
Effectiveness of the enhanced recovery after surgery (ERAS) program after lobectomy for lung cancer: a single-center observational study using propensity score matching in Vietnam
Highlight box
Key findings
• The study demonstrates that implementing enhanced recovery after surgery (ERAS) protocols significantly reduces postoperative length of stay (LOS) and 30-day readmission rates in patients undergoing lobectomy for non-small cell lung cancer (NSCLC).
• The ERAS group showed a median postoperative LOS of 4.6 days compared to 5.1 days in the routine care group, and a readmission rate of 1.6% vs. 14.3%.
What is known and what is new?
• Lobectomy for NSCLC is often associated with significant postoperative complications and extended LOS, particularly in developing countries with limited resources.
• This paper provides evidence that ERAS protocols are effective in reducing LOS and readmission rates without increasing postoperative complications or re-operation rates in a Vietnamese healthcare setting.
What is the implication, and what should change now?
• The adoption of ERAS protocols can improve surgical outcomes, reduce healthcare costs, and minimize the economic burden on healthcare systems in developing countries.
• There is a need for broader implementation and strict adherence to ERAS protocols in surgical practices, particularly in resource-limited settings, to enhance patient recovery and optimize healthcare resource utilization.
Introduction
Lung cancer remains the leading cause of cancer-related deaths worldwide. Early stages are primarily treated with lobectomy, particularly in the 80% of cases identified as non-small cell lung cancer (NSCLC) (1). Despite advancements, this surgical intervention is often associated with significant postoperative complications, with an incidence of 20–29% (2-4). Additionally, lung cancer surgery in general, and lobectomy in particular, often results in an extended length of stay (LOS), exacerbating healthcare and economic burdens, particularly in developing countries with limited resources (5-7). These challenges emphasize the need for strategies to enhance recovery and minimize complications. Therefore, the enhanced recovery after surgery (ERAS) protocols have been developed and have become increasingly prevalent across various surgical fields (8).
In 2019, the ERAS Society updated its guidelines to include specific recommendations for thoracic surgeries, including lung lobectomy (9). Innovations in surgical care and management have been shown to enhance the prognosis and outcomes of lung cancer surgery, yielding positive results (10). Despite their demonstrated benefits, the implementation of ERAS protocols varies significantly across healthcare settings, influenced by local resources, institutional policies, and the specific needs of patient populations (8,11). In developing countries, where certain healthcare settings may experience higher complication rates and resource limitations, the benefits of the ERAS program could be especially substantial (7,12). However, the practical application of these protocols to improve surgical outcomes in lung cancer treatments is significantly hindered.
At our center, we have integrated the ERAS guidelines into our treatment protocol for surgical patients since 2019. This initiative is essential for improving surgical results and serves as a model for similar healthcare settings facing resource limitations. Our study aims to assess the effect of the ERAS program on patients with lung cancers undergoing lobectomy. We seek to add to the evidence supporting the efficacy of these practices and provide insights to promote their wider adoption nationally. Our study hypothesized that implementing the ERAS protocol would reduce postoperative LOS as well as complications, re-operation, and re-admission rates. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1053/rc).
Methods
Study settings and participants
This observational study was conducted at the University Medical Center Ho Chi Minh City (UMC HCMC) and included patients who underwent lobectomy for NSCLC. This study compared the outcomes between two groups: one undergoing the ERAS protocol from February 2022 to December 2023 and a routine care group prior to the implementation of the ERAS protocol, from January 2018 to December 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee in Biomedical Research of the University of Medicine and Pharmacy at Ho Chi Minh City on February 21th, 2022 (No. 2294-DHYD) and individual consent for this retrospective analysis was waived.
The study involved patients diagnosed with NSCLC via pathology who were undergoing anatomical lung lobectomy. Exclusion criteria included patients transferred to other facilities for postoperative care. For the ERAS care group, patients diagnosed with a lung tumor but without a confirmed pathology result were scheduled for surgery and underwent intraoperative frozen section biopsy. These patients were initially included in the ERAS program. However, if the intraoperative frozen section biopsy results did not confirm NSCLC, they were excluded from the study.
