|
Original Article
Effects of antidiabetic drug metformin on the migration and invasion abilities of human pulmonary adenocarcinoma A549 cell line in vitro
Ning Wu, Hong-Jun Gu, Qiang Li
Department of Respiratory Medicine (Drs Wu and Li), Changhai Hospital, Second Military Medical University, Shanghai 200433; Department of Internal Medicine (Dr Gu), Soochow Medical Center of People's Liberation Army 101 Hospital, Soochow 215007, PR China
Corresponding to: Dr Qiang Li, MD, Department of Respiratory Medicine, Changhai Hospital, Second Military Medical University, No. 168, Changhai Rd, Shanghai 200433, PR China. Tel: +86-21-81871114, fax:+86- 21-65492727. Email: liqressh@yahoo.com.cn.
|
|
Abstract
Background and purpose: There is growing evidence that metformin, a clinically widely used drug in the treatment
of type II diabetes, may impede the growth of human tumors. However in a recent study it was found that metformin
treatment might result in promotion of the angiogenic phenotype and promote early tumorigenic progression. In order
to evaluate the relevance between metformin and tumor metastases, we investigated the effects of metformin on
the migration and invasion abilities of human pulmonary adenocarcinoma cell line A549 in vitro and explored the
possible underlying mechanisms.
Methods: A549 cells were treated with 0.5 mmol/L, 2 mmol/L and 8 mmol/L metformin for 72h. The laterad-migration
and invasion abilities of the cells in vitro were evaluated by scratch assay and Boyden-Chamber assay, respectively.
Expressions of MMP2 and MMP9 mRNA of the cells before and after metformin treatment were measured by
Real-Time PCR.
Results: The migration rate of A549 cells was increased after metformin treatment at the concentration of 8mmol/L.
The invasion ability was also significantly increased from 37.4±4.6 to 59.8±7.2(P<0.05) by 8mmol/L metformin
treatment. No significant difference of the migration and invasion abilities was observed between the Group 0.5mmol/
L, 2mmol/L and the Control. The expressions of MMP2 and MMP9 mRNA were both up-regulated after metformin
treatment, while in the 8mmol/L Group the genes changes were the most significant.
Conclusion: Metformin can increase the migration speed and enhance invasion abilities of A549 cells in vitro,
which may be attributed to the up-regulation of MMP2 and MMP9.
Key words
pulmonary adenocarcionma; metformin; migration; invasion ability; MMP2; MMP9
J Thorac Dis 2010;2:76-80. DOI: 10.3978/j.issn.2072-1439.2010.02.02.008
|
|
Introduction
As a euglycemic agent, metformin can reduce the
hepatic gluconeogenesis and increase peripheral tissues’
sugar intake and utilization, so that blood sugar would
be reduced efficiently. From the 1950s, it has been
widely applied for treating type Ⅱ diabetes ( 1). Through
researches in the recent years, it was found that metformin
might be efficient on anti-tumor, which had been verified in treating some animentary and reproductive system
tumors. However, a recent animal trial about breast cancer
showed that though metformin treatment could slow down
the growing of transplanted tumor, vessels in tumor were
significantly increased, which implied that metformin
might promote neoangiogenesis ( 2). If this presumption
was right, then, metformin would lead to a risk of
promoting tumorous metastases. This research studied
the effect of metformin on the migration and invasion of
human pulmonary adenocarcinoma cell line A549 in vitro;
moreover, it evaluated the related mechanisms.
|
|
Materials and Methods
Materials
Pulmonary adenocarcinoma A549 cell lines were
obtained from cell bank, Chinese Academy of Sciences.
Fetal bovine serum, double-antibody and RPMI1640
medium we reobtained from Hyclone Australia. Metformin was purchased from Sigma Corporation of
America. Millicell cell culture inserts were from Millipore
Corp (United States). Matrigel Basement Membrane
Matrix was from BD Bioscience, USA and Trizol from
Invitrogen Coperation, USA. Reverse Transcriptase RNA
Kit and SYRB Realtime-PCR Kit were obtained from
Takara Biotechnology (Da Lian) Co., ltd. PCR premier was
synthesized by Shanghai SBS Genetech Technology Co.,
Ltd.. Real-time PCR machine was type 7500, from Applied
Biosystems Inc, USA.
