
Development and validation of a nomogram for incorporating 18F-FDG PET/CT spleen uptake for predicting prognosis in elderly esophageal cancer patients treated with radiotherapy
Highlight box
Key findings
• The study developed a nomogram that incorporates spleen:liver ratio (SLR), length of visual tumor (Lv), and clinical tumor, node, metastasis (cTNM) staging to predict overall survival in elderly patients with esophageal squamous cell carcinoma (ESCC) undergoing radiotherapy. The model demonstrated superior predictive power compared to traditional TNM staging.
What is known and what is new?
• Prognostic models, such as the TNM staging system, are commonly used to estimate survival outcomes.
• This study developed a prognostic nomogram for elderly patients with ESCC undergoing radiotherapy by incorporating 18F-fluorodeoxyglucose positron emission tomography-computed tomography metabolic parameters, specifically the SLR and Lv, with cTNM staging.
What is the implication, and what should change now?
• The nomogram provides a reliable tool for risk stratification and personalized treatment planning, which could enhance clinical outcomes for elderly patients with ESCC. This model should be considered in future clinical practice for this patient population.
Introduction
Esophageal cancer is the eighth most common cancer globally and the sixth leading cause of cancer-related death. Among its histological types, esophageal squamous cell carcinoma (ESCC) accounts for approximately 90% of cases worldwide, particularly in high-incidence regions such as East Asia and parts of Africa (1). The management of ESCC involves a multimodal approach, including surgery, chemotherapy, and radiotherapy, either alone or in combination (2). For elderly patients, treatment decisions are complicated by age-related factors such as decreased physiological reserves and the presence of multiple comorbidities (3).
Previous studies have developed various prognosis prediction models for elderly patients with esophageal cancer undergoing radiotherapy. These models typically use predictors such as clinical staging, tumor size, and metabolic activity. However, many of these models have limitations in terms of predictive accuracy and applicability to elderly patients, who often have unique physiological characteristics that impact treatment outcomes. The tumor, node, metastasis (TNM) staging system, although widely used, does not sufficiently account for the biological behavior of tumors or patient-specific factors, which are crucial in elderly patients (4,5). This limitation underscores the need for supplementary prognostic markers.
In recent years, metabolic imaging with 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET/CT) has emerged as a valuable tool in oncology, providing insights into tumor biology beyond anatomical information (6). Metrics derived from 18F-FDG PET/CT, such as standardized uptake values (SUV), metabolic tumor volume (MTV), and total lesion glycolysis (TLG), have shown promise as prognostic indicators in various cancers (7). The metabolic activity of the tumor and surrounding tissues, including the spleen and liver, has been proposed as a potential prognostic factor (8). The spleen:liver ratio (SLR) of 18F-FDG uptake is a novel parameter reflecting the systemic inflammatory response and tumor-host interactions. Studies have suggested that a higher SLR is associated with poorer outcomes in several cancers, but its prognostic value in elderly patients with ESCC remains underexplored (9-11).
For elderly patients with ESCC, there is a significant clinical need for a prognostic model that can provide personalized treatment planning due to the challenges presented by age-related physiological decline and comorbidities. Developing a model that integrates imaging data and clinical information is essential to improve treatment decisions and outcomes in this patient population.
In this context, this study investigated the prognostic significance of SLR and tumor length as measured by 18F-FDG PET/CT in elderly patients with ESCC undergoing radiotherapy. Tumor length was determined using visual analysis, where the extent of the tumor was identified by regions with a radiotracer uptake higher than that of the surrounding normal esophageal tissue. The longitudinal axis of the tumor was then measured and defined as length of visual tumor (Lv). The study aimed to establish whether these parameters can serve as reliable markers for overall survival (OS) and to develop a composite prognostic model incorporating these variables.
Despite the potential of metabolic imaging markers, there is a paucity of data on their prognostic utility in elderly patients with ESCC. The integration of SLR and Lv into a composite score has not been thoroughly examined, leaving a gap in personalized prognostic models tailored for this specific patient population. This study sought to fulfill this need by validating these markers and developing a comprehensive prognostic tool.
