
Serum sestrin 2 and fetuin-A are associated with early pleural involvement in patients with pulmonary tuberculosis: a cross-sectional study
Highlight box
Key findings
• High sestrin 2 and low fetuin-A serum levels can indicate early pleural involvement in patients with pulmonary tuberculosis.
What is known and what is new?
• Tuberculous pleuritis is the second most common extrapulmonary tuberculosis. Its diagnosis is a clinical challenge.
• A high serum sestrin 2 level and a low serum fetuin-A level were associated with early pleural involvement in pulmonary tuberculosis patients.
What is the implication, and what should change now?
• In patients with pulmonary tuberculosis, clinicians can use serum sestrin 2 and fetuin-A levels to assess early pleural involvement.
Introduction
Tuberculosis is a contagious bacterial infection from Mycobacterium tuberculosis (M. tuberculosis). It is a global public health concern, especially in developing countries (1). In addition to the lungs being the most commonly infected site, M. tuberculosis frequently affects other organs to cause extrapulmonary tuberculosis. The diagnosis and management of patients with pulmonary tuberculosis should consider the possibility of extrapulmonary tuberculosis, since the antituberculosis treatment regimens and durations as well as the follow-up monitoring might be different (2).
After M. tuberculosis infection, bacteria can enter the alveoli through the respiratory tract. Macrophages, as the most common host cells of M. tuberculosis, can engulf the bacteria via endocytosis, with the subsequent activation of multiple inflammatory pathways, release of various cytokines, and recruitment of other immune cells (3). Pulmonary tuberculosis can involve the pleural space. Tuberculous pleuritis is the second most common extrapulmonary tuberculosis, with its prevalence reaching up to 31.4% in all tuberculosis patients (4). Without appropriate management, tuberculosis with pleural involvement can lead to serious outcomes, such as pleural thickening, effusion, and adhesion, or even respiratory restriction and distress (5). Nevertheless, the diagnosis of tuberculosis with early pleural involvement can be a challenge (6). Its determination is often completed by pleural biopsy or thoracentesis to look for M. tuberculosis in the tissue samples or pleural fluid. However, the procedures are invasive and not appropriate for every patient, such as those without an adequate amount of pleural effusion for thoracentesis. Thoracic imaging studies, including X-ray and computed tomography (CT) scans, can be used to evaluate pleural involvement in patients with tuberculosis (7). But it must be noted that X-ray has a low sensitivity to detect pleural lesions, and CT scanning is relatively expensive and carries the risk of radiation exposure, which makes it inappropriate to be used repeatedly to monitor for pleural invasion in patients with tuberculosis (8). Although ultrasound is a noninvasive test that is commonly used to guide interventional procedures, its application in diagnosing pleural tuberculosis is still under investigation (9). Therefore, a simple and easy test to provide an accurate diagnosis of pleural tuberculosis is highly desired.
The pleural involvement of pulmonary tuberculosis can be a result of direct bacterial invasion or a type IV hypersensitivity inflammatory response to mycobacterial antigens (6). Various inflammatory biomarkers have been studied during active tuberculosis infection; for example, adenosine deaminase and interferon-gamma are two of the most commonly reported biomarkers (10). However, there is no consensus on their clinical applications. Stress-inducing protein 2 (sestrin 2) is a cellular stress response protein that is mainly involved in autophagy and oxidative stress response (11). Sestrin 2 can interact with multiple signaling pathways to regulate biological functions (12). In addition, it has shown its potential importance in a variety of inflammatory and lung diseases (13,14). Fetuin-A is a multifunctional protein belonging to the cysteine protease inhibitor supergene family. It is mainly synthesized in the liver and secreted into the serum (15). A previous studu has demonstrated that fetuin-A is an acute-phase response protein that is associated with a variety of inflammatory diseases (16). Therefore, it was hypothesized that the sestrin 2 and fetuin-A levels might be significantly altered in patients with tuberculosis.