Protocols
The ERAS program followed ERAS Society guidelines (9) and recommendations from the French Society of Anaesthesia and Intensive Care Medicine for pulmonary lobectomy (13). Its development involved assessments of preoperative, intraoperative, and postoperative patient care, facility conditions, and medical staff practices guided by the hospital’s Scientific Council. Components of ERAS protocol are shown in Figure 1. Details of ERAS activities are presented in Table S1.
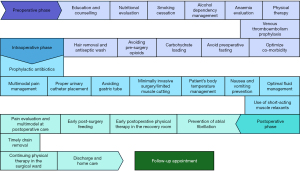
For pain management, we use a combination of 1–3 non-opioid analgesics such as intravenous paracetamol, ketorolac, and nefopam, administered towards the end of surgery. Thoracotomy patients typically receive thoracic epidural analgesia (TEA) and thoracic paravertebral block (TPVB) preoperatively, continued until chest drainage ceases. In video-assisted thoracoscopic surgery (VATS), patients get either a single-shot TPVB or erector spinae plane block (ESPB), or three non-opioids. Intravenous opioids like tramadol or morphine are reserved for rescue analgesia in VATS when pain exceeds a visual analogue scale score of 4/10. Proactive intravenous opioid use is considered when VATS converts to open surgery. Postoperatively, oral non-opioids such as paracetamol, diclofenac, celecoxib, and pregabalin are used as soon as feasible.
Hospital discharge criteria included: (I) pleural drainage tube removed, (II) no signs of infection, (III) pain control with oral analgesics, (IV) the X-ray indicated well-expanded lungs with no significant effusion or pneumothorax, and (V) able to oral feedings and did not require nasal cannula oxygen. These criteria have been standardized in the hospital procedures since the implementation of the hospital’s lobectomy procedures.
Data collection
Data were extracted from a computerized database of patients hospitalized for lobectomy in the treatment of NSCLC from January 2018 to December 2023.
Demographic and clinical characteristics variables involved age, gender, body mass index (BMI), smoking status, comorbidities, American Society of Anesthesiologists (ASA) physical status classification, tumour location, tumour-node-metastasis (TNM) staging, histological cancer features, and cellular differentiation.
Variables for treatment process included operation method (open surgery, VATS, and VATS converted to open surgery), operation time (minutes), blood loss (mL), duration in post anesthesia care unit (hours), duration of thoracic drainage (days), postoperative fasting time (hours), urinary catheter retention time (hours).
Outcomes
The primary outcome, postoperative LOS, was determined by counting the days from the end of surgery until discharge. Similarly, perioperative LOS was determined by totalling the days from patient admission to discharge.
Secondary outcomes were postoperative complications, re-operation, and re-admission. Complications are categorized into five levels using the Clavien-Dindo classification: no complications, grade I, grade II, grade IIIa, grade IIIb, grade IVa, grade IVb, and grade V (14). Re-operation and re-admission within 30 days and detailed reasons for these events were collected.
Propensity score matching (PSM) analysis
PSM analysis was conducted to adjust for differences between the two groups. The following covariates were considered: age, sex, BMI, ASA classification, smoking status, cancer stage, and operation method. Individual propensity scores were calculated using a logistic regression model, and patients from the two groups were matched using the nearest-neighbor matching algorithm at a 1:1 ratio, with a caliper width of 0.1 standard deviations (SDs) of the propensity score.
Statistical analysis
Statistical analyses were performed using R software (version 4.3.2, R Foundation for Statistical Computing, Austria). All patient characteristics and outcomes were summarized using frequency and percentage for categorical variables, mean and SD for numeric variables with normal distribution, and median and interquartile range (IQR) for numeric variables without normal distribution. Differences between the two groups were tested using the Chi-square test or Fisher’s exact test for categorical variables, two-sample t-test for numeric variables with normal distribution, and Wilcoxon rank-sum test for numeric variables without normal distribution. To estimate the central tendency difference in LOS between two groups after matching, the mean difference was used for a normally distributed variable and the Hodges-Lehmann estimate was used for a non-normally distributed variable. All tests were two-sided and statistical significance was defined when P value was less than 0.05.
Results
Demographic and clinical characteristics
A total of 197 patients were enrolled in the study, with 98 patients assigned to the ERAS group and 99 to the routine care group. Prior to matching, differences were observed in age, ASA classification, and smoking status. After PSM, each group comprised 63 patients. All baseline characteristics were more balanced, including age, gender, BMI and ASA classification, comorbidities, smoking status, tumor location, lung cancer cell types, and differentiation, as detailed in Table 1.