This retrospective study included 118 elderly patients with ESCC, all of whom underwent 18F-FDG PET/CT imaging prior to radiotherapy. Key demographic and clinical characteristics, including age, sex, tumor location, and clinical staging, were documented. Univariate and multivariate Cox regression analyses were performed to identify independent prognostic factors for OS. The prognostic value of SLR and Lv was assessed using Kaplan-Meier survival analysis, and a composite score (S-L grade) was developed by combining these parameters. The predictive performance of the S-L grade and other prognostic indicators was evaluated through receiver operating characteristic (ROC) curves and decision curve analysis (DCA).
A nomogram incorporating the S-L grade and clinical TNM (cTNM) stage was constructed to predict the 1-, 2-, and 3-year OS. Calibration plots and the concordance index (C-index) were used to validate the nomogram’s accuracy. Patients were stratified into risk groups based on nomogram scores, and survival differences among these groups were analyzed.
The overall aims of this work are to provide a robust prognostic tool for elderly patients with ESCC, enabling personalized treatment planning and improved clinical outcomes. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1698/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved the Institutional Review Board of Fujian Cancer Hospital (No. K2024-488-01) and informed consent was taken from all the patients. The study included elderly patients diagnosed with ESCC at Fujian Cancer Hospital between November 2011 and June 2021 who received radiotherapy or chemoradiotherapy and underwent a pretreatment 18F-FDG PET/CT scan. The inclusion criteria were as follows: (I) a histological or cytological diagnosis of ESCC; (II) age between 70 and 90 years; (III) treatment with either radiotherapy alone or chemoradiotherapy; and (IV) completion of a whole-body 18F-FDG PET/CT scan within 2 weeks prior to treatment. Meanwhile, the exclusion criteria were as follows: (I) presence of other primary tumors; (II) multifocal esophageal cancer; (III) distant metastases beyond the supraclavicular or abdominal lymph nodes; (IV) prior immunotherapy, targeted therapy, or other antitumor treatments; and (V) severe comorbidities affecting the heart, lungs, or brain. cTNM staging was conducted according to the eighth edition of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) esophageal cancer staging system.
Sample size estimation
Sample size estimation was conducted using the formula for survival analysis, taking into consideration the expected event rate, statistical power, and significance level. A minimum of 100 patients was required to ensure adequate power for detecting significant differences in OS.
Data collection of baseline clinical factors
Baseline clinical factors (e.g., age, sex, tumor location, and clinical staging) were collected from each patient. Each patient underwent 18F-FDG PET/CT scanning before treatment, and all data were recorded in the hospital’s electronic medical record system. The data were subsequently anonymized to ensure patient privacy. Key clinical factors were used to develop the prognostic model in combination with PET/CT imaging variables.
Treatment and follow-up
Patients included in this study underwent radiotherapy with an average dose of 59.43 Gy (ranging from 40 to 67.65 Gy), administered in fractions of 1.8–2.2 Gy per session. Of the 118 patients, 65 (55.1%) received chemotherapy, with 24 (20.3%) receiving oral S-1 chemotherapy, and the remaining 41 (63.1%) receiving platinum- or taxane-based chemotherapy, with a median of 2 cycles. The primary endpoint of the study was OS, defined as the time from histological diagnosis to death from any cause or the last follow-up date. Specifically, follow-up evaluations were performed every 3 months during the first 2 years post-radiotherapy, every 6 months between the third and fifth years, and annually thereafter. For patients unable to attend follow-up appointments in person, their status was ascertained via telephone or online communication. The last follow-up for this study was conducted in June 2024.