In the present study, we studied the association between the serum sestrin 2 and fetuin-A levels with pleural involvement in patients with pulmonary tuberculosis. Moreover, we explored the diagnostic performance of these two serum biomarkers, with the purpose of providing a simple and rapid test for clinicians to diagnose pleural involvement in pulmonary tuberculosis patients. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-989/rc).
Methods
Study design and participant selection
We performed a cross-sectional study in consecutive patients with pulmonary tuberculosis admitted to Hunan Chest Hospital between October 2020 and October 2022. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Hunan Chest Hospital (No. LS2021051807). All study participants signed the written informed consent form.
The inclusion criteria were as follows: (I) patient age ≥18 years old; (II) first-time diagnosis of pulmonary tuberculosis based on the clinical symptoms and positive M. tuberculosis result on either a sputum smear or culture (17); and (III) having chest CT scan images to determine the presence of early pleural involvement (infiltrative tuberculosis resulting in local pleural inflammatory changes but without pleural effusion, hypertrophy, or adhesion) (17). The exclusion criteria were as follows: (I) patients with pulmonary or pleural disease other than tuberculosis; (II) other concurrent infectious disease from other pathogens or tubertuculosis in organs other than the respiratory system; (III) long-term treatment with an immunosuppressor; (IV) malignancy; (V) history of drug abuse, or (VI) pregnant or lactating women.
Data collection
Patient baseline information, including age, sex, weight, height, history of smoking or alcohol consumption, hypertension, diabetes, duration of tuberculosis, and clinical symptoms (e.g., pleuritic chest pain, fever, and cough), was recorded. The body mass index (BMI) was calculated.
A chest CT scan was performed to determine early pleural involvement, which was defined as infiltrative tuberculosis resulting in local pleural inflammatory changes but without pleural effusion, hypertrophy, or adhesion (Figure 1) (17).
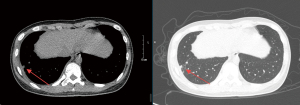
Peripheral blood samples were collected on the same day as the chest CT scan. The serum sestrin 2 and fetuin-A levels were detected by enzyme-linked immunosorbent assays, according to the manufacturer’s instructions (Cloud-Clone Corporation, Wuhan, China). In addition, the white blood cell count, lymphocyte percentage, erythrocyte sedimentation rate, and C-reactive protein levels were also determined in the hospital laboratory.
Statistical analysis
The patients were assigned into either the pleural involvement group or the nonpleural involvement group. The continuous data were presented as the mean ± standard deviation or the median with the interquartile range, and the data were compared by the Student’s t test or the Mann-Whitney test, depending on the normality test result. The categorical data were presented as numbers with percentages and the interquartile range, and they were compared by the Chi-squared or Fisher’s exact test. Multivariate logistic regression analysis was performed to evaluate the relationships between the serum sestrin 2 and fetuin-A levels with pleural involvement. Receiver operating characteristic (ROC) curve analysis was conducted to assess the diagnostic values of the serum sestrin 2 and fetuin-A levels on pleural involvement. The area under the curve (AUC), 95% confidence interval (CI), sensitivity, and specificity were reported. All statistical analyses were performed by using SPSS (version 23.0, IBM, New York, USA). A P value <0.05 was considered statistically significant.
Results
Baseline characteristics of the study participants
A total of 136 patients, including 79 (58.1%) males, with pulmonary tuberculosis were included in the present study. The mean age was 49.9±7.9 (range, 33–66) years, and the mean BMI was 21.0±4.4 kg/m2. The mean values of sestrin 2 and fetuin-A were 18.3±4.3 ng/mL and 321.0±58.1 µg/mL, respectively. The distributions in the study participants are shown in Figure 2. There were 47 patients without pleural involvement (nonpleural involvement group) and 89 patients with pleural involvement (pleural involvement group). The baseline characteristics were comparable between the two groups, except for the sputum bacterial detection (Table 1).