Table 1
| Variables | Unmatched cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| Routine (n=99) | ERAS (n=98) | P value | Routine (n=63) | ERAS (n=63) | P value | ||
| Age (years), mean ± SD | 64.8±9.9 | 61.1±10.7 | 0.02 | 61.6±9.2 | 62.7±9.7 | 0.37 | |
| Male, n (%) | 53 (53.5) | 43 (43.9) | 0.20 | 33 (52.4) | 31 (49.2) | 0.86 | |
| BMI (kg/m2), mean ± SD | 22.5±2.6 | 22.2±2.2 | 0.45 | 22.3±2.6 | 22.1±2.3 | 0.38 | |
| ASA classification, n (%) | <0.001 | 0.20 | |||||
| I | 4 (4.0) | 6 (6.1) | 2 (3.2) | 6 (9.5) | |||
| II | 79 (79.8) | 49 (50.0) | 49 (77.8) | 41 (65.1) | |||
| III | 16 (16.2) | 43 (43.9) | 12 (19.0) | 16 (25.4) | |||
| Comorbidities, n (%) | |||||||
| Hypertension | 47 (47.5) | 51 (52.0) | 0.57 | 25 (39.7) | 34 (54.0) | 0.15 | |
| Asthma/COPD | 5 (5.1) | 4 (4.1) | >0.99 | 4 (6.3) | 2 (3.2) | 0.68 | |
| Diabetes | 16 (16.2) | 13 (13.3) | 0.69 | 12 (19.0) | 10 (15.9) | 0.82 | |
| Dyslipidemia | 2 (2.0) | 1 (1.0) | >0.99 | 2 (3.2) | 1 (1.6) | >0.99 | |
| Coronary artery disease | 9 (9.1) | 11 (11.2) | 0.65 | 6 (9.5) | 7 (11.1) | >0.99 | |
| Heart failure | 1 (1.0) | 2 (2.0) | 0.62 | 0 (0.0) | 1 (1.6) | >0.99 | |
| Chronic kidney disease | 1 (1.0) | 0 (0.0) | >0.99 | 1 (1.6) | 0 (0.0) | >0.99 | |
| Sequelae of stroke | 1 (1.0) | 2 (2.0) | 0.62 | 0 (0.0) | 1 (1.6) | >0.99 | |
| Thyroid dysfunction | 3 (3.0) | 6 (6.1) | 0.33 | 2 (3.2) | 3 (4.8) | >0.99 | |
| Anemia | 3 (3.0) | 1 (1.0) | 0.62 | 3 (4.8) | 1 (1.6) | 0.62 | |
| Extrapulmonary cancers | 2 (2.0) | 2 (2.0) | >0.99 | 2 (3.2) | 1 (1.6) | >0.99 | |
| Other diseases | 13 (13.1) | 9 (9.2) | 0.50 | 8 (12.7) | 6 (9.5) | 0.79 | |
| Smoking status, n (%) | 0.005 | 0.93 | |||||
| Non-smoker | 62 (62.6) | 70 (71.4) | 43 (68.3) | 42 (66.7) | |||
| Current smoker | 27 (27.3) | 10 (10.2) | 10 (15.9) | 9 (14.3) | |||
| Former smoker | 10 (10.1) | 18 (18.4) | 10 (15.9) | 12 (19.0) | |||
| Tumor location, n (%) | 0.27 | 0.43 | |||||
| Left upper lobe | 27 (27.3) | 21 (21.4) | 15 (23.8) | 12 (19.0) | |||
| Left lower lobe | 12 (12.1) | 21 (21.4) | 8 (12.7) | 14 (22.2) | |||
| Right upper lobe | 30 (30.3) | 21 (21.4) | 20 (31.7) | 13 (20.6) | |||
| Right lower lobe | 18 (18.2) | 22 (22.4) | 11 (17.5) | 14 (22.2) | |||
| Right middle lobe | 12 (12.1) | 13 (13.3) | 9 (14.3) | 10 (15.9) | |||
| TNM staging, n (%) | 0.19 | 0.88 | |||||
| Stage I | 39 (39.4) | 35 (35.7) | 28 (44.4) | 25 (39.7) | |||
| Stage II | 50 (50.5) | 44 (44.9) | 27 (42.9) | 30 (47.6) | |||
| Stage III | 10 (10.1) | 19 (19.4) | 8 (12.7) | 8 (12.7) | |||
| Histological features, n (%) | 0.65 | >0.99 | |||||
| Adenocarcinoma | 78 (78.8) | 81 (82.7) | 51 (81.0) | 51 (81.0) | |||
| Squamous cell carcinoma | 20 (20.2) | 17 (17.3) | 11 (17.5) | 12 (19.0) | |||
| Large cell carcinoma | 1 (1.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | |||
| Cell differentiation, n (%) | 0.12 | 0.12 | |||||
| Well-differentiated | 4 (4.0) | 10 (10.2) | 2 (3.2) | 7 (11.1) | |||
| Moderately differentiated | 88 (88.9) | 77 (78.6) | 58 (92.1) | 50 (79.4) | |||
| Poorly differentiated | 7 (7.1) | 11 (11.2) | 3 (4.8) | 6 (9.5) | |||
ERAS, enhanced recovery after surgery; SD, standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; TNM, tumour-node-metastasis.
