
Esophageal perforation mimicking an acute inferior myocardial infarction: a case report
Highlight box
Key findings
• This case report highlights how esophageal perforation can mimic acute inferior myocardial infarction (AMI) on electrocardiography (ECG), leading to misdiagnosis and delayed treatment.
• The absence of reciprocal ST-segment depression in lateral leads and lack of abnormal myocardial necrosis markers should suggest alternative diagnoses, such as an esophageal perforation, especially in patients with Q waves and ST-segment elevation in the inferior ECG leads.
What is known and what is new?
• Esophageal perforation is a rare but life-threatening condition with high mortality, often presenting with nonspecific clinical manifestations. Previous case reports have described esophageal perforation mimicking AMI on ECG.
• This case report highlights esophageal perforation presenting with Q waves and ST-segment elevation in inferior leads without reciprocal changes or abnormal myocardial necrosis markers. It emphasizes the importance of considering esophageal perforation in the differential diagnosis for patients with ECG changes suggestive of AMI, particularly when clinical presentation is consistent with an esophageal injury such as a recent history of forceful vomiting or esophageal instrumentation.
What is the implication, and what should change now?
• Emergency physicians should maintain high suspicion for esophageal perforation in patients presenting with ECG changes suggestive of AMI, especially with atypical clinical presentation and diagnostic findings.
• Contrast-enhanced chest computed tomography should be considered as a key diagnostic tool for confirming suspected esophageal perforation.
Introduction
Esophageal perforation can occur spontaneously or be induced by the ingestion of a foreign body or instrumentation. Despite its low incidence, esophageal perforation is associated with a high mortality rate of approximately 50% (1). Delayed treatment beyond 48 hours is associated with increased risk of mortality, up to 60%, due to the associated morbidity of multiple organ failure either before reaching the hospital (2) or during interhospital transfer (3). Clinical manifestations such as pain (4) and vomiting (5) are nonspecific and can be easily mistaken for acute coronary syndrome (6). Patients with Boerhaave’s syndrome, characterized by spontaneous esophageal rupture, are particularly at risk of treatment delay due to dynamic ST-T changes, which can mimic cardiac conditions. Boerhaave’s syndrome was first described by Hermann Boerhaave in 1724, after observing a man’s death from spontaneous esophageal perforation following vomiting. Boerhaave’s syndrome accounts for 15% of all esophageal perforations (7) and is more prevalent in male drinkers (8). We present this article in accordance with the CARE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1616/rc).
Case presentation
A 65-year-old male, with history of hypertension and alcohol use, was seen by emergency medical services (EMS) with complaints of persistent oppressive pain in the anterior and posterior regions of the chest on December 28, 2019, following half an hour of exercise. The dull pain was located in the precardiac area and radiated to the shoulder and back. There was no history of gagging, vomiting, or retching prior to the onset of symptoms. The patient reported no history of digestive tract-related diseases but endorsed a 40-year history of alcohol use, averaging 2 bottles of beer and approximately 100 mL of liquor per day. There was no history of esophageal disease or gastrointestinal surgeries. The patient was living with his daughter. The medical history was obtained from both the patient and his daughter upon admission.
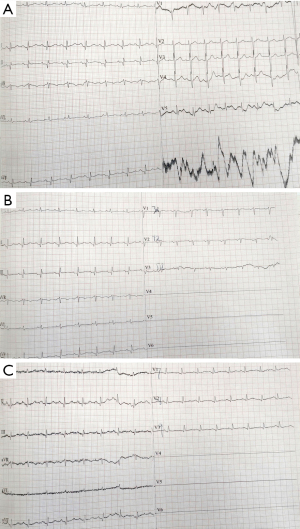
In transit to our hospital (Affiliated Hospital of Liaoning University of Traditional Chinese Medicine), an electrocardiogram (ECG) revealed Q-wave and ST-segment elevation of approximately 0.1 mV in lead II and III, along with findings consistent with an arteriovenous fistula (Figure 1). Sublingual administration of 2 capsules of nitroglycerin provided no relief. His vital signs demonstrated a blood pressure of 180/100 mmHg, and the patient confirmed a 10-year history of hypertension, but was not taking any medication in the days preceding the event and had never been hospitalized. Physical examination revealed no upper abdominal pain on deep palpation, and chest and heart examinations were unremarkable. Repeat blood pressure was 130/90 mmHg, heart rate was 90 beats/min, peripheral oxygen saturation was 90%, and body temperature was 36.5 ℃.