Grouping and management of cells
Cells were conventionally cultured in RPMI1640
medium containing 10% fetal bovine serum, 100U/ml
penicillin and 100ng/ml streptomycin, at 37℃ in 5%
CO2. Experimental groups included control group,
0.5mmol/L metformin group, 2mmol/L metformin
group and 8mmol/L met formingroup. Cells in
logarithm phrase were collected and inoculated for
24hours, after their adherence, each group would be
kept in RPMI1640 medium, which contains metformin,
through which, the final metformin concentration
would respectively reach at 0.5mmol/L, 2mmol/L and
8mmol/L, besides, PBS in the same volume was added
to control group medium.
Cell scratch test
After trypsinization and collection for the cells
treated with metformin for 72h of each group, they
were inoculated in a six-well plate at 5×105 cells for
each well, and cultured in a conventional way for 24
hours. When cells of each group had achieved 80-90%
integration, the medium was abandoned. After cleaning
the cells with sterile PBS for once, 200ul transferpettor
tip was applied to draw a straight line along the Y
direction in the center of each well in the plate. Cells
were rinsed tenderly for twice with sterile PBS so as
to remove scratched f loating cells. Draw five parallel
lines in 0.5-1cm distance with marker pen over the
vertical cells in the bottom of each plate well, which
were observed under microscope. Appropriate serumfree
medium was added into each well for conventional
culture so that to observe the cells over the scratching
line to check their growth at 0h, 8h, 16h and 24h under
microscope and photos were taken. For each group, 5
visual fields and 3 repeated holes were detected, and
the experiment would be repeated for three times.
Evaluation of cell in vitro invasion
Boyden-Chamber assay was applied. Millicell cell
culture inserts with 8um diameter PET membrane were put
into a 24-well culture plate, and then cover the bottom of Millicell cell culture inserts with Matrigel Basement
Membrane Matrix 50ul/well, keep the min 37℃
for a whole night to make them become gelatinous.
A549 cells, which had been treated with metformin
with dif ferent concent rat ions for 72h, would get
try psinization and be collected. Then, R IMP1640
culture solution with 1% fetal bovine ser um was
applied to make cell suspension, and regulate the
cell density into 5×105/ml. Inoculate over insert at
100ul/well, besides, add 600ul culture medium which
contains 10% fetal bovine serum to insert. There were
3 repeated holes in each cell group. After conventional
culture for 30 hours, fetch Millicell and remove the
cells over top of microporous membranes carefully
with cotton swabs, then fix with 4% paraformaldehyde
for 10minutes, stain with methyl violet for 10 minutes.
Take upper, lower, left, right and central five visional
f ields u nder light microscope (×40), cou nt lower
membrane cells, and calculate the average value.
MMP2 and MMP9 mRNA expression detected by Real-Time PCR
After interfering cells with metformin for 48 hours, extract
total RNA of each group by Trizol, then cDNA would be
get from 500ng RNA reverse transcription, then take cDNA
product as format to evaluate MMP2 and MMP9 mRNA
expression by Real-Time PCR. MMP9 premier: upstream:
5’-AACTCACGCGCCAGTAGAAG-3’; downstream: 5’
-GAGGTGGACCGGATGTTCC-3’; product length was
105bp, and the annealing temperature was 60℃. MMP2
premier: upstream: 5’-GCCCAAGAATAGATGCTGACTG-3’
; downstream: 5’-TGAAAGGAGAAGAGCCTGAAGTG-3’
; product length was 165bp, and the annealing
temperature was 56℃. GAPDH premier: upstream: 5’
-GCACCGTCAAGGCTGAGAAC-3’; downstream: 5’
-ATGGTGGTGAAGACGCCAGT-3’; product length was
142bp, and the annealing temperature was 56℃. Amplification
conditions of Realtime-PCR: 95℃ 30s; 95℃ 15s, 60℃ 15s,
72℃ 45s, there were 40 cycles in total and the experiment
was repeated for three times. GAPDH was taken as internal
reference, and Rotor-Gene 6000 Series Software 1.7 was
employed for result analysis, the relative qualification (RQ) of
target gene =2-ΔΔCT. We would take mean value of target
gene mRNA of control group as 1, and calculate mRNA
relative qualification of other groups.