Table 1
| Characteristic | Nonpleural involvement group (n=47) | Pleural involvement group (n=89) | t/χ2 | P |
|---|---|---|---|---|
| Age, years | 50.3±7.6 | 49.7±8.0 | 0.406 | 0.69 |
| Sex | 0.065 | 0.80 | ||
| Male | 28 (59.6) | 51 (57.3) | ||
| Female | 19 (40.4) | 38 (42.7) | ||
| BMI, kg/m2 | 20.6±4.9 | 21.2±4.2 | 0.637 | 0.50 |
| Duration of tuberculosis, months | 3.9±1.2 | 4.2±1.0 | 1.479 | 0.14 |
| History of smoking | 2.139 | 0.14 | ||
| Yes | 19 (40.4) | 25 (28.1) | ||
| No | 28 (59.6) | 64 (71.9) | ||
| History of alcohol drinking | 0.742 | 0.39 | ||
| Yes | 16 (34.0) | 24 (27.0) | ||
| No | 31 (66.0) | 65 (73.0) | ||
| Hypertension | 11 (23.4) | 20 (22.5) | 0.015 | 0.90 |
| Diabetes | 7 (14.9) | 12 (13.5) | 0.051 | 0.82 |
| Clinical symptoms | ||||
| Pleuritic chest pain | 9 (19.1) | 16 (18.0) | 0.028 | 0.87 |
| Fever | 3 (6.4) | 8 (9.0) | 0.040 | 0.84 |
| Cough | 16 (34.0) | 35 (39.3) | 0.366 | 0.55 |
| Bacterial detection | 35.317 | <0.001 | ||
| Positive | 6 (12.8) | 59 (66.3) | ||
| Negative | 41 (87.2) | 30 (33.7) |
Data are presented as mean ± standard deviation or number (percentage). BMI, body mass index.
Associations of different laboratory data with early pleural involvement
As shown in Table 2, the patients in the pleural involvement group had a significantly higher serum sestrin 2 level, but a lower serum fetuin-A level, compared with the patients in the nonpleural involvement group (20.3±3.7 vs. 11.6±2.4 ng/mL and 267.2±26.6 vs. 392.4±35.8 µg/mL, respectively, both P<0.001). However, all P values in the inter-group comparions of the white blood cell count, lymphocyte percentage, erythrocyte sedimentation rate, or C-reactive protein level were >0.05 (Table 2).
Table 2
| Serum level | Nonpleural involvement group (n=47) | Pleural involvement group (n=89) | t/Z | P |
|---|---|---|---|---|
| White blood cell count, ×109 | 7.6±2.0 | 7.3±1.9 | 0.843 | 0.40 |
| Lymphocyte, % | 31.3±9.2 | 32.2±8.8 | 0.549 | 0.58 |
| Erythrocyte sedimentation rate, mm/h | 52 [31–89] | 58 [36–87] | 0.396 | 0.69 |
| C-reactive protein, mg/mL | 58.1 [26.9–87.9] | 55.7 [23.0–88.5] | 0.309 | 0.76 |
| Sestrin 2, ng/mL | 11.6±2.4 | 20.3±3.7 | 14.638 | <0.001 |
| Fetuin-A, μg/mL | 392.4±35.8 | 267.2±26.6 | 23.086 | <0.001 |
All data are presented as the mean ± standard deviation, except for the erythrocyte sedimentation rate and C-reactive protein level, which are presented as the median [interquartile range].
Since the sputum bacterial detection rate was different between the two groups (66.3% in the pleural involvement group versus 12.8% in the nonpleural involvement group, P<0.001; Table 1), we performed multivariate logistic regression analysis, with pleural involvement as the dependent variable and the serum sestrin 2 and fetuin-A levels as well as the presence of sputum bacteria as the independent variables (Table 3). The results showed that all three independent variables were closely associated with pleural involvement in the pulmonary tuberculosis patients. A positive sputum bacterial test result, a higher sestrin 2 level, and a lower fetuin-A level were associated with a high likelihood of patients with pulmonary tuberculosis having pleural involvement (P=0.006, <0.001, and <0.001, respectively).