18F-FDG PET/CT examination
Patients fasted for at least 6 hours prior to the examination, with blood glucose levels maintained below 10.0 mmol/L. An 18F-FDG dose of 4.4 to 7.4 MBq/kg was administered according to patient body weight and followed by a 60- to 70-minute rest period before PET/CT imaging (Gemini TF II PET/CT, Philips, The Netherlands). The 18F-FDG was synthesized automatically by the F3003E module using the 18F produced by the HM-10 cyclotron (Sumitomo, Tokyo, Japan), with a radiochemical purity exceeding 98%. PET imaging conditions included a scan time of 1 minute per bed position, covering the range from the cranial vertex to the midthighs. The CT scan parameters were as follows: field of view (FOV), 250 mm; voltage, 140 kV; current, 250 mA; pitch, 0.75; and single rotation time, 0.8 s. CT scans were performed first, followed by PET acquisition.
18F-FDG PET/CT image analysis
All 18F-FDG PET/CT images were processed using a Medical Image Storage and Transmission System MEMRS (Version V4.0, Manufacturer: Beijing Medisys Technology Co., Ltd.), with software automatically delineating lesion boundaries. The esophageal cancer primary lesions were included in the volume of interest (VOI) using the 40% SUVmax threshold method, based on axial, sagittal, and coronal images. The software automatically calculated the maximum standardized uptake value (SUVmax), MTV, and TLG. Experienced nuclear medicine physicians determined the tumor extent using visual analysis, with tumor boundaries being defined as areas where the radiotracer uptake was higher than surrounding normal esophageal tissue, which was confirmed with concurrent CT images. The longitudinal axis length of the tumor was defined as Lv, and the maximum diameter of the tumor in the axial plane was defined as diameter of visual tumor (Dv). Splenic uptake was measured by delineating a 2-cm diameter region of interest in the spleen, and hepatic uptake was measured with a 3-cm diameter region of interest in the liver. The SLR was calculated by dividing the splenic SUVmax by the hepatic SUVmax, thus normalizing splenic uptake.
Statistical methods
Statistical analyses were performed using R version 4.2.1 (The R Foundation of Statistical Computing; http://www.r-project.org). The optimal thresholds for continuous variables predicting survival were determined using the surv_cutpoint function. Cox proportional hazards regression was used for univariate and multivariate analyses of baseline factors to identify independent prognostic factors for OS. Kaplan-Meier methods estimated OS rates, and differences between groups were assessed using the log-rank test. The predictive power of independent prognostic factors was evaluated using the C-index and the area under the ROC curve, with C-index calculations based on 40-fold sampling repeated 800 times. For a satisfactory predictive model, a C-index value above 0.7 and an AUC value above 0.75 were considered acceptable. A prognostic nomogram model was constructed using S-L grading combined with cTNM staging to predict the 1-, 2-, and 3-year OS rates. Model accuracy was validated by plotting calibration curves. DCA based on net benefit was used to assess the clinical utility of the model. Statistical significance was set at a two-sided P<0.05. Finally, based on the total scores derived from the model, the 118 patients were categorized into high-risk, intermediate-risk, and low-risk groups via the quantile function. Group differences were assessed using the log-rank test.
Results
Clinical characteristics and optimal thresholds for continuous variables
The clinical characteristics of the 118 patients included in this study are summarized in Table 1. Among these patients, 76 (64.4%) were male, and 42 (35.6%) were female. A total of 94 patients (79.7%) reached the endpoint event during the follow-up period, with an average follow-up duration of 26.6 months. The primary tumor was located in the cervical esophagus in 4 patients (3.4%), in the upper thoracic esophagus in 35 patients (29.7%), in the middle thoracic esophagus in 56 patients (47.5%), and in the lower thoracic esophagus in 23 patients (19.5%). The majority of primary tumors were classified as T3 (68 patients, 57.6%) or T4 (40 patients, 33.9%), with only 10 patients (8.5%) classified as T2. Supraclavicular lymph node metastasis (M1) was observed in 20 patients (16.9%). Of the total cohort, 53 (44.9%) patients received radiotherapy alone, while 65 (55.1%) patients received chemoradiotherapy.