Statistical analysis
Experimental data was expressed as Mean±SD,
and SPSS 11.5 statistical software was applied, onefactor
analysis of variance was carried out by multi
group comparison. LSD-t test was performed between
two groups, we would take it to be with statistical significance when P<0.05.
|
|
Results
Effects of metformin to A549 cell lateral migration ability
After cell scratch for 16 hours, compare it with control
group, A549 cell scratch of 8mmol/L metformin group
was obvious narrowed, which indicated lateral migration
speed of this cell group had been significantly improved
( Figure 1), however, there was no big speed difference
over metformin 0.5mmol/L group and 2mmol/L when
comparing with control group.
Effect of metformin to A549 cell in vitro invasion
After conventional culture in Millicell cell culture
inserts for 30 hours, the count of cells crossed Matrigel
Basement Membrane Matrix contained membrane in
control group and 8mmol/L metformin group were
respectively 37.4±4.6 and 59.8±7.2, the difference between
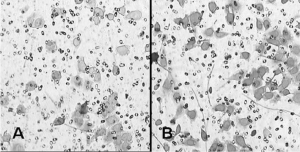
which showed statistical significance (P<0.05) ( Figure 2).
The counts of 0.5mmol/L and 2mmol/L metformin groups were respectively 36.5±2.8 and 40.6±4.9, both of which had
no obvious difference when comparing with that of control
group.
Effect of metform to MMP2 and MMP9 mRNA
expression in A549 cells
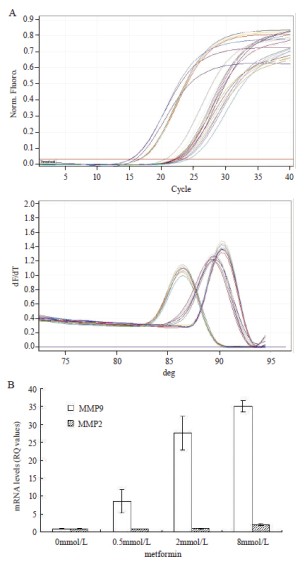
Realtime-PCR result showed that after metformin
interference, MMP9 mRNA expression in A549 cells of
each group had been heightened, which had significant
statistical difference comparing with control group
(P<0.05), besides, the mRNA expression level would
increase as the concentration of metformin increases.
MMP2 expression in cells of metformin 8mmol/L group
was obviously higher than that of control group (P<0.05),
however, MMP2 mRNA expression in cells of 0.5mmol/
L and 2mmol/L metformin groups had no statistical
difference with that of control group ( Table1, Figure 3).
|
|
Discussion
Metformin is a safe and effective hypoglycemic drug widely applied in clinical. It has been verified through
research that mainly through activating AMPK (AMPactivated
protein kinase) signal in cells, metformin would
establish transduction pathway, so as to reduce intrahepatic
gluconegenesis, meanwhile increase sugar absorption and
utilization of skeletal muscle and so on, so that peripheral
blood sugar would be reduced ( 3). In researches in
recent years, it had been shown that activation of AMPK
signaling pathway had significant effect on inhibiting
tumor occurrence and development ( 4, 5), as an effective
AMPK activator, potential anti-tumor ability of metformin
had been recognized. According to many researches,
metformin has obvious inhibition to breast cancer, prostate
cancer, ovarian cancer and colorectal cancer ( 2, 5-8).
However, a recent research to animals with breast cancer
has showed that though metformin could inhibit the growth
of transplanted tumor in mice, it meanwhile increases
VEGF (vascular endothelial growth factor) expression in
estrogen receptor α negative breast cancer tissues, and it
also increases intratumoral microvessel density, which
indicates that metformin might lead to invasion and
increased transfer activity for some tumors ( 2). It has been
shown in our preliminary researches that metformin has
a strong inhibition to human pulmonary adenocarcinoma
A549 cells ( 9), and in this research, migration in vitro
and invasive activity of A549 cells under metformin
interference were detected, it had been found that after
metformin interference in a certain concentration,
migration in vitro and invasive activity had been increased. Tumor metastases means tumor cells adhere and
pass through extracellular matrix, survive and migrate
beyond primary foci. The key step for cancer cell
metastases lies in tumor cells migration and their
invasion to surrounding tissues and vessels, occurrence
and development of which requires the combined effect
of change of cell proliferation, differentiation and
locomotion related gene and their expression regulation
mode and external factors for promoting cell locomotion
( 10). MMPs (matrix metalloproteinases), as a kind of
Zn+ dependent endogenous proteinase, containing at
least 25 members, can almost degrade all components
of extracellular matrix except for polysaccharide, and it
is involved in tumor progression, metastases and other
various pathophysiological processes ( 11). Among MMPs
secreted by tumor cells, MMP2 and MMP9 are the most
important degradable collagenases, both of which have
significant effect of tumor neovascularization, tumor cell
invasion and the progress of metastasis formation. It has
been verified by lots of studies that high expression level
of MMP2 and MMP9 has a close relationship with lung
cancer metastases, moreover, expression increase of these
two protease would be more obvious in small cell lung cancer with early stage metastases ( 12, 13). This research
has compared MMP2 and MMP9 mRNA expression in
A549 cells before and after metformin interference, whose
result shows that after medicine treatment, both of these
two gene expressions have been significantly increased,
and especially for MMP9, it has been indicated that upregulation
of MMP2 and MMP9 expression might be
one of the mechanisms for A549 cell migration and
strengthened invasive ability after metformin interference.