Table 3
| Variable | Coefficient | Standard error | Wald χ2 | P | Odds ratio (95% confidence interval) |
|---|---|---|---|---|---|
| Sputum bacterial detection | 1.847 | 0.678 | 7.421 | 0.006 | 6.338 (1.679–23.929) |
| Sestrin 2 | 0.400 | 0.099 | 16.337 | <0.001 | 1.491 (1.229–1.810) |
| Fetuin-A | −0.027 | 0.006 | 19.225 | <0.001 | 0.973 (0.961–0.985) |
Both sestrin 2 and fetuin-A were entered into the analysis as continuous data, and sputum bacterial detection was entered as categorical data (positive =1, negative =0).
ROC curve analysis
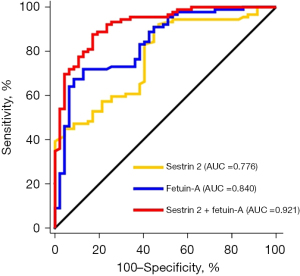
Next, we performed ROC curve analyses. The results showed that both sestrin 2 and fetuin-A alone had a high sensitivity to detect pleural involvement (at the threshold of 16.1 ng/mL, sestrin had an AUC of 0.776, with a 95% CI of 0.697–0.843, a sensitivity of 90.1%, and a specificity of 55.3%. At the threshold of 330.6 µg/mL, fetuin-A had an AUC of 0.840, with a 95% CI of 0.768–0.898, a sensitivity of 71.9%, and a specificity of 87.2%), but their combination had the best performance to detect pleural involvement (AUC: 0.921, 95% CI: 0.863–0.961, sensitivity: 87.6%, and specificity: 83.0%, Table 4 and Figure 3).
Table 4
| Serum marker | Area under the curve | 95% confidence interval | Threshold | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Sestrin 2 | 0.776 | 0.697–0.843 | 16.1 ng/mL | 90.1 | 55.3 |
| Fetuin-A | 0.840 | 0.768–0.898 | 330.6 μg/mL | 71.9 | 87.2 |
| Sestrin 2 + fetuin-A | 0.921 | 0.863–0.961 | 87.6 | 83.0 |
Discussion
Tuberculous pleuritis is the second most common extrapulmonary tuberculosis (4). In the present study, we demonstrated that pulmonary tuberculosis patients with a high serum sestrin 2 level or a low serum fetuin-A level could have a high likelihood of having pleural involvement. A test that combined serum sestrin 2 and fetuin-A determination could provide the best performance in diagnosing early tuberculous pleuritis. Similar study results have never been reported previously.
Pulmonary tuberculosis is a chronic respiratory infectious disease caused by M. tuberculosis. The pleura of the chest can be involved when the pleura is infected through M. tuberculosis dissemination or a type IV hypersensitivity inflammatory response to mycobacterial antigens (6). Without prompt diagnosis and appropriate treatment, patients can develop serious outcomes, such as pleural effusion and respiratory distress. Therefore, it is important to closely monitor potential pleural involvement in patients with pulmonary tuberculosis. Chest imaging, such as X-ray and CT scans, is the most common examination to rule out pleural diseases. However, X-ray has a low sensitivity for pleural illness, and CT scanning carries the risk of radiation exposure and a high medical cost. CT scanning is especially not appropriate for repeated chest examinations during the clinical follow-up visits of patients with pulmonary tuberculosis as it can increase the financial burden and cause a high cumulative radiation dose (18). A serum biomarker that accurately evaluates pleural involvement can be especially important and useful in this clinical situation. Of note, as shown in the present study, commonly used blood tests, such as the white blood cell count, lymphocyte percentage, erythrocyte sedimentation rate, and C-reactive protein, had no statistically significant differences between the patients with or without pleural involvement. Therefore, a new biomarker is needed.
