
Treating deep sternal wound infection with pectoralis major flap transposition: a systemic factor analysis of efficacy and safety
Highlight box
Key findings
• Pectoralis major flap transposition (PMFT) is a safe and effective method for treating deep sternal wound infection (DSWI) after cardiac surgery.
What is known, and what is new?
• Many methods are used to treat DSWI; however, to date, no consensus has been reached as to the efficacy of these methods.
• PMFT was shown to be a safe and effective method for treating DSWI after cardiac surgery. Our models also revealed the risk factors related to DSWI.
What is the implication, and what should change now?
• The risk factors identified in the preoperative model and operation-related model deserve more attention. Prospective studies need to be conducted to explore the relationships among the novel risk factors and DSWI.
Introduction
Median sternotomy, a widely used incision for cardiac surgeries, provides direct access to the heart and clearly shows its morphology. However, if the incision becomes infected and develops into deep sternal wound infection (DSWI), it is challenging to treat. Delayed wound healing (WH) or non-WH prolongs the length of stay, and increases the pain and the economic burden placed on patients. If the efficacy of the debridement alone following median sternotomy wound infection is poor, the unhealed incision may eventually develop into DSWI. Phoon et al. reported that the incidence of DSWI ranges from 0.2–3.0% (1). Currently, the methods used to treat DSWI include hyperbaric oxygen therapy, negative pressure-assisted wound therapy, tissue engineering therapy, gene and stem cell therapy (2), muscle flap transposition (3) and nitinol clips application (4). However, no consensus has been reached as to the efficacy of these treatment modalities. Therefore, this study aimed to retrospectively analyze the effectiveness of pectoralis major flap transposition (PMFT) in treating DSWI, and to develop predictive models related to delayed wound healing or death (DWHD) after PMFT. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1490/rc).
Methods
Subjects
A total of 785 consecutive patients at Beijing Anzhen Hospital who suffered from DSWI after cardiac surgery were enrolled in the study from January 2010 until December 2020. All the patients were diagnosed with DSWI according to definitions of surgical wound infections from Centers for Disease Control (5), and all of the patients were treated with PMFT. The patients were divided into the following two groups based on their results following PMFT treatment: (I) the WH group, which comprised patients who achieved WH without any further surgery; and (II) the DWHD group, which comprised patients who experienced DWHD. The baseline and operation-related information of the patients are shown in Table 1. All the diabetic patients underwent strict glycemic control with insulin during the perioperative period of PMFT. In addition, the patients were treated with β-lactam antibiotics or vancomycin until their bacterial culture and drug sensitivity test results were obtained. All the patients were followed up for 1 year.
Table 1
| Variables | Overall | WH | DWHD | P |
|---|---|---|---|---|
| Overall | 785 (100.0) | 709 (90.3) | 76 (9.7)§ | |
| Group | 0.03* | |||
| Aorta | 90 (11.46) | 78 (11.00) | 12 (15.79) | |
| CABG | 334 (42.55) | 299 (42.17) | 35 (46.05) | |
| Complicated | 213 (27.13) | 189 (26.66) | 24 (31.58) | |
| Valve | 148 (18.85) | 143 (20.17) | 5 (6.58) | |
| Age (years) | 59.00 [50.00, 66.00] | 60.00 [51.00, 67.00] | 57.50 [49.00, 65.25] | 0.22 |
| Sex: female | 295 (37.58) | 271 (38.22) | 24 (31.58) | 0.31 |
| Diabetes mellitus | 370 (47.13) | 323 (45.56) | 47 (61.84) | 0.01* |
| Obesity | 414 (52.74) | 363 (51.20) | 51 (67.11) | 0.01* |
| Smoker | 440 (56.05) | 386 (54.44) | 54 (71.05) | 0.008** |
| COPD | 338 (43.06) | 300 (42.31) | 38 (50.00) | 0.24 |
| Creatinine clearance, mL/min | <0.001***∆ | |||