Table 1
| Characteristics | Overall (n=118) |
|---|---|
| Gender | |
| Male | 76 (64.4) |
| Female | 42 (35.6) |
| Tumor location | |
| Cervical | 4 (3.4) |
| Upper thoracic | 35 (29.7) |
| Middle thoracic | 56 (47.5) |
| Lower thoracic | 23 (19.5) |
| Clinical T stage | |
| T2 | 10 (8.5) |
| T3 | 68 (57.6) |
| T4 | 40 (33.9) |
| Clinical N stage | |
| N0 | 40 (33.9) |
| N1 | 55 (46.6) |
| N2 | 20 (16.9) |
| N3 | 3 (2.5) |
| Clinical M stage | |
| M0 | 98 (83.1) |
| M1 | 20 (16.9) |
| 8th AJCC/UICC stage | |
| II | 29 (24.6) |
| III | 36 (30.5) |
| IVA | 34 (28.8) |
| IVB | 19 (16.1) |
| Chemotherapy | |
| No | 53 (44.9) |
| Yes | 65 (55.1) |
| SLR | |
| ≤0.9 | 68 (57.6) |
| >0.9 | 50 (42.4) |
| Lv (cm) | |
| ≤4.7 | 70 (59.3) |
| >4.7 | 48 (40.7) |
| Dv (cm) | |
| ≤2.2 | 35 (29.7) |
| >2.2 | 83 (70.3) |
| SUVmax | |
| ≤7.3 | 15 (12.7) |
| >7.3 | 103 (87.3) |
| MTV (cm3) | |
| ≤13.1 | 70 (59.3) |
| >13.1 | 48 (40.7) |
| TLG | |
| ≤28.1 | 20 (16.9) |
| >28.1 | 98 (83.1) |
Data are presented as n (%). AJCC, American Joint Committee on Cancer; UICC, International Union Against Cancer; SLR, spleen:liver ratio; Lv, length of visual tumor; Dv, diameter of visual tumor; SUVmax, maximum standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis.
The optimal thresholds for the continuous variables calculated via the surv_cutpoint function were as follows: SUVmax, 7.3; MTV, 13.1; TLG, 28.1; Lv, 4.7 cm; Dv, 2.2 cm; and SLR, 0.9. The S-L grade was defined based on SLR and Lv using the following criteria: S-L grade 1 was assigned when SLR was ≤0.9 and Lv was ≤4.7 cm. S-L grade 2 was assigned if either SLR or Lv exceeded these thresholds, and S-L grade 3 was assigned when both parameters exceeded their respective thresholds.
Univariate and multivariate cox regression analysis
Univariate Cox regression analysis identified cT stage, cN stage, cTNM stage, SUVmax, MTV, TLG, Lv, Dv, and SLR as significant prognostic factors for elderly patients with esophageal cancer (P<0.05). In the multivariate Cox regression analysis, SLR [hazard ratio (HR) 2.062, 95% confidence interval (CI): 1.283–3.313; P=0.003] and Lv (HR 1.835, 95% CI: 1.032–3.264; P=0.04) were determined to be independent prognostic factors for elderly patients with esophageal cancer (Table 2).
Table 2
| Characteristics | Total (n=118) | HR (95% CI) | P |
|---|---|---|---|
| Clinical T stage | |||
| T2 | 10 | Reference | |
| T3 | 68 | 2.138 (0.580–7.883) | 0.25 |
| T4 | 40 | 2.756 (0.651–11.673) | 0.17 |
| Clinical N stage | |||
| N0 | 40 | Reference | |
| Nl | 55 | 1.360 (0.638–2.896) | 0.43 |
| N2 | 20 | 2.177 (0.886–5.349) | 0.09 |
| N3 | 3 | 0.450 (0.080–2.526) | 0.36 |
| 8th AJCC/UICC stage | |||
| II | 29 | Reference | |
| Ill | 36 | 0.791 (0.288–2.176) | 0.65 |
| IVA | 34 | 0.995 (0.301–3.286) | 0.99 |
| IVB | 19 | 1.570 (0.537–4.591) | 0.41 |
| SLR | |||
| ≤0.9 | 68 | Reference | |
| >0.9 | 50 | 2.062 (1.283–3.313) | 0.003 |
| Lv (cm) | |||
| ≤4.7 | 70 | Reference | |
| >4.7 | 48 | 1.835 (1.032–3.264) | 0.04 |
| Dv (cm) | |||
| ≤2.2 | 35 | Reference | |
| >2.2 | 83 | 1.005 (0.498–2.030) | 0.99 |
| SUVmax | |||
| ≤7.3 | 15 | Reference | |
| >7.3 | 103 | 1.579 (0.505–4.940) | 0.43 |
| MTV (cm3) | |||
| ≤13.1 | 70 | Reference | |
| >13.1 | 48 | 1.202 (0.683–2.115) | 0.52 |
| TLG | |||
| ≤28.1 | 20 | Reference | |
| >28.1 | 98 | 1.050 (0.425–2.591) | 0.92 |
HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; UICC, International Union Against Cancer; SLR, spleen:liver ratio; Lv, length of visual tumor; Dv, diameter of visual tumor; SUVmax, maximum standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis.