Sestrin 2, also known as hypoxia-inducible gene 95, has antioxidant activity. It contributes to the human antioxidant defense system and plays multiple roles in cellular inflammatory responses, including offering antioxidation, maintaining metabolic balance, and inhibiting cell overproliferation (19). In pulmonary tuberculosis, the invasion of M. tuberculosis can induce oxidative stress in the host cells (20). It has been reported that sestrin 2 can reduce oxidative stress and decrease reactive oxygen species production by inhibiting nicotinamide adenine dinucleotide phosphate oxidase (21). Previous studies also have demonstrated that serum sestrin 2 can be used as an important biomarker for respiratory diseases (13,14). Our results showed that the serum sestrin 2 level in the pleural involvement group was greater than that in the nonpleural involvement group, indicating that serum sestrin 2 could be related to the invasion of the pleura in patients with pulmonary tuberculosis. The underlying mechanism might be the activation of oxidative stress following the spread and pleural invasion of M. tuberculosis, leading to an increase in free oxygen radicals and reactive oxygen species, with humoral or cellular mediated immune responses. The human body can increase sestrin 2 production in response to oxidative stress (22). In addition, it has been reported that pleural mesothelial cells can secrete sestrin 2 (23). Pleural mesothelial cells are the most common cells in the chest pleural space. They are the main type of cells to initiate responses when exposed to noxious stimuli (24). In patients with pulmonary tuberculosis, infection or inflammation involving the pleural space might directly activate the pleural mesothelial cells to secrete sestrin 2, thus increasing its level in the circulatory blood. Our results showed a relatively high AUC, sensitivity, and specificity of serum sestrin 2 for assessing pleural invasion. Therefore, it has value in clinical practice to assess pleural involvement in patients with pulmonary tuberculosis.
Fetuin-A is a multifunctional glycoprotein that plays an important role in regulating inflammation and immune responses (15). It functions as a potent anti-inflammatory cytokine to inhibit the production of cytokines and interleukins, thereby balancing the inflammatory process (25). In patients with chronic obstructive pulmonary disease, the expression of serum fetuin-A has been reported to be significantly less than that in healthy controls (26). It has been suggested that inflammation can downregulate hepatic synthesis, causing a reduced level of fetuin-A. A previous study has found lower serum fetuin-A levels in patients with active tuberculosis than in healthy people (27). Other research also has shown that the serum fetuin-A level is negatively correlated with the levels of airway inflammatory cytokines, such as tumor necrosis factor alpha, interleukin 1 beta, and interleukin 12 (28). Fetuin-A can deactivate macrophages and reduce inflammatory responses. In patients with tuberculosis infection, the balance of cytokines and cytokines is crucial to control the disease. Changes in the serum fetuin-A level may reflect the body’s inflammatory regulation process in the fight against tuberculosis and the repair of the corresponding injured tissues. In the present study, we found that the serum fetuin-A level in patients with pleural involvement was less than that in patients without pleural involvement. The underlying mechanism might involve the host response to infection by downregulating fetuin-A secretion, thus increasing the immune response to M. tuberculosis invasion. The AUC of serum fetuin-A was 0.840, which is greater than the threshold (>0.8) considered to be a useful biomarker applicable in clinical practice (29).
We further analyzed the performance of the combined measurements of the sestrin 2 and fetuin-A levels. The results showed that the AUC increased to 0.921, reaching an excellent level (≥0.9) for a diagnostic test (29). The sensitivity and specificity of the combined measurements were 87.6% and 83.0%, respectively, which was not significantly less than that for sestrin 2 and fetuin-A individually. Therefore, we believe that the combined measurements of both the sestrin 2 and fetuin-A levels could provide a better diagnostic accuracy than the individual tests alone in determining early pleural involvement in patients with pulmonary tuberculosis.