| >85 | 417 (53.12) | 367 (51.76) | 50 (65.79) | |
| 50–85 | 345 (43.95) | 326 (45.98) | 19 (25.00) | |
| <50 | 18 (2.29) | 13 (1.83) | 5 (6.58) | |
| Dialysis | 5 (0.64) | 3 (0.42) | 2 (2.63) | |
| Abnormal liver function | 81 (10.32) | 64 (9.03) | 17 (22.37) | 0.001** |
| NYHA | 0.63 | |||
| NYHA II | 481 (61.27) | 432 (60.93) | 49 (64.47) | |
| NYHA III | 304 (38.73) | 277 (39.07) | 27 (35.53) | |
| Hypoalbuminemia | 544 (69.30) | 483 (68.12) | 61 (80.26) | 0.040* |
| Anemia | 314 (40.00) | 270 (38.08) | 44 (57.89) | 0.001** |
| Atrial fibrillation | 229 (29.17) | 212 (29.90) | 17 (22.37) | 0.22 |
| Extracardiac arteriopathy | 277 (35.29) | 243 (34.27) | 34 (44.74) | 0.09 |
| EuroSCORE II | 2.39 [1.45, 4.33] | 2.38 [1.45, 4.22] | 2.51 [1.52, 4.78] | 0.40 |
| Chronic infection | 144 (18.34) | 123 (17.35) | 21 (27.63) | 0.041* |
| Immune disease | 42 (5.35) | 30 (4.23) | 12 (15.79) | <0.002** |
| Secondary surgery† | 14 (1.78) | 11 (1.55) | 3 (3.95) | 0.15∆ |
| Salvage surgery† | 24 (3.06) | 12 (1.69) | 12 (15.79) | <0.002** |
| Graft type† | 0.21∆ | |||
| None | 238 (30.32) | 221 (31.17) | 17 (22.37) | |
| Vein | 326 (41.53) | 292 (41.18) | 34 (44.74) | |
| Vein + BIMA | 4 (0.51) | 3 (0.42) | 1 (1.32) | |
| Vein + SIMA | 217 (27.64) | 193 (27.22) | 24 (31.58) | |
| Operation time 1† (min) | 299.00 [276.00, 353.00] | 299.00 [278.00, 353.00] | 294.00 [266.00, 349.25] | 0.42 |
| Low temperature† | 29 (3.69) | 18 (2.54) | 11 (14.47) | <0.001***∆ |
| Osteoporosis | 236 (30.06) | 208 (29.34) | 28 (36.84) | 0.22 |
| Transfusion† | 394 (50.19) | 350 (49.37) | 44 (57.89) | 0.20 |
| IABP† | 8 (1.02) | 4 (0.56) | 4 (5.26) | 0.004**∆ |
| ECMO† | 11 (1.40) | 2 (0.28) | 9 (11.84) | <0.001***∆ |
| Redo† | 12 (1.53) | 6 (0.85) | 6 (7.89) | <0.002** |
| Ventilation time† (hour) | 13.00 [11.00, 16.00] | 13.00 [11.00, 16.00] | 14.00 [11.00, 21.50] | 0.10 |
| ICU stay† (hour) | 22.00 [20.00, 25.00] | 22.00 [20.00, 25.00] | 23.00 [20.00, 30.00] | 0.052 |
| Low cardiac output syndrome† | 14 (1.78) | 3 (0.42) | 11 (14.47) | <0.002** |
| Hospital acquired pneumonia† | 46 (5.86) | 35 (4.94) | 11 (14.47) | 0.002** |
| Operation time 2‡ (min) | 95.00 [80.00, 112.00] | 94.00 [80.00, 113.00] | 97.50 [79.00, 109.75] | 0.60 |
| Gap time (day) | 20.00 [15.00, 27.00] | 20.00 [14.00, 25.00] | 30.50 [22.00, 34.25] | <0.001*** |
| Hospitalized time‡ (day) | 11.00 [9.00, 15.00] | 11.00 [9.00, 15.00] | 12.00 [9.00, 16.00] | 0.11 |
| Bacterial culture | 0.89∆ | |||
| Candida | 11 (1.40) | 11 (1.55) | 0 | |
| Coagulase-negative gram-positive bacteria | 16 (2.04) | 14 (1.97) | 2 (2.63) | |
| Corynebacterium | 7 (0.89) | 7 (0.99) | 0 | |
| Escherichia coli | 38 (4.84) | 33 (4.65) | 5 (6.58) | |
| Fungi | 12 (1.53) | 10 (1.41) | 2 (2.63) | |
| Klebsiella | 18 (2.29) | 16 (2.26) | 2 (2.63) | |
| Pseudomonas aeruginosa | 74 (9.43) | 66 (9.31) | 8 (10.53) | |
| Staphylococcus aureus | 489 (62.29) | 443 (62.48) | 46 (60.53) | |
| Staphylococcus epidermidis | 120 (15.29) | 109 (15.37) | 11 (14.47) |
The categorical data are reported as n (%), and the continuous data are reported as median [IQR]. P: χ2 test with the exact method for the categorical variables, and t-test or Mann-Whitney U test for the continuous variables. P value <0.05 indicates a significant statistical difference. †, the information related to the previous cardiac surgery; ‡, the information related to the debridement and pectoralis major flap transposition; §, among the 76 patients, 29 (38.16%) died of severe infection, cardiovascular diseases, multiorgan failure, and so on. In addition, 47 (61.84%) patients needed extra procedures to achieve wound healing after debridement and PMFT; ∆, Fisher exact test. *, P<0.05; **, P<0.01; ***, P<0.001. WH, wound healing group; DWHD, delayed wound healing or death group; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association; BIMA, bilateral internal mammary arteries; SIMA, single internal mammary arteries; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range.