An 18-lead ECG performed within 10 minutes of presentation to the hospital revealed Q-waves in the inferior leads and low-amplitude T-waves in all leads (Figure 2). Dynamic changes on the ECG were observed, with the depth of the Q wave suggesting myocardial infarction (9). Although acute coronary syndrome was initially suspected and diagnosed, dynamic evolution of the ST segment was inconsistent with changes in the markers of myocardial injury (10). Myocardial injury markers were monitored dynamically, with initial results at admission showing a cardiac troponin I (CTNI) level <0.01 ng/mL and a myoglobin (MYO) level of 20.5 ng/mL. Reexamination after two hours showed a CTNI level <0.01 ng/mL and a MYO level of 53.3 ng/mL, all of which were lower than reference values. Although not all acute myocardial infarctions are accompanied by mirror changes (11), most ST-elevation myocardial infarctions (STEMIs) are. No abnormal markers of myocardial necrosis or changes in reciprocal leads on the ECG were found, leading to the exclusion of acute coronary syndrome.
Physicochemical results included a D-dimer level of 0.64 mg/mL, with routine blood test results showing the following: white blood cell count, 10.62×109/L [normal range: (4.0–10.0)×109/L]; neutrophil percentage, 78.6% (normal range: 50–70%); lymphocyte percentage, 10.3% (normal range: 20–40%); red blood cell count, 4.65×1012/L [normal range: (4.5–5.5)×1012/L for men]; hemoglobin level, 149 g/L (normal range: 135–175 g/L for men); and platelet count, 332×109/L [normal range: (150–450)×109/L].
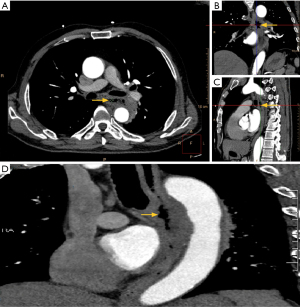
Due to concerns for an aortic dissection, aortic enhanced computed tomography (CT) was performed, revealing wall thickening of the thoracic esophagus. We decided to proceed directly with an aortic enhanced CT scan without performing a chest X-ray because even though the D-dimer was negative, we still could not rule out esophageal rupture or aortic dissection. The left margin of the thoracic esophagus was approximately at the level of the fifth to sixth thoracic vertebra, with a discontinuous wall and an irregular, mixed-density shadow of the soft tissue beside it. Gas density shadow was observed in the esophagus, indicating communication of gas with the esophageal lumen due to esophageal rupture (Figure 3). The patient was diagnosed with esophageal perforation and transferred to a hospital with surgical capabilities on December 28, 2019. Unfortunately, the patient died the following morning due to his esophageal rupture and hemorrhage while waiting for surgery. The patient’s family declined an autopsy for post-mortem examination.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s family members for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
International multidisciplinary team (iMDT) discussion
Discussion among physicians from the Affiliated Hospital of Liaoning University of Traditional Chinese Medicine
Department of Emergency Medicine
Here we present a case of mortality secondary to esophageal perforation, which was initially misdiagnosed as acute myocardial infarction due to ECG findings of ST-segment elevation in the inferolateral leads. This misdiagnosis ultimately led to a delay in the diagnosis and definitive management of the esophageal perforation. We initially suspected foreign body ingestion, but the patient’s family member denied this possibility. The patient’s long-term history of alcohol consumption reminds that it is more likely a case of spontaneous perforation. Unfortunately, without an autopsy, we cannot definitively determine the exact cause. This study highlights the importance of maintaining a broad differential diagnosis and avoiding diagnostic anchoring bias when faced with an atypical presentation.
Department of Cardiology
The presence of ischemic symptoms and ST-segment elevation on ECG commonly indicates myocardial infarction (12). Initially, the patient in this case was suspected to be experiencing acute myocardial infarction. Treatment for acute myocardial infarction typically involves dual antiplatelet therapy before coronary angiography and heparinization, both of which can increase the risk of bleeding in cases of esophageal perforation. A study reports severe esophageal hematoma resulting from the use of heparin in patients with esophageal rupture presenting with chest pain (13).
While ST-segment elevation on ECG due to esophageal rupture is rare, there are a few reported cases in the literature. One report described a 76-year-old woman with a middle-esophageal perforation and ST-segment elevation in the anterior leads without any coronary lesions or increased myocardial enzyme markers (14). Another case involved a 69-year-old man with ST-segment elevation in the inferoposterior leads, initially misdiagnosed as acute inferoposterior myocardial infarction, but a later CT scan indicated pleural effusion and perforation of the lower esophagus (15).