Association of Lv, SLR, and S-L grade on OS
The median OS time for the 118 patients was 20 months, with 1-, 2-, and 3-year OS rates of 70.3%, 44.5%, and 31.4%, respectively. Patients with an Lv ≤4.7 cm had a 3-year OS rate of 44.5%, compared to 11.4% for those with an Lv >4.7 cm (Figure 1A; P<0.001). Similarly, patients with an SLR ≤0.9 had a 3-year OS rate of 46.2%, while those with an SLR >0.9 had a 3-year OS rate of 11.67% (Figure 1B, P<0.001). In the stratification of patients by S-L grade, those in grade 1 had a 3-year OS rate of 58.3%, those in grade 2 has a rate of 17.6%, and those in grade 3 has a rate of 7.4% (Figure 1C; all P values <0.001).

ROC curve analysis of Lv, SLR, and S-L grade for OS prediction
Figure 2 illustrates the ROC curve analysis of Lv, SLR, and S-L grade for predicting OS. The area under the curve (AUC) for Lv in predicting the 1-, 2-, and 3-year OS was 0.621 (95% CI: 0.524–0.718), 0.672 (95% CI: 0.589–0.756), and 0.708 (95% CI: 0.631–0.785), respectively. For SLR, the AUCs for predicting 1-, 2-, and 3-year OS were 0.684 (95% CI: 0.592–0.777), 0.685 (95% CI: 0.601–0.770), and 0.710 (95% CI: 0.628–0.792), respectively. S-L grade showed superior predictive power, with AUCs of 0.718 (95% CI: 0.628–0.807), 0.749 (95% CI: 0.666–0.832), and 0.798 (95% CI: 0.724–0.873) for predicting 1-, 2-, and 3-year OS, respectively, all of which were higher than those of Lv and SLR. The predictive capability of Lv, SLR, and S-L grade was further assessed using the C-index. The C-index for S-L grade was 0.671 (95% CI: 0.647–0.695), which was higher than that of Lv (C-index 0.607; 95% CI: 0.581–0.633) and SLR (C-index 0.635; 95% CI: 0.610–0.660) (Table 3).

Table 3
| Factor | AUC (95% CI) | C-index (95% CI) | ||
|---|---|---|---|---|
| 1-year OS | 2-year OS | 3-year OS | ||
| Lv (cm) | 0.621 (0.524–0.718) | 0.672 (0.589–0.756) | 0.708 (0.631–0.785) | 0.607 (0.581–0.633) |
| SLR | 0.684 (0.592–0.777) | 0.685 (0.601–0.770) | 0.710 (0.628–0.792) | 0.635 (0.610–0.660) |
| S-L grade | 0.718 (0.628–0.807) | 0.749 (0.666–0.832) | 0.798 (0.724–0.873) | 0.671 (0.647–0.695) |
AUC, area under the curve; CI, confidence interval; OS, overall survival; Lv, length of visual tumor; SLR, spleen:liver ratio; S-L, a scoring system based on SLR and Lv, dividing patients into three levels for prognosis assessment.