Definitions
The definitions of most of the variables in this study were adopted from the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II) (6). According to the clinical practice guideline (“Obesity in adults”) (7), obesity was defined as a body mass index >30 kg/m2. Patients who had persistent abnormal blood liver tests in the preoperative period were diagnosed with abnormal liver function. Notably, some patients in this study had comorbid immune diseases, such as Behçet’s disease, Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and systemic lupus erythematosus. These patients were treated with glucocorticoids, immunosuppressants, immunomodulators, or biologics before undergoing cardiac surgery. Among the preoperative (before PMFT) variables, hypoalbuminemia was defined as albumin in the serum <3.5 g/dL, and anemia was defined as a hemoglobin (Hb) concentration <120 g/L in women and a Hb concentration <130 g/L in men based on the criteria of the World Health Organization (8).
“Operation time 1” was defined as the total time of the first cardiac surgery, while “operation time 2” was defined as the total time of the debridement and PMFT. A “redo” was defined as a patient having to undergo repeat cardiac surgery for any appropriate reason. Hypothermia was defined as the core temperature of the patient dropping to <36 ℃ after cardiac surgery (9).
Osteoporosis was defined as a diagnosis of low bone mineral density, which was confirmed by dual-energy X-ray absorptiometry (10). Postoperative low cardiac output syndrome was defined as three consecutive cardiac indexes <2.0 L/min/m2 after cardiac surgery despite the adequate preload, afterload, and proper use of a positive inotropic pharmacological therapeutic or intra-aortic balloon pump (IABP). “Gap time” was defined as the period from the time point of the patient undergoing cardiac surgery until a definitive diagnosis of DSWI was confirmed. Hospitalization time was defined as the time of the latest hospitalization, which indicates the whole treatment course of DSWI.
It is important to note that incisional infections are usually classified as superficial or deep. The former usually only involves skin and subcutaneous tissue, while the latter involves fascia or muscle with or without sternal and mediastinal infections. In the present study, all the patients enrolled had deep wound sternal infections. The primary composite endpoint in the study was defined as all-cause death and failure to achieve WH without any other extra procedures after undergoing PMFT.
PMFT technique
After general anesthesia and sterilization, the PMFT procedure (Figure 1) was performed along the previous incision of the last cardiac surgery. It should be noted that the PMFT incision extended beyond the entire sternum to ensure good exposure. The necrotic skin, peripheral hyperplastic tissue, and muscle were cleaned out, and the sternal wires or sternal fixators were removed entirely. After the entire surface of the sternum was visible, the infected sternum was manually scraped until a fresh wound was observed. Next, the wound was irrigated with hydrogen peroxide or diluted iodine, and then repeatedly rinsed with sterile saline. Once the debridement was finished, the pectoralis major muscle was separated from the midline of the sternum. Usually, intercostal vessels originating from the internal mammary artery were observed at a distance of 10 cm from the midline of the sternum. It should be noted that the intercostal vessels, which supplies blood for the pectoralis major muscle, were preserved. When approaching the humerus, the pectoralis converges into the tendon, a marker of release. Once the tension of the pectoralis was released, it was easy to rotate the muscle and expose the vessels related to the pectoralis. To ensure satisfactory blood supply to the pectoralis, at least one vessel in each part of the muscle was split. After separating the flaps, the pedicled muscle was drawn and curled to the wound surface to cover the sternum defect. If the defect was minor, unilateral PMFT sufficed; otherwise, bilateral PMFT was necessary. If the defect could not be eliminated, a rectus abdominis or omental flap was used to fill the residual defect. Postoperative antibiotics were administered based on microbiological culture results of wound secretions.