Treatment characteristics
The compliance rates for the activities within the ERAS protocol are presented in Figure 2. Most activities achieved a compliance rate of over 90%, demonstrating strong adherence to the protocol. The lowest compliance rates were observed for intraoperative multimodal pain management (62.2%), pain evaluation and multimodal at postoperative care (62.2%), minimally invasive surgery/limited muscle cutting (76.5%), proper urinary catheter placement (76.5%).
Table 2 illustrates the intraoperative and postoperative care of patients. Before matching, the routine care group had a thoracotomy rate of 21.2%, higher than the 8.2% observed in the ERAS group. After PSM, this rate was balanced between the two groups (14.3% vs. 11.1%). The ERAS group exhibited a shorter postoperative fasting time (median: 10 vs. 12 hours, P<0.001) and a reduced urinary catheter retention time (17 vs. 20 hours, P=0.046).
Table 2
| Variables | Unmatched cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| Routine (n=99) | ERAS (n=98) | P value | Routine (n=63) | ERAS (n=63) | P value | ||
| Operation method n (%) | 0.02 | 0.72 | |||||
| Thoracotomy | 21 (21.2) | 8 (8.2) | 9 (14.3) | 7 (11.1) | |||
| VATS | 68 (68.7) | 71 (72.4) | 46 (73.0) | 45 (71.4) | |||
| VATS conversion to thoracotomy | 10 (10.1) | 19 (19.4) | 8 (12.7) | 11 (17.5) | |||
| Operation time (minutes) | 170 (130–195) | 170 (150–190) | 0.15 | 170 (140–198) | 180 (152–209) | 0.18 | |
| Blood loss (mL) | 50 (30–125) | 100 (50–100) | 0.003 | 50 (30–100) | 100 (50–100) | 0.003 | |
| Duration in PACU (hours) | 26 (21–40) | 24 (21–30) | 0.23 | 27 (22–40) | 25 (22–30) | 0.30 | |
| Duration of thoracic drainage (days) | 3 (3–4) | 3 (2–4) | 0.02 | 3 (2–4) | 3 (2–4) | 0.32 | |
| Postoperative fasting time (hours) | 12 (10–16) | 10 (8–12) | <0.001 | 12 (10–16) | 10 (8–12) | <0.001 | |
| Urinary catheter retention time (hours) | 20 (17–24) | 17 (14–20) | 0.001 | 20 (17–24) | 17 (15–21) | 0.046 | |
Data are expressed as n (%) or median (25th–75th percentiles). ERAS, enhanced recovery after surgery; VATS, video-assisted thoracoscopic surgery; PACU, post-anesthesia care unit.
Primary outcome
Figure 3A compares postoperative LOS between the ERAS and routine care groups before and after PSM. The results indicate that both before and after PSM, the ERAS group had a shorter postoperative LOS compared to the routine care group. After PSM, the median postoperative LOS for the ERAS group was 4.6 days, which was significantly shorter than the 5.1 days observed in the routine care group (P=0.01). The difference between postoperative LOS between two group, using the Hodges-Lehmann method, was 0.736 days [95% confidence interval (CI): 0.126 to 1.122].