Nomogram construction
A nomogram was constructed using the S-L grade and cTNM staging system to predict 1-, 2-, and 3-year OS rates. Initially, internal validation was performed to assess the predictive accuracy of the nomogram model. The AUCs for the nomogram in predicting the 1-, 2-, and 3-year OS were 0.771 (95% CI: 0.678–0.865), 0.763 (95% CI: 0.673–0.852), and 0.815 (95% CI: 0.734–0.896), respectively. The discriminative ability of the nomogram model was evaluated via the calculation of the C-index, which was found to be 0.707 (95% CI: 0.680–0.735) (Figure 3). DCA was used to assess the clinical utility of the nomogram, and the net clinical benefit of the nomogram was found to be greater than that of the eighth edition AJCC/UICC staging and the S-L grade (Figure 4).


Risk stratification based on the nomogram model
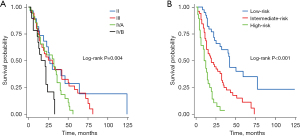
Using the scores calculated from the nomogram model, 118 patients were stratified into three risk subgroups via the quantile function (Figure 5). Specifically, 39 patients (33.1%) with scores <0.911 were classified as the low-risk group, 51 patients (43.2%) with scores between 0.911 and 1.775 as the intermediate-risk group, and 28 patients (23.7%) with scores >1.775 as the high-risk group; the 3-year survival rates for these three risk groups were 60.3%, 25.0%, and 3.6%, respectively, with a C-index of 0.678 (95% CI: 0.653–0.703). In comparison, when the cohort was stratified according to the eighth edition AJCC/UICC staging into stages II, III, IVA, and IVB, and the proportions of patients in each stage were 24.58%, 30.51%, 28.81%, and 16.10%, respectively. The 3-year survival rates for these groups were 49.6%, 23.3%, 27.9%, and 26.3%, respectively, with a C-index of 0.598 (95% CI: 0.563–0.632) (Figure 5). These results indicate that the nomogram model had a significantly better discriminative ability in terms of the C-index.
Discussion
Our study identified two key prognostic factors in elderly patients with ESCC undergoing radiotherapy: SLR and Lv as measured by 18F-FDG PET/CT. The univariate Cox regression analysis revealed several significant prognostic factors, but the multivariate analysis identified SLR >0.9 and Lv >4.7 cm as independent indicators of OS. Specifically, a higher SLR and greater tumor length were associated with poorer survival outcomes. The integration of these two parameters into a composite score, the S-L grade, demonstrated enhanced prognostic accuracy, surpassing the predictive performance of either parameter alone. Our developed nomogram, which incorporates the S-L grade and the cTNM stage, provided a robust predictive tool for OS, demonstrating strong agreement between predicted and observed survival probabilities and showing higher net benefit in the DCA.
A key limitation of treating elderly patients with ESCC is their inability to tolerate intensive therapies, such as chemotherapy or combined chemoradiotherapy, due to physiological limitations and multiple comorbidities. In our cohort, 44% of patients did not receive chemotherapy, which underscores the difficulty of escalating treatment for patients with poor prognoses. This limitation often leads to under-treatment of elderly patients, which ultimately affects outcomes. Thus, having a robust prognostic model, like the one developed in this study, may facilitate better-informed clinical decisions by accurately identifying which patients are at higher risk and potentially require closer monitoring or supportive care.
The findings of this study underscore the significant prognostic value of metabolic imaging markers in elderly patients with ESCC. The relationship between SLR and tumor length and their combined prognostic value was a central finding in this study. SLR >0.9 and Lv >4.7 cm were identified as independent predictors of poorer OS in multivariate Cox regression analysis. This aligns with previous studies that have emphasized the importance of metabolic activity and tumor burden in cancer prognosis (9,12,13). For instance, the study by Wong et al. demonstrated that a pretreatment SLR >1.1 was associated with poor outcomes after ipilimumab in advanced melanoma (9). Similar studies have noted that high metabolic activity as measured by PET/CT scans is correlated with worse outcomes in various cancers, including ESCC (14-16).