The case presented here underscores the importance of avoiding diagnostic anchoring bias and considering a broad differential diagnosis when faced with an atypical presentation. Accidental ingestion of foreign bodies, particularly sharp objects, can result in esophageal perforation with increased morbidity and mortality. Early diagnosis is crucial for a positive prognosis (4,16). Performing an esophagram at the presenting site can prevent unnecessary transfers and decrease management delays (3). In addition, a dynamic review of the chest CT scan can also be beneficial, not only for the initial identification of segmental esophageal injury but also for prioritizing subsequent treatment strategies (17).
Although the Pittsburgh Severity Score has been suggested to predict outcomes in esophageal perforation, particularly for Boerhaave’s syndrome (18,19), no scoring system is currently available for diagnosis. Angiographic right heart hypermobility has been proposed as a sign of esophageal perforation in the context of ST-elevation (6,20). ECG may also be useful in distinguishing between “pseudo” myocardial infarction characterized by ST-segment elevation without other laboratory findings of true myocardial infarction or Takotsubo cardiomyopathy when gastrointestinal symptoms are present without concurrent chest pain (21).
Several issues on the diagnosis and treatment of this patient were further discussed as follows
Question 1: in cases where esophageal perforation mimics acute myocardial infarction on ECG, what are the key diagnostic criteria that can help differentiate between the two conditions and guide appropriate treatment?
Expert opinion 1: Dr. Sofoklis Mitsos
The differentiation between esophageal perforation and AMI can be challenging and requires a combination of detailed clinical history, physical examination, imaging studies, and careful interpretation of ECG and biomarkers. The presence of risk factors for esophageal perforation, mediastinal air, or atypical ECG changes should raise suspicion and prompt further diagnostic evaluation. Early intervention in esophageal perforation is crucial to prevent life-threatening complications.
Key diagnostic criteria include:
- Clinical history and symptoms. Awareness of specific patient populations and risk factors is key to raising suspicion for esophageal perforation when symptoms suggest AMI. Esophageal perforation often follows a history of recent endoscopy, vomiting, or trauma. Sudden onset of severe and sharp chest pain, usually following these events, is typical. While for AMI, chest pain is typically retrosternal, described as heavy, and may radiate to the left arm, neck, or jaw.
- Physical examination. Subcutaneous emphysema in the neck or chest is a classic finding of esophageal perforation. Fever, tachycardia, and hypotension may be present, indicating systemic inflammatory response or sepsis. Nevertheless, in AMI, the physical examination may reveal signs of heart failure or cardiogenic shock.
- ECG findings. ECG changes in esophageal perforation can mimic those of AMI, such as ST-segment elevation, T-wave changes, or even low-voltage combinations of Q wave, R wave, and S wave. However, the changes are often atypical and do not correlate with a coronary artery territory. On the other hand, ST-segment elevation or depression during AMI typically corresponds with specific coronary artery territories. Reciprocal changes in opposing leads may also be seen.
- Imaging. A chest X-ray or CT scan may show pneumomediastinum, subcutaneous emphysema, pleural effusion, or widened mediastinum in esophageal perforation. Contrast esophagography or CT with oral contrast can confirm the diagnosis by showing extravasation of contrast from the esophagus. Abnormal radiological findings include extraluminal contrast, mediastinal air, periesophageal fluid collection, pleural effusion, esophageal thickening and communication of the air-filled esophagus with a contiguous mediastinal air-fluid collection. Moreover, endoscopy can be used cautiously to confirm the diagnosis by directly visualizing the perforation. However, coronary angiogram can confirm the diagnosis of AMI by demonstrating coronary artery occlusion.
- Biomarkers. Elevated white blood cell count and inflammatory markers, and sometimes metabolic acidosis can be present in the esophageal perforation. Cardiac biomarkers might be mildly elevated due to stress or inflammation but are not typically as high as in AMI. On the contrary, significantly elevated cardiac biomarkers are a hallmark of myocardial injury and serial measurements showing a rising trend are typical in AMI.
Expert opinion 2: Dr. Jules Lin
The clinical presentation should help to guide the need for further diagnostic studies. If there is suspicion for an esophageal perforation based on the patient’s history including recent forceful vomiting, foreign body ingestion, or esophageal instrumentation, the patient should undergo an oral-contrast study such as a barium esophagram or a chest and abdominal CT with oral contrast.
