregnancy-Related Acute Aortic Dissection. JAMA Cardiol 2021;6:58-66. [Crossref] [PubMed]
- Li Q, Dimopoulos K, Liu T, et al. Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease. Eur J Prev Cardiol 2019;26:1067-76. [Crossref] [PubMed]
- Yang M, Wang J, Zhang X, et al. Incidence and long-term outcomes of pregnant women complicated with pulmonary arterial hypertension during different pregnancies: A prospective cohort study from China. Int J Cardiol 2021;326:178-83. [Crossref] [PubMed]
- Sarkar MS, Desai PM. Pulmonary hypertension and cardiac anesthesia: Anesthesiologist's perspective. Ann Card Anaesth 2018;21:116-22. [Crossref] [PubMed]
- Meng ML, Arendt KW, Banayan JM, et al. Anesthetic Care of the Pregnant Patient With Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2023;147:e657-73. [Crossref] [PubMed]
- Afify H, Kong A, Bernal J, et al. Pulmonary Hypertension in Pregnancy: Challenges and Solutions. Integr Blood Press Control 2022;15:33-41. [Crossref] [PubMed]
- Siu SC, Lam M, Le B, et al. Morbidity in Pregnant Women with a Prosthetic Heart Valve. Am J Obstet Gynecol MFM 2020;2:100105. [Crossref] [PubMed]
- Wichert-Schmitt B, Grewal J, Malinowski AK, et al. Outcomes of Pregnancy in Women With Bioprosthetic Heart Valves With or Without Valve Dysfunction. J Am Coll Cardiol 2022;80:2014-24. [Crossref] [PubMed]
- You Y, Liu S, Wu Z, et al. Cardiac surgery under cardiopulmonary bypass in pregnancy: report of four cases. J Cardiothorac Surg 2021;16:268. [Crossref] [PubMed]