The prognostic significance Lv is also consistent with that reported in the literature. Tumor size has long been recognized as a critical factor in cancer staging and prognosis (17,18). In ESCC, larger tumors are associated with higher stages and worse outcomes due to the increased likelihood of invasion and metastasis (19-21). Our study confirmed that Lv >4.7 cm is a strong independent predictor of poor OS, reinforcing the relevance of tumor dimensions in the prognostic assessment of elderly patients with ESCC.
The combination of SLR and Lv into the S-L grade provided a more refined prognostic stratification. This composite score better captured the heterogeneity of the patient population, allowing for more precise risk assessment. The S-L grade’s superior predictive accuracy, as evidenced by AUC values in ROC analysis, suggests that it is a valuable tool for clinicians in decision-making processes. This approach aligns with the trend in oncology toward more personalized prognostic models that incorporate multiple factors to provide a comprehensive assessment of patient risk.
Our study offers a novel contribution by integrating SLR and Lv into a single composite score. This integration addresses the limitations of the TNM staging system, which does not adequately account for biological tumor behavior and patient-specific factors. By incorporating metabolic activity and tumor length, the S-L grade provides a more holistic view of the patient’s prognosis, especially in the older adult population for whom the traditional staging may fall short.
The nomogram developed in this study further enhances the clinical utility of our findings. By combining the S-L grade with cTNM stage, the nomogram offers a practical tool for predicting 1-, 2-, and 3-year OS. The high C-index values and well-calibrated plots indicate that the nomogram is both accurate and reliable. The DCA supported its clinical value, demonstrating that it provides greater net benefit across a range of threshold probabilities compared to using cTNM stage or S-L grade alone. This reinforces the importance of integrating multiple prognostic factors into a single predictive model to effectively guide clinical decisions.
In summary, our study provides robust evidence supporting the use of SLR and Lv as prognostic markers in elderly patients with ESCC. The integration of these markers into a composite score and nomogram enhances prognostic accuracy and offers a practical tool for personalized treatment planning. Recent advancements in PET-guided radiotherapy have provided a more targeted approach, which could potentially improve treatment outcomes in patients with esophageal cancer (22).
These findings align with and expand upon the existing literature, emphasizing the need for comprehensive prognostic models that incorporate both metabolic and anatomical information.
Our findings provide several important academic contributions. First, we have established the prognostic significance of the SLR and Lv in elderly patients with ESCC, demonstrating that these markers are reliable predictors of OS. Second, the study introduced the S-L grade, a novel composite score that combines SLR and Lv, which exhibited superior predictive accuracy compared to the individual parameters. This composite score enhances the ability to stratify patients based on their risk and guides personalized treatment planning. Third, the development of a nomogram that incorporates the S-L grade and the cTNM stage provides a practical and robust tool for predicting 1-, 2-, and 3-year OS. The high C-index and well-calibrated plots validate the accuracy and reliability of the nomogram, making it a valuable resource for clinicians. Finally, the DCA demonstrated the clinical utility of the nomogram, highlighting its potential to improve decision-making and optimize treatment outcomes in elderly patients with ESCC.
Although our study provides significant insights, there are several limitations that need to be addressed. To begin, the retrospective nature of the study might have introduced selection bias, and the findings need to be validated in prospective, multicenter cohorts to confirm their generalizability. Additionally, the study population was limited to elderly patients with ESCC undergoing radiotherapy, which may limit the applicability of the findings to other patient groups or treatment modalities. Another limitation was the lack of consideration for other potential prognostic factors, such as genetic and molecular markers, which could further enhance the prognostic model. Future studies should explore the integration of these markers with metabolic and anatomical parameters to develop even more comprehensive prognostic tools. Furthermore, the study did not evaluate the impact of different treatment regimens on the prognostic value of SLR and Lv. It would be beneficial to investigate how various therapeutic approaches, such as surgery, chemotherapy, and combination treatments, influence the prognostic significance of these markers. This would provide a more nuanced understanding of their utility across different clinical scenarios. Additionally, the follow-up period in our study was relatively short, with a median duration of 26.6 months. Longer follow-up is necessary to assess the long-term prognostic value of SLR and Lv and to determine their impact on OS and quality of life in elderly patients with ESCC. Finally, while the nomogram demonstrated good predictive performance, its clinical implementation requires validation in real-world settings. Future research should focus on integrating the nomogram into clinical practice and evaluating its impact on clinical decision-making and patient outcomes.