Similarly, after PSM, the total LOS, including pre-, intra-, and post-operative periods, was shorter in the ERAS group compared to the routine care group. The median LOS for the ERAS group was 8.1 days, while it was 12.1 days for the routine care group (P<0.001), as illustrated in Figure 3B.
Secondary outcomes
After PSM, complications were reported in 17.5% of patients in the ERAS group, compared to 12.7% in the routine care group, P=0.62 (Table 3). The re-operation rates were low and similar between the groups (1.6% in ERAS vs. 3.2% in routine care, P>0.99). However, the 30-day postoperative re-admission rate was significantly lower in the ERAS group (1.6% vs. 14.3%, P=0.02), primarily due to a reduction in pneumonia cases (0.0% vs. 11.1%).
Table 3
| Variables | Unmatched cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| Routine (n=99) | ERAS (n=98) | P value | Routine (n=63) | ERAS (n=63) | P value | ||
| Complications, n (%) | 0.12 | 0.62 | |||||
| Absence | 80 (80.8) | 86 (87.8) | 55 (87.3) | 52 (82.5) | |||
| Presence | 19 (19.2) | 12 (12.2) | 8 (12.7) | 11 (17.5) | |||
| Clavien-Dindo I | 4 (4.0) | 7 (7.1) | 1 (1.6) | 7 (11.1) | |||
| Clavien-Dindo II | 9 (9.1) | 4 (4.1) | 4 (6.3) | 3 (4.8) | |||
| Clavien-Dindo IIIa | 4 (4.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | |||
| Clavien-Dindo IIIb | 2 (2.0) | 1 (1.0) | 2 (3.2) | 1 (1.6) | |||
| Re-operation, n (%) | >0.99 | >0.99 | |||||
| Absence | 97 (98.0) | 97 (99.0) | 61 (96.8) | 62 (98.4) | |||
| Presence | 2 (2.0) | 1 (1.0) | 2 (3.2) | 1 (1.6) | |||
| Bronchopleural fistula repair | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (1.6) | |||
| Chest tube placement surveillance | 1 (1.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | |||
| Thoracotomy for hemostasis | 1 (1.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | |||
| Re-admission, n (%) | 0.001 | 0.02 | |||||
| Absence | 86 (89.6) | 97 (99.0) | 54 (85.7) | 62 (98.4) | |||
| Presence | 13 (13.1) | 1 (1.0) | 9 (14.3) | 1 (1.6) | |||
| Pneumonia | 10 (10.1) | 0 (0.0) | 7 (11.1) | 0 (0.0) | |||
| Pleural effusion | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (1.6) | |||
| Respiratory failure | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Cerebral infarction | 1 (1.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | |||
| Hemoptysis | 1 (1.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | |||
ERAS, enhanced recovery after surgery.
Discussion
This study thoroughly evaluates the ERAS protocols at the UMC HCMC for patients undergoing lobectomy due to NSCLC. One of the problems in Vietnam is the prolonged hospital stay after surgery and the high rate of complications (15). This context is similar to that of other developing countries (16). In Vietnam, ERAS protocols have not been extensively adopted, primarily because their effectiveness has not been widely studied and acknowledged. Additionally, hospitals lack the resources and knowledge regarding these protocols.
The ERAS protocols have effectively reduced LOS for lobectomy patients, as evidenced by our study’s reduction from 5.1 to 4.6 days postoperatively and 12.1 to 8.1 days perioperatively. This aligns with Laohathai’s research in a similar developing country setting, which recorded an average LOS reduction of 2.40 days (95% CI: 0.65–4.45) (12). A meta-analysis by Li et al., covering 21 studies and 6,480 patients, reported a postoperative LOS range of 3.0 to 8.9 days, fitting within the global observations of 4.0 to 18.9 days (17). Our study further confirms the global efficacy of ERAS in lung lobectomy.
Although our study observed a statistically significant reduction in postoperative LOS of 0.736 days between two groups, the clinical significance of this modest improvement remains uncertain. This highlights the need for future research to establish the minimal clinically important difference (MCID) for lobectomy patients, particularly in the absence of relevant meta-analyses. Nonetheless, we consider this result acceptable for a developing country that is in the initial stages of implementing the ERAS protocol, given the limitations in resources and the numerous challenges faced.