Expert opinion 3: Dr. Christina M. Stuart
When differentiating between AMI and esophageal perforation a careful history taking is critical. Patients should be interviewed thoroughly to uncover history that may indicate esophageal perforation including foreign body ingestion, caustic ingestion, trauma (either blunt or penetrating), violent emesis, Valsalva maneuvers including cough, heavy lifting or childbirth, history of severe esophagitis, ulcer disease, etc. Patients should then be carefully examined. Those with esophageal perforation may have vital sign derangement including tachycardia, hypoxia or hypotension, as well as subcutaneous emphysema with associated chest or neck swelling. The later findings are unlikely in patients with AMI. Laboratory values may reveal elevated white blood cells or anemia. However, patients with esophageal perforation may also present clinically stable, without obvious clinical signs or symptomatology. Chest X-ray may demonstrate pneumomediastinum or pleural effusions both of which should raise suspicion for esophageal perforation over AMI.
Question 2: given the high mortality rate associated with delayed diagnosis of esophageal perforation, should emergency departments consider implementing a standardized diagnostic protocol for patients exhibiting chest pain and ECG changes suggestive of acute myocardial infarction to rule out esophageal perforation?
Expert opinion 1: Dr. Sofoklis Mitsos
Unfortunately, the rarity of this pathological condition and its nonspecific presentation can lead to delay in diagnosis in more than 50% of patients. The overlap in symptoms between esophageal perforation and AMI can lead to misdiagnosis, and in the case of esophageal perforation, delayed treatment significantly worsens outcomes. In fact, the mortality ranges from 10% to 25% when therapy starts within 24 hours but increases up to 60% when treatment is delayed beyond 48 hours. It would be prudent for emergency departments to consider implementing a standardized diagnostic protocol. Such a protocol may include: detailed clinical history with identification of risk factors that could predispose a patient to esophageal perforation, physical examination: looking for signs like subcutaneous emphysema, which is a hallmark of esophageal perforation but is absent in AMI, imaging: Incorporating contrast-enhanced CT scans and finally early multidisciplinary consultation: Involving both cardiology and thoracic surgery teams early in cases where the diagnosis is uncertain can lead to faster and more accurate decision-making. Implementation of a protocol could improve diagnostic accuracy and lead to earlier treatment of esophageal perforation, reducing the high mortality associated with delayed diagnosis. This would be particularly important in patients where there is a clinical suspicion that chest pain might not be cardiac in origin.
Expert opinion 2: Dr. Jules Lin
Most patients with an esophageal perforation will have a clinical presentation that should raise suspicion for an esophageal perforation, and a diagnostic protocol should not be needed for all patients with chest pain and ECG changes in the inferior leads. However, a diagnostic protocol could be considered for patients where there is suspicion for an esophageal perforation based on a history of recent forceful vomiting, foreign body ingestion, or esophageal instrumentation, which should be a relatively small subset of patients.
Expert opinion 3: Dr. Christina M. Stuart
The majority of patients with esophageal perforation will have the frank clinical signs and history suggestive of perforation as discussed above. The presentation described in this case is rare, with the predominant finding being an abnormal ECG. Given the rarity of this presentation, abnormal ECG should not prompt routine esophagram or CT in the absence of a detailed history or adjunctive studies suggestive of esophageal perforation. However, a patient presenting with abnormal ECG but a lack of cardiac enzymes should suggest alternative diagnosis. In short, we do not recommend routine esophagram or CT in the evaluation of patients with abnormal ECG.
Question 3: what is the role of novel imaging techniques, such as contrast-enhanced CT or magnetic resonance imaging (MRI), in the early detection and characterization of esophageal perforation?
Expert opinion 1: Dr. Sofoklis Mitsos
Early and accurate imaging is essential for guiding treatment decisions and improving patient outcomes in cases of esophageal perforation. Novel imaging techniques play a critical role in the early detection, offering significant advantages over traditional methods.
Contrast-enhanced CT is the gold standard for diagnosis and is currently the imaging modality of choice for diagnosing esophageal perforation. It offers high sensitivity and specificity, allowing for rapid identification of perforation. It can detect pneumomediastinum, pleural effusions, and extraluminal contrast leaks, which are indicative of esophageal rupture. It also helps in assessing the extent of the perforation, mediastinal contamination, and any associated complications. Moreover, contrast-enhanced CT provides a detailed anatomic assessment, is widely available, and allows for prompt diagnosis and treatment planning.