In conclusion, this study highlights the significant prognostic value of the SLR and Lv in elderly patients with ESCC undergoing radiotherapy. The development of the S-L grade and the associated nomogram represents a major advancement in personalized prognostic models for this patient population. However, further research is needed to validate these findings, explore additional prognostic factors, and assess the clinical utility of the nomogram in diverse settings. By addressing these limitations, future studies can build upon our work to improve the prognostic assessment and treatment planning for elderly patients with ESCC.
Conclusions
The study highlights the significant prognostic value of spleen uptake in 18F-FDG PET/CT, as measured by the SLR and Lv in elderly patients with esophageal cancer undergoing radiotherapy. The composite S-L grade serves as a more accurate predictor of survival compared to either parameter alone. The developed nomogram, incorporating S-L grade and cTNM stage, provides a reliable tool for personalized survival prediction and risk stratification, which may guide clinical decision-making and treatment optimization in this patient population.
Acknowledgments
Funding: This study was supported in part by grants from
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1698/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1698/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1698/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1698/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved the Institutional Review Board of Fujian Cancer Hospital (No. K2024-488-01) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Conway E, Wu H, Tian L. Overview of Risk Factors for Esophageal Squamous Cell Carcinoma in China. Cancers (Basel) 2023;15:5604. [Crossref] [PubMed]
- Morgan E, Soerjomataram I, Rumgay H, et al. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology 2022;163:649-658.e2. [Crossref] [PubMed]
- Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018;154:360-73. [Crossref] [PubMed]
- Liu M, Zhang P, Wang S, et al. Comparation between novel online models and the AJCC 8th TNM staging system in predicting cancer-specific and overall survival of small cell lung cancer. Front Endocrinol (Lausanne) 2023;14:1132915. [Crossref] [PubMed]
- Hu D, Liu Z, Chen S, et al. Assessment of the Novel, Practical, and Prognosis-Relevant TNM Staging System for Stage I-III Cutaneous Melanoma. Front Oncol 2022;12:738298. [Crossref] [PubMed]
- Li H, Li L, Liu Y, et al. Predictive value of CT and (18)F-FDG PET/CT features on spread through air space in lung adenocarcinoma. BMC Cancer 2024;24:434. [Crossref] [PubMed]
- Dong X, Wang R, Ying X, et al. Construction and validation of an (18)F-FDG-PET/CT-based prognostic model to predict progression-free survival in newly diagnosed multiple myeloma patients. Hematology 2024;29:2329029. [Crossref] [PubMed]
- Wang Y, Li Y, Jiang H, et al. Elevated splenic 18F-fluorodeoxyglucose positron emission tomography/computed tomography activity is associated with 5-year risk of recurrence in non-metastatic invasive ductal carcinoma of the breast. Br J Radiol 2024;97:237-48. [Crossref] [PubMed]
- Wong A, Callahan J, Keyaerts M, et al. (18)F-FDG PET/CT based spleen to liver ratio associates with clinical outcome to ipilimumab in patients with metastatic melanoma. Cancer Imaging 2020;20:36. [Crossref] [PubMed]
- Oliveira M, Lasnon C, Nganoa C, et al. Comprehensive analysis of the influence of G-CSF on the biodistribution of (18)F-FDG in lymphoma patients: insights for PET/CT scheduling. EJNMMI Res 2019;9:79. [Crossref] [PubMed]
- Prigent K, Lasnon C, Ezine E, et al. Assessing immune organs