Our current study showed that a high sestrin 2 level and a low fetuin-A level in the peripheral blood sample indicate the presence of early pleural involvement in patients with pulmonary tuberculosis. These results might provide an early biomarker to monitor the local dissemination of pulmonary tuberculosis and determine the anti-tuberculosis treatment duration. In addition, our current results also provide a noninvasive way to stratify tuberculosis patients with or without early pleural involvement, which could facilitate the targeted management and research in specific patient populations.
The strength of this study was that it is the first study to explore the role of sestrin 2 and fetuin-A as novel biomarkers to assess pleural involvement in patients with pulmonary tuberculosis. We selected patients with early pleural involvement without pleural effusion. This patient population has been rarely studied previously. Our study results could provide an easy way to monitor pulmonary tuberculosis patients for potential pleural invasion. The limitations of this study include its single-center design and the small sample size. The associations between the serum sestrin 2 and fetuin-A levels with the tuberculosis patient outcomes were also unknown. We used CT scanning to identify pleural lesions and sputum smear or culture to determine the presence of M. tuberculosis. We could not rule out pleural lesions from other causes. The high levels of sestrin 2 and fetuin-A might be due to a more severe pulmonary infection in addition to pleural involvement. Thus, further multi-center research studies with a large sample size and a long-term follow-up duration are required.
Conclusions
In conclusion, in patients with pulmonary tuberculosis, a high serum sestrin 2 level and a low serum fetuin-A level can be applied to assess potential pleural involvement. The combination of serum sestrin 2 and fetuin-A provides a better performance to detect pleural involvement compared with each individual test alone.
Acknowledgments
We thank Medjaden Inc. for providing scientific editing of this manuscript.
Funding: This project was supported by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-989/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-989/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-989/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-989/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Hunan Chest Hospital (No. LS2021051807). All study participants signed the written informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Tuberculosis. Available online: https://wwwwhoint/news-room/fact-sheets/detail/tuberculosis (Accessed on 2023) [Cited 2024 June 10].
- Sharma SK, Mohan A, Kohli M. Extrapulmonary tuberculosis. Expert Rev Respir Med 2021;15:931-48. [Crossref] [PubMed]
- Ahmad F, Rani A, Alam A, et al. Macrophage: A Cell With Many Faces and Functions in Tuberculosis. Front Immunol 2022;13:747799. [Crossref] [PubMed]
- Chan KKP, Lee YCG. Tuberculous pleuritis: clinical presentations and diagnostic challenges. Curr Opin Pulm Med 2024;30:210-6. [Crossref] [PubMed]
- Krishna R, Antoine MH, Alahmadi MH, et al. Pleural Effusion. Treasure Island (FL): StatPearls Publishing; 2024.
- Shaw JA, Koegelenberg CFN. Pleural Tuberculosis. Clin Chest Med 2021;42:649-66.
- Rea G, Sperandeo M, Lieto R, et al. Chest Imaging in the Diagnosis and Management of Pulmonary Tuberculosis: The Complementary Role of Thoraci Ultrasound. Front Med (Lausanne) 2021;8:753821. [Crossref] [PubMed]
- Shaw JA, Diacon AH, Koegelenberg CFN. Tuberculous pleural effusion. Respirology 2019;24:962-71. [Crossref] [PubMed]
- Möller K, Löwe A, Jenssen C, et al. Comments and Illustrations of Ultrasound Findings in Extrapulmonary Tuberculosis Manifestations. Diagnostics (Basel) 2024;14:706. [Crossref] [PubMed]