Although MRI is not typically the first-line modality for esophageal perforation due to its lower availability and longer acquisition times compared to CT, it can be useful in specific scenarios. MRI offers excellent soft tissue contrast, which can be beneficial in assessing mediastinal structures and potential complications, such as mediastinitis or abscess formation. Moreover, MRI is a safer option in certain populations (e.g., pregnant patients) due to involve ionizing radiation.
Expert opinion 2: Dr. Jules Lin
If there is suspicion for an esophageal perforation based on the patient’s history including recent forceful vomiting, foreign body ingestion, or esophageal instrumentation, the patient should undergo an oral-contrast study such as a barium esophagram or a chest and abdominal CT with oral contrast. These tests will be more available and accessible and would be recommended over an MRI.
Expert opinion 3: Dr. Christina M. Stuart
We recommend obtaining dynamic esophagram in evaluation of suspected esophageal perforation, however this may not be readily obtainable in smaller hospital settings. CT with oral contrast can be used in this setting, with an appropriate sensitivity/specificity. In prior work we have extensively explored alternative imaging strategies, and defined our preference and recommendations (3).
Question 4: are there any specific patient populations or risk factors that should prompt a higher degree of suspicion for esophageal perforation when signs and symptoms suggestive of acute myocardial infarction are present? How can emergency physicians be better educated to recognize these atypical presentations?
Expert opinion 1: Dr. Sofoklis Mitsos
There are specific patient populations and risk factors that should prompt a higher degree of suspicion for esophageal perforation. Recognizing these factors is crucial to avoid delayed diagnosis, which can significantly impact patient outcomes. Most esophageal perforations are caused by diagnostic and therapeutic interventions, followed by spontaneous rupture, foreign body ingestion, trauma and malignancy. Esophageal diagnostic and therapeutic interventions, such as endoscopy, esophageal dilation, or biopsy are known to increase the risk of esophageal perforation, particularly in patients with underlying esophageal conditions like strictures or achalasia. Spontaneous perforation can occur after forceful vomiting or retching (Boerhaave syndrome) particularly in patients with a history of heavy alcohol use or eating disorders. Another high-risk group are patients with esophageal pathologies, such as Barrett’s esophagus, esophagitis, or malignancy. These conditions weaken the esophageal wall and increase the risk of perforation, especially if invasive procedures or trauma are involved. Injuries to the chest or neck, whether from accidents or other blunt or penetrating chest trauma, can lead to esophageal perforation and should be considered when chest pain is present. Finally immunocompromised patients, patients on chemotherapy, with human immunodeficiency virus, or on chronic corticosteroids may be at higher risk of esophageal perforation due to infections or weaker tissue integrity.
Better education of emergency physicians through targeted training, checklists, and a multidisciplinary approach can improve recognition of these atypical presentations and ultimately enhance patient outcomes. It would be useful to enhance training on non-cardiac causes of chest pain by incorporating modules on esophageal perforation into continuing medical education for emergency physicians, highlighting how its presentation can mimic AMI, implementing checklists in emergency departments that flag high-risk populations and emphasizing in recognition of subtle signs of esophageal perforation. Moreover, emergency physicians could be educated on the importance of early imaging, such as contrast-enhanced CT, in high-risk populations presenting with chest pain, even when initial ECG findings suggest AMI. We should always encourage a multidisciplinary approach and seek early consultation with thoracic surgery, gastroenterology or upper gastrointestinal surgery in ambiguous cases.
Expert opinion 2: Dr. Jules Lin
The clinical presentation should help guide the need for further diagnostic studies. If there is suspicion for an esophageal perforation based on a history of recent forceful vomiting, foreign body ingestion, or esophageal instrumentation in a patient with ST-elevation in the inferior ECG leads, the patient should undergo an oral-contrast study such as a barium esophagram or a chest and abdominal CT with oral contrast, especially in the absence of reciprocal changes in the lateral ECG leads or other laboratory findings of myocardial infarction.
Expert opinion 3: Dr. Christina M. Stuart
As always, the first step in evaluation of a patient should be a careful history taking, including history of foreign body ingestion, trauma and forceful vomiting or Valsalva. Patients presenting with abnormal EKG, especially those with signs of infection and respiratory compromise who have an absence of abnormal cardiac enzymes, should be screened for these histories. If a positive history is uncovered, or alternative suggestive imaging findings such as pleural effusion or pneumomediastinum above are discovered, patients should undergo formal esophagram or “gulp-and-go” oral contrasted CT to evaluate for perforation.