Most activities in our study achieved a compliance rate of over 90%, significantly exceeding the typical ERAS adherence rates in developing countries, which range from 15.5% to 61.8% depending on the component (18). This high compliance demonstrates that implementing ERAS programs in such settings is feasible and can lead to substantial improvements across various phases, including reductions in smoking rates, thoracotomy rates, fasting times, and catheter retention times. Our findings suggest that positive outcomes are attainable. However, domains such as intraoperative pain management, postoperative pain evaluation, minimally invasive surgical techniques, and urinary catheter placement demonstrated lower compliance rates, suggesting that these areas warrant further attention and enhancement in practice.
The incidence of postoperative pneumonia was significantly lower in the ERAS group (0.0%) compared to the routine care group (11.1%). This finding was similar to results from a systematic review and meta-analysis of randomized controlled trials, which demonstrated that using ERAS protocols in VATS lobectomy significantly reduced the rates of postoperative pulmonary complications (relative risk 0.43, P<0.001) (19). These outcomes enhance patient satisfaction and reduce the risk of nosocomial infections, contributing to considerable cost savings for healthcare systems. This is especially critical for developing countries like Vietnam, where resources are scarce, and the economic impact of postoperative respiratory complications is considerable, affecting both individuals and the broader national economy (7). Physical therapy and early mobilization are two critical components of the ERAS program, playing a key role in reducing postoperative pneumonia complications. Respiratory physical therapy includes deep breathing exercises and controlled coughing techniques, which help clear the airways, enhance ventilation, and reduce mucus retention, thereby lowering the risk of pneumonia. Measures such as the use of positive pressure breathing devices can also improve lung function and maintain airway patency (20). Early mobilization, another crucial factor in ERAS, significantly promotes the recovery of lung function. Encouraging patients to move shortly after surgery enhances blood circulation and improves gas exchange, reducing fluid retention in the lungs. A study has shown that patients participating in early mobilization programs have lower rates of respiratory complications and shorter hospital stays compared to those not encouraged to mobilize (20).
Regarding resources, we invested in four key areas as per the 3M + T formula: manpower, material, money, and time. For manpower, the implementation of ERAS involved surgeons, anesthesiologists, nurses, technicians, and other healthcare staff. They were provided extensive training on the ERAS process, from preoperative patient preparation to postoperative monitoring and care. For materials, we utilized medical equipment, support tools, and necessary infrastructure. Devices for controlling body temperature, managing postoperative pain, and anesthesia tools were required to meet high standards and were regularly maintained. Money and time were essential for sustaining staff training and the rollout of the ERAS program. In developing countries, barriers in education, multidisciplinary teams, and communication often restrict the effective implementation of ERAS protocols (18).
The strict adherence to ERAS protocols—from preoperative counselling through postoperative care—highlights a solid commitment to these procedures, significantly enhancing surgical outcomes (21). However, widespread adoption and compliance with ERAS protocols remain limited, especially in developing countries with constrained resources. These challenges underline the critical need for better support systems to promote the comprehensive implementation of ERAS practices. Given the substantial benefits observed in this study, there is a strong case for the rapid introduction and strict observance of ERAS in developing countries to achieve improved patient outcomes. Achieving these benefits requires patience and sustained effort to realize long-term gains for individuals, regions, and countries (12).
This study employed PSM to mitigate confounding factors in the observational study but has some limitations. Firstly, the sample size both before and after PSM was indeed limited, potentially leading to residual confounders as not all relevant factors could be matched. Secondly, it was an ambispective observational study with retrospectively collected routine group data, raising concerns about missing value biases and information biases. Thirdly, the observational nature might constrain the generalizability of results across varied healthcare environments. Further research is needed to explore the individual components of ERAS protocols more thoroughly and determine which elements contribute most significantly to improved outcomes. Additionally, studies should investigate the implementation challenges and strategies for overcoming barriers in different healthcare systems, especially in developing countries.
Conclusions
ERAS protocols significantly improve postoperative outcomes in lobectomy patients by reducing the LOS and re-admission rates without increasing complications or re-operation rates. Consequently, ERAS should be considered an alternative protocol for routine use in developing countries to minimize healthcare and economic burdens.