Data and statistical analysis
The data distribution was analyzed by the Shapiro-Wilk test, and the test results were visualized using normal Q-Q plots (Figures S1-S8). The categorical variables are expressed as the number of patients with the percentage, and the continuous variables are expressed as the mean ± standard deviation, or the median with the interquartile range (IQR; the range between the first and the third quartile). All the variables in this study were compared using the chi-square (χ2) or Fisher’s exact test for the categorical variables, and the Student’s t-test or Mann-Whitney U test for the continuous variables as appropriate. A P value <0.05 indicated a statistically significant difference.
In this study, a systemic factor analysis was carried out using logistic regression. First, a univariate logistic regression model was established to identify any possible associations between different prognoses (WH and DWHD) and the independent positive variables. Next, a multivariate logistic regression model was constructed based on the previous univariate logistic regression results. First, all the covariables with P values <0.10 were included in the model, and the remaining covariables were then added step by step. Finally, the model results were checked, and the covariables that exerted significant bias were removed to ensure they did not affect the model’s fitness (overfitting or underfitting). The odds ratio (OR) with a 95% confidence interval (95% CI) was computed for each variable in the univariate and multivariate logistic regression models. The systemic factor analysis established two multivariate logistic models (the preoperative and operation-related models). The preoperative model included the variables before cardiac surgery, and the operation-related model included those related to cardiac surgery and PMFT. Once the models were established, the discrimination power of both models was assessed using the receiver operating characteristic (ROC) curve, and the area under the curve (AUC) of the ROC was calculated. The accuracy of the model prediction power was defined as low (AUC: 0.5–0.7), moderate (AUC: 0.7–0.9), or high (AUC: 0.9–1) (11). Multiple imputation methods were used to manage any missing values. All the statistical analyses were conducted with R (version 4.1.2 for Mac OS, Vienna, Austria, https://www.R-project.org).
Ethical statement
Written informed consent was obtained from all the patients enrolled in the study, and the study was approved by the ethics committee of Beijing Anzhen Hospital (No. 2022191X). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
Composite primary endpoint events
The flow of participants through the study is shown in Figure 2. In total, 76 (9.68%) DSWI patients experienced DWHD in this study. Among these 76 patients, 29 (38.16%) died from severe infection, cardiovascular diseases, multiorgan failure, etc., and 47 (61.84%) underwent extra procedures to achieve WH after debridement and PMFT (Table 1). Per El Oakley and Wright’s classification (12), there are 50 patients with mediastinitis type I, 147 patients with mediastinitis type II, and 572 patients with mediastinitis type III (139 with type IIIA and 433 with type IIIB). Furthermore, there are 14 patients with mediastinitis type IVA and 5 patients with mediastinitis type V.