Conclusions
Our report suggests that esophageal rupture should be considered in patients with necrotic Q waves and ST-segment elevation in the inferior leads on ECG, especially when there are no dynamic changes in reciprocal leads and no abnormal markers of myocardial necrosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1616/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1616/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1616/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s family members for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kassem MM, Wallen JM. Esophageal Perforation and Tears. 2024.
- Kotsis L, Kostic S, Zubovits K. Multimodality treatment of esophageal disruptions. Chest 1997;112:1304-9. [Crossref] [PubMed]
- Madsen HJ, Stuart CM, Wojcik BM, et al. Esophagram should be performed to diagnose esophageal perforation before inter-hospital transfer. J Thorac Dis 2023;15:2984-96. [Crossref] [PubMed]
- Qin C, Yang Y, Lu Y. Perforation of the esophagus: an overlooked cause of chest pain as a complication of esophageal foreign bodies. J Zhejiang Univ Sci B 2023;24:455-7. [Crossref] [PubMed]
- Pate JW, Walker WA, Cole FH Jr, et al. Spontaneous rupture of the esophagus: a 30-year experience. Ann Thorac Surg 1989;47:689-92. [Crossref] [PubMed]
- Hendry C, Srivastava V, Kumar S, et al. Angiographic right heart hypermobility as a sign of non-cardiac pathology in the setting of ST elevation. J Cardiol Cases 2013;8:e36-8. [Crossref] [PubMed]
- Brinster CJ, Singhal S, Lee L, et al. Evolving options in the management of esophageal perforation. Ann Thorac Surg 2004;77:1475-83. [Crossref] [PubMed]
- Haba Y, Yano S, Akizuki H, et al. Boerhaave syndrome due to excessive alcohol consumption: two case reports. Int J Emerg Med 2020;13:56. [Crossref] [PubMed]
- Wilkins ML, Maynard C, Annex BH, et al. Admission prediction of expected final myocardial infarct size using weighted ST-segment, Q wave, and T wave measurements. J Electrocardiol 1997;30:1-7. [Crossref] [PubMed]
- Jurlander B, Holmvang L, Galatius S, et al. "Mirror-lake" serial relationship of electrocardiographic and biochemical indices for the detection of reperfusion and the prediction of salvage in patients with acute myocardial infarction. Am Heart J 2003;146:757-63. [Crossref] [PubMed]
- Huang L, Song L. Clinical Interpretation Of ECG.1st Edition. Beijing: Chemical Industry Press, 2009: 42-82.
- Ibánez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed) 2017;70:1082. [Crossref] [PubMed]
- Vyas H, Desal D, Abraham P, et al. Heparin therapy for mistaken cardiac diagnosis in Boerhaave's syndrome. Indian J Gastroenterol 2004;23:72-3. [PubMed]
- Mosseri M, Eliakim R, Mogle P. Perforation of the esophagus electrocardiographically mimicking myocardial infarction. Isr J Med Sci 1986;22:451-4. [PubMed]
- Guo RW, Li L. Analysis of 4 cases of spontaneous esophageal perforation misdiagnosed as coronary heart disease. Chinese Journal of Integrative Medicine on Cardio Cerebrovascular Disease 2006;4:845-6.
- Kaman L, Iqbal J, Kundil B, et al. Management of Esophageal Perforation in Adults. Gastroenterology Res 2010;3:235-44. [PubMed]
- Tan N, Luo YH, Li GC, et al. Presentation of Boerhaave's syndrome as an upper-esophageal perforation associated with a right-sided pleural effusion: A case report. World J Clin Cases 2022;10:6192-7. [Crossref] [PubMed]
- Wigley C, Athanasiou A, Bhatti A, et al. Does the Pittsburgh Severity Score predict outcome in esophageal perforation? Dis Esophagus 2019;32:doy109. [Crossref] [PubMed]
- Surendran S, Victor C, Yacob M, et al. Clinical profile and treatment outcomes of Boerhaave's syndrome: A 13-year experience from an upper gastrointestinal surgical unit. Turk J Surg 2023;39:177-89. [Crossref] [PubMed]
- Li YM, Jia YH, Tsauo JY, et al. Case Report: ST-Segment Elevation in a Man With Acute Pericarditis. Front Cardiovasc Med 2020;7:609691. [Crossref] [PubMed]
- Jolobe OMP. Differential diagnosis of the association of gastrointestinal symptoms and ST segment elevation, in the absence of chest pain. Am J Emerg Med 2021;49:137-41. [Crossref] [PubMed]