Acknowledgments
The authors extend their gratitude to the nurses, physicians, and participants at the University Medical Center Ho Chi Minh City, where the study was conducted.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1053/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1053/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1053/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1053/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee in Biomedical Research of the University of Medicine and Pharmacy at Ho Chi Minh City on February 21th, 2022 (No. 2294-DHYD) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Cancer Facts & Figures 2024. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf
- Motono N, Ishikawa M, Iwai S, et al. Individualization of risk factors for postoperative complication after lung cancer surgery: a retrospective study. BMC Surg 2021;21:311. [Crossref] [PubMed]
- Jing R, He S, Dai H, Lin F, Ge W, Tao G, et al. Incidence and risk factors of postoperative pulmonary complications after thoracic surgery for early non-small cell lung cancer. Int J Clin Exp Med 2018;11:285-94.
- Bevilacqua Filho CT, Schmidt AP, Felix EA, et al. Risk factors for postoperative pulmonary complications and prolonged hospital stay in pulmonary resection patients: a retrospective study. Braz J Anesthesiol 2021;71:333-8. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin 2012;62:283-98. [Crossref] [PubMed]
- Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol 2013;14:e152-7. [Crossref] [PubMed]
- Hanh BM, Long KQ, Anh LP, et al. Respiratory complications after surgery in Vietnam: National estimates of the economic burden. Lancet Reg Health West Pac 2021;10:100125. [Crossref] [PubMed]
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Comacchio GM, Monaci N, Verderi E, et al. Enhanced recovery after elective surgery for lung cancer patients: analysis of current pathways and perspectives. J Thorac Dis 2019;11:S515-22. [Crossref] [PubMed]
- Clet A, Guy M, Muir JF, et al. Enhanced Recovery after Surgery (ERAS) Implementation and Barriers among Healthcare Providers in France: A Cross-Sectional Study. Healthcare (Basel) 2024;12:436. [Crossref] [PubMed]
- Laohathai S, Sadad Z, Suvarnakich K, et al. Efficacy of the Enhanced Recovery After Surgery program for thoracic surgery in a developing country. Indian J Thorac Cardiovasc Surg 2023;39:476-83. [Crossref] [PubMed]
- Berna P, Quesnel C, Assouad J, et al. Guidelines on enhanced recovery after pulmonary lobectomy. Anaesth Crit Care Pain Med 2021;40:100791. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Tat Bang H, Thanh Vy T, Tap NV. Length of Postoperative Hospital Stay and Related Factors After Lobectomy for Lung Cancer: A Pre-enhanced Recovery After Surgery (ERAS) Single Center Assessment. Cureus 2024;16:e54724. [Crossref] [PubMed]
- Oodit R, McQueen K. ERAS for Low- and Middle-Income Countries. In: Ljungqvist O, Francis NK, Urman RD. editors. Enhanced Recovery After Surgery: A Complete Guide to Optimizing Outcomes. Cham: Springer International Publishing; 2020:623-30.
- Li R, Wang K, Qu C, et al. The effect of the enhanced recovery after surgery program on lung cancer surgery: a systematic review and meta-analysis. J Thorac Dis 2021;13:3566-86. [Crossref] [PubMed]
- Özbay T, Şanlı D, Springer JE. An investigation on the compliance of perioperative practices using ERAS protocols and barriers to the implementation of the ERAS protocols in colorectal surgery. Acta Chir Belg 2024;124:396-405. [Crossref] [PubMed]
- Bellas-Cot Ín S, Casans-Franc S RN, Ib Í. Implementation of an ERAS program in patients undergoing thoracic surgery at a third-level university hospital: an ambispective cohort study. Braz J Anesthesiol 2023;73:16-24. [Crossref] [PubMed]
- Bertani A, Ferrari P, Terzo D, et al. A comprehensive protocol for physiokinesis therapy and enhanced recovery after surgery in patients undergoing video-assisted thoracoscopic surgery lobectomy. J Thorac Dis 2018;10:S499-511. [Crossref] [PubMed]
- Forster C, Doucet V, Perentes JY, et al. Impact of Compliance With Components of an ERAS Pathway on the Outcomes of Anatomic VATS Pulmonary Resections. J Cardiothorac Vasc Anesth 2020;34:1858-66. [Crossref] [PubMed]