Systemic factor analysis of patients suffering from DWHD
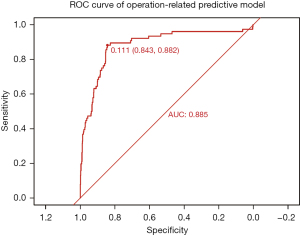
According to the logistic regression results of this study, the univariate logistic regression model revealed many factors related to DWHD in DSWI patients (Figure 3). However, the potential relationship between the different prognoses remained unclear. Therefore, we separated all the variables with P values <0.1 from the univariate regression model into two groups: the first group contained the baseline characteristics of the patients; the second group included the information associated with the previous cardiac surgery and the variables related to the whole therapeutic course of DSWI. As some variables, such as low cardiac output syndrome and bacterial culture results, had unexplainable outliers, we excluded these variables (Tables S1-S3). We established the multivariable logistic regression models of both groups’ variables. The preoperative multivariate logistic regression model indicated that patients with diabetes mellitus (OR: 2.99, 95% CI: 1.56–5.96, P=0.001), obesity (OR: 2.01, 95% CI: 1.12–3.70, P=0.02), a history of smoking (OR: 2.24, 95% CI: 1.26–4.09, P=0.007), abnormal liver function (OR: 3.99, 95% CI: 1.61–9.79, P=0.003), anemia (OR: 2.35, 95% CI: 1.35–4.14, P=0.003), chronic infection (OR: 1.93, 95% CI: 1.00–3.62, P=0.045), and immune disease (OR: 5.25, 95% CI: 2.08–12.97, P<0.001) were more likely to experience DWHD (Figure 4). The operation-related multivariate logistic regression model showed that patients who underwent salvage surgery (OR: 9.24, 95% CI: 2.39–33.74, P=0.001), had hypothermia (OR: 4.26, 95% CI: 1.16–14.58, P=0.02), required extracorporeal membrane oxygenation (ECMO) assistance (OR: 31.14, 95% CI: 2.59–517.33, P=0.009), had a longer gap time (OR: 1.2, 95% CI: 1.15–1.25, P<0.001), and required redo cardiac surgery (OR: 7.44, 95% CI: 1.16–45.59, P=0.03) were more likely to experience DWDH (Figure 5). The discrimination power of both models was assessed with the ROC curve, and the AUCs were 0.811 and 0.885 for the preoperative and operation-related predictive models, respectively (Figures 6,7).




Discussion
DSWI after cardiac surgery is relatively rare and difficult to treat. In addition to the emotional and physical pain it causes, this condition is life-threatening if not managed properly (13). Phoon et al. noted that the incidence of DSWI ranges from 0.2–3.0% (1), and while the incidence of DSWI is low, the mortality of DSWI ranges from 3–50% (12,14-16). In our study, the mortality rate of DSWI after PMFT was approximately 3.7% (29/785), which showed that DSWI patients treated with PMFT after cardiac surgery can achieve a satisfactory result with low mortality. In the systemic factor analysis of the present study, we found some risk factors similar to those reported in previous studies (17-20), including diabetes mellitus, obesity, a history of smoking, and anemia.
Interestingly, our study included some patients (42/785, 5.35%) with comorbid immune diseases. These patients had different phenotypes and were treated with various medications (e.g., glucocorticoids, immunosuppressants, immunomodulators, and biologics) to control the progression of the immune diseases. This scenario made it challenging to design the therapeutic plan for the whole perioperative period. Previous studies have shown that long-term glucocorticoid or biological administration can affect surgical WH (21-26). Otrocka-Domagała et al. examined the potential reason for glucocorticoid injury skeletal muscle regeneration in a porcine model (27). WH is a process that includes the inflammatory phase, proliferative phase, and epithelialization phase. The interaction of cellular (e.g., immunocytes) and humoral factors (e.g., chemokines) in WH is complex (28,29). If DSWI patients have a comorbid immune disease, their wound microenvironment differs to that of other patients. This might be the reason for the unsatisfactory efficacy of debridement and PMFT in such patients. Moreover, opportunistic infections are another issue for these patients, regardless of whether or not they undergo cardiac surgery. Therefore, transcatheter therapeutic methods (e.g., transcatheter heart valve replacement, and percutaneous coronary interventions), which could reduce the risk of incision infections, might be the best choice for this special group of patients. Moreover, multidisciplinary teamwork (e.g., with relevant departments, such as the rheumatology, dermatology, gastroenterology departments) is necessary to assess and adjust the medications of these patients during the perioperative phase.
We found that patients with abnormal liver function during the perioperative phase of PMFT were more likely to meet the primary composite endpoint. We also found that the patients with valvular disease (especially mitral and tricuspid valvular disease) were more likely to suffer from abnormal liver function than those without valvular disease. Some of these patients experienced congestive heart failure before cardiac surgery; thus, their liver function might be injured as the valvular disease develops. The liver, which cannot be replaced by any other organs, plays a significant role in catabolic and metabolic functions. Abnormal liver function can result in abnormal coagulation and hypoalbuminemia. Hemorrhage or thromboembolism may be followed by abnormal coagulation after cardiac surgery, and these events can result in life-threatening strokes and infractions of other organs. Hypoalbuminemia can delay WH and increase the risk of wound dehiscence. Thus, more attention should be paid to the liver function of DSWI patients undergoing valvular surgery.