As to metformin’s influence to angiogenesis in pulmonary
adenocarcinoma tissues and its correlation with tumor
metastases, experiment in vivo is required for further
verification. This research shows that metformin can promote
human pulmonary adenocarcinoma A549 cell lines in
vitro migration and the strengthening of invasive activity,
it potentially promotes tumor metastases, which might be
relevant to metformin’s inducing expression up-regulation
of MMP2 and MMP9 in tumor cells.
|
|
References
- Bailey CJ, Turner RC. Metformin. N Engl J Med 1996;334:574-9.[LinkOut]
- Hadad SM, Appleyard V, Thompson AM. Therapeutic metformin/
AMPK activation promotes the angiogenic phenotype in the
ERalpha negative MDA-MB-435 breast cancer model. Breast
Cancer Res Treat 2009;114:391.[LinkOut]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al.
Role of AMP-activated protein kinase in mechanism of metformin
action. J Clin Invest 2001;108:1167-74.[LinkOut]
- Li J, Jiang P, Robinson M, Lawrence TS, Sun Y. AMPK-beta1
subunit is a p53-independent stress responsive protein that inhibits
tumor cell growth upon forced expression. Carcinogenesis 2003;24:827-34.[LinkOut]
- Hadad SM, Fleming S, Thompson AM. Targeting AMPK: A new
therapeutic opportunity in breast cancer . Crit Rev Oncol Hematol
2008;67:1-7.[LinkOut]
- Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti
P, Auberger P, et al. The antidiabetic drug metformin exerts an
antitumoral effect in vitro and in vivo through a decrease of cyclin
D1 level. Oncogene 2008;27:3576-86.[LinkOut]
- Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis
RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug
metformin selectively impairs p53-deficient tumor cell growth.
Cancer Res 2007;67:6745-52.[LinkOut]
- Gotlieb WH, Saumet J, Beauchamp MC, Gu J, Lau S, Pollak MN,
Bruchim I. In vitro metformin anti-neoplastic activity in epithelial
ovarian cancer. Gynecol Oncol 2008;110:246-50.[LinkOut]
- Wu N, Gu HJ, Yin HJ, Li Q. Effects of antidiabetic drug metformin
on human lung adenocarcinoma cell A549 proliferation and
apoptosis in vitro. China Oncology 2009;19:21-4.
- Erik S. Mechanisms of cancer cell invasion. Curr Opin Genet Dev
2005;15:87-96.[LinkOut]
- Chambers AF, Matrisian LM. Changing views of the role of
matrix metalloproteinases in metastasis. J Natl Cancer Inst
1997;89:1260-70.[LinkOut]
- González-Avila G, Iturria C, Vadillo F, Terán L, Selman M,
Pérez-Tamayo R. 72-kD (MMP-2) and 92-kD (MMP-9) type IV
collagenase production and activity in different histologic types of
lung cancer cells. Pathobiology 1998;66:5-16.[LinkOut]
- Ylisirniö S, Höyhtyä M, Turpeenniemi-Hujanen T. Serum matrix
metalloproteinases -2, -9 and tissue inhibitors of metalloproteinases
-1, -2 in lung cancer--TIMP-1 as a prognostic marker. Anticancer
Res 2000;20:1311-6.[LinkOut]
Cite this article as: Wu N, Gu HJ, Li Q. Effects of antidiabetic drug metformin on the migration and invasion abilities of human pulmonary adenocarcinoma A549 cell line in vitro. J Thorac Dis 2010;2(2):76-80. doi: 10.3978/j.issn.2072-1439.2010.02.02.008
|