- Aggarwal AN, Agarwal R, Dhooria S, et al. Comparative accuracy of pleural fluid unstimulated interferon-gamma and adenosine deaminase for diagnosing pleural tuberculosis: A systematic review and meta-analysis. PLoS One 2021;16:e0253525. [Crossref] [PubMed]
- Wang LX, Zhu XM, Yao YM. Sestrin2: Its Potential Role and Regulatory Mechanism in Host Immune Response in Diseases. Front Immunol 2019;10:2797. [Crossref] [PubMed]
- Ala M, Eftekhar SP. Target Sestrin2 to Rescue the Damaged Organ: Mechanistic Insight into Its Function. Oxid Med Cell Longev 2021;2021:8790369. [Crossref] [PubMed]
- Zhang DW, Wei YY, Ji S, et al. Correlation between sestrin2 expression and airway remodeling in COPD. BMC Pulm Med 2020;20:297. [Crossref] [PubMed]
- Chae HS, Gil M, Saha SK, et al. Sestrin2 Expression Has Regulatory Properties and Prognostic Value in Lung Cancer. J Pers Med 2020;10:109. [Crossref] [PubMed]
- Chekol Abebe E, Tilahun Muche Z. The structure, biosynthesis, and biological roles of fetuin-A: A review. Front Cell Dev Biol 2022;10:945287. [Crossref] [PubMed]
- Rudloff S, Jahnen-Dechent W, Huynh-Do U. Tissue chaperoning-the expanded functions of fetuin-A beyond inhibition of systemic calcification. Pflugers Arch 2022;474:949-62. [Crossref] [PubMed]
- National Health Commission of the People's Republic of China. Diagnosis of pulmonary tuberculosis. Available online: http://wwwnhcgovcn/wjw/s9491/201712/a452586fd21d4018b0ebc00b89c06254shtml (cited 2024 June 11).
- Brambilla M, Vassileva J, Kuchcinska A, et al. Multinational data on cumulative radiation exposure of patients from recurrent radiological procedures: call for action. Eur Radiol 2020;30:2493-501. [Crossref] [PubMed]
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett 2022;27:2. [Crossref] [PubMed]
- Tiwari D, Martineau AR. Inflammation-mediated tissue damage in pulmonary tuberculosis and host-directed therapeutic strategies. Semin Immunol 2023;65:101672. [Crossref] [PubMed]
- Zhou XR, Ru XC, Xiao C, et al. Sestrin2 is involved in the Nrf2-regulated antioxidative signaling pathway in luteolin-induced prevention of the diabetic rat heart from ischemia/reperfusion injury. Food Funct 2021;12:3562-3571. [Crossref] [PubMed]
- Rongjin H, Feng C, Jun K, et al. Oxidative Stress-Induced Protein of SESTRIN2 in Cardioprotection Effect. Dis Markers 2022;2022:7439878. [Crossref] [PubMed]
- Howlader S, Sumi KR, Sarkar S, et al. Effects of dietary replacement of fish meal by soybean meal on growth, feed utilization, and health condition of stinging catfish, Heteropneustes fossilis. Saudi J Biol Sci 2023;30:103601. [Crossref] [PubMed]
- Wu YY, Hsu YL, Huang YC, et al. Characterization of the pleural microenvironment niche and cancer transition using single-cell RNA sequencing in EGFR-mutated lung cancer. Theranostics 2023;13:4412-29. [Crossref] [PubMed]
- Icer MA, Yıldıran H. Effects of fetuin-A with diverse functions and multiple mechanisms on human health. Clin Biochem 2021;88:1-10. [Crossref] [PubMed]
- Małujło-Balcerska E, Kumor-Kisielewska A, Śmigielski W. Leptin, resistin and fetuin a concentration as the potential useful biomarkers in stable COPD - An exploratory study. Cytokine 2023;169:156275. [Crossref] [PubMed]
- Keicho N, Matsushita I, Tanaka T, et al. Circulating levels of adiponectin, leptin, fetuin-A and retinol-binding protein in patients with tuberculosis: markers of metabolism and inflammation. PLoS One 2012;7:e38703. [Crossref] [PubMed]
- Tanaka T, Sakurada S, Kano K, et al. Identification of tuberculosis-associated proteins in whole blood supernatant. BMC Infect Dis 2011;11:71. [Crossref] [PubMed]
- Çorbacıoğlu ŞK, Aksel G. Receiver operating characteristic curve analysis in diagnostic accuracy studies: A guide to interpreting the area under the curve value. Turk J Emerg Med 2023;23:195-8. [Crossref] [PubMed]