Unlike other surgeries, many cardiac surgeries must be facilitated by cardiopulmonary bypass, which can trigger hypothermia after surgery. Postoperative hypothermia was another risk factor found to be related to DWHD in this study. Intraoperative hypothermia provides a clean surgical field without blood during the cardiac procedure; however, blood stored in peripheral circulation might affect the blood supply of the incision. Thus, when patients suffer from hypothermia after cardiac surgery, the ideal WH environment (moist, clean, and warm) is difficult to establish, and coagulation factors have difficulty playing their roles. Therefore, warming strategies should be used to avoid hypothermia.
Notably, some DSWI patients did not achieve WH after undergoing PMFT. We retrospectively analyzed the data of these patients and found that a large number of these patients underwent debridement during the gap period, and incomplete debridement and improper strategies for treating DSWI made the chest hostile. When these patients were admitted to our centers, the conditions of their chest walls were more complicated than others, and some had become resistant to many antibiotics. Thus, the mechanical and antibiotic treatments provided ineffective, making WH difficult. As mentioned above, DSWI patients can only achieve WH if they undergo proper and complete debridement and PMFT. Further, numerous factors affect WH after cardiac surgery. If a patient has any of the above-mentioned risk factors, a proper treatment plan needs to be established based on this information to avoid DSWI. If a patient suffers from DSWI after cardiac surgery, the patient can be safely and effectively treated with PMFT.
Study limitations
The retrospective, descriptive nature of the present study is its primary limitation. The sample size of the study was relatively small, which limits the generalizability of our conclusions. Further, this study did not evaluate the potential contribution of other DWHD risk factors, such as the volume of the residual defect, antibiotic prophylaxis strategies, and patient preparation before cardiac surgery and PMFT. In summary, multicentered prospective studies on therapeutic strategies for DSWI after cardiac surgery need to be conducted. A reasonable clinical trial design, uniform diagnostic criteria, and more detailed variable analysis might be directions for future research.
Conclusions
PMFT was proven to be a safe and effective method in the treatment of DSWI after cardiac surgery. Most patients with DSWI can achieve WH after a single PMFT, although some patients with DWHD may benefit from staged debridement and vacuum-assisted closure (VAC) assessment with PMFT. Managing blood glucose levels, chronic infection, and immune disease activity, as well as adjusting liver function and hemoglobin levels before cardiac surgery, may reduce the occurrence of DSWI. It is advisable to prevent hypothermia following cardiac surgery, as this may help reduce the need for additional surgeries and minimize unsatisfactory results after PMFT in DSWI patients. Prospective studies need to be conducted in the future to explore the relationships among the novel risk factors associated with DSWI.
Acknowledgments
Funding: This research was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1490/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1490/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1490/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1490/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from all the patients enrolled in the study, and the study was approved by the ethics committee of Beijing Anzhen Hospital (No. 2022191X). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Phoon PHY, Hwang NC. Deep Sternal Wound Infection: Diagnosis, Treatment and Prevention. J Cardiothorac Vasc Anesth 2020;34:1602-13. [Crossref] [PubMed]
- Kolimi P, Narala S, Nyavanandi D, et al. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022;11:2439. [Crossref] [PubMed]
- Kaul P. Sternal reconstruction after post-sternotomy mediastinitis. J Cardiothorac Surg 2017;12:94. [Crossref] [PubMed]
- Saltarocchi S, Chourda E, D'Abramo M, et al. Vacuum-assisted wound closure with instillation followed by nitinol clips application to treat deep sternal wound infections after cardiac surgery: evolution of a two-step approach. Wounds 2023;35:E63-8. [Crossref] [PubMed]
- Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 1992;20:271-4. [Crossref] [PubMed]
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734-44; discussion 744-5. [Crossref] [PubMed]
- Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ 2020;192:E875-91. [Crossref] [PubMed]
- World Health Organization (WHO). Hemoglobin concentrations for the diagnosis of anemia and assessment of severity. 2011. Available online: http://www.who.int/vmnis/indicators/hemoglobin/en/(2011)
- Rauch S, Miller C, Bräuer A, et al. Perioperative Hypothermia-A Narrative Review. Int J Environ Res Public Health 2021;18:8749. [Crossref] [PubMed]
- Rentzeperi E, Pegiou S, Tsakiridis I, et al. Diagnosis and Management of Osteoporosis: A Comprehensive Review of Guidelines. Obstet Gynecol Surv 2023;78:657-81. [Crossref] [PubMed]
- Swets JA. Measuring the accuracy of diagnostic systems. Science 1988;240:1285-93. [Crossref] [PubMed]
- El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg 1996;61:1030-6. [Crossref] [PubMed]
- Niclauss L, Delay D, Stumpe F. Right ventricular rupture due to recurrent mediastinal infection with a closed chest. Interact Cardiovasc Thorac Surg 2010;10:470-2. [Crossref] [PubMed]
- Abdelnoor M, Vengen ØA, Johansen O, et al. Latitude of the study place and age of the patient are associated with incidence of mediastinitis and microbiology in open-heart surgery: a systematic review and meta-analysis. Clin Epidemiol 2016;8:151-63. [Crossref] [PubMed]
- Vitartaitė M, Vaičiulytė D, Venclovienė J, et al. Risk Factors Associated with an Increased Risk of Deep Sternal Wound Infections in Patients After Coronary Artery Bypass Grafting and Heart Defect Surgery. Heart Surg Forum 2021;24:E741-5. [Crossref] [PubMed]
- Cha YK, Choi MS, Bak SH, et al. Incidence and risk factors for sternal osteomyelitis after median sternotomy. J Thorac Dis 2022;14:962-8. [Crossref] [PubMed]
- Diez C, Koch D, Kuss O, et al. Risk factors for mediastinitis after cardiac surgery - a retrospective analysis of 1700 patients. J Cardiothorac Surg 2007;2:23. [Crossref] [PubMed]
- Risnes I, Abdelnoor M, Almdahl SM, et al. Mediastinitis after coronary artery bypass grafting risk factors and long-term survival. Ann Thorac Surg 2010;89:1502-9. [Crossref] [PubMed]
- Gatti G, Dell'Angela L, Barbati G, et al. A predictive scoring system for deep sternal wound infection after bilateral internal thoracic artery grafting. Eur J Cardiothorac Surg 2016;49:910-7. [Crossref] [PubMed]
- Gundestrup L, Florczak CK, Riber LPS. Factors associated with deep sternal wound infection after open-heart surgery in a Danish registry. Am Heart J Plus 2023;31:100307. [Crossref] [PubMed]
- Ismael H, Horst M, Farooq M, et al. Adverse effects of preoperative steroid use on surgical outcomes. Am J Surg 2011;201:305-8; discussion 308-9. [Crossref] [PubMed]
- Billioud V, Ford AC, Tedesco ED, et al. Preoperative use of anti-TNF therapy and postoperative complications in inflammatory bowel diseases: a meta-analysis. J Crohns Colitis 2013;7:853-67. [Crossref] [PubMed]
- Yang ZP, Hong L, Wu Q, et al. Preoperative infliximab use and postoperative complications in Crohn's disease: a systematic review and meta-analysis. Int J Surg 2014;12:224-30. [Crossref] [PubMed]
- Bakkour W, Purssell H, Chinoy H, et al. The risk of post-operative complications in psoriasis and psoriatic arthritis patients on biologic therapy undergoing surgical procedures. J Eur Acad Dermatol Venereol 2016;30:86-91. [Crossref] [PubMed]
- Fassihi SC, Gu A, Perim DA, et al. Chronic preoperative corticosteroid use is not associated with surgical site infection following revision total knee arthroplasty. J Orthop 2020;20:173-6. [Crossref] [PubMed]
- Busti AJ, Hooper JS, Amaya CJ, et al. Effects of perioperative antiinflammatory and immunomodulating therapy on surgical wound healing. Pharmacotherapy 2005;25:1566-91. [Crossref] [PubMed]
- Otrocka-Domagała I, Paździor-Czapula K, Gesek M. Dexamethasone-induced impairment of post-injury skeletal muscle regeneration. BMC Vet Res 2019;15:56. [Crossref] [PubMed]
- Julier Z, Park AJ, Briquez PS, et al. Promoting tissue regeneration by modulating the immune system. Acta Biomater 2017;53:13-28. [Crossref] [PubMed]
- Shanley LC, Mahon OR, Kelly DJ, et al. Harnessing the innate and adaptive immune system for tissue repair and regeneration: Considering more than macrophages. Acta Biomater 2021;133:208-21.
(English Language Editor: L. Huleatt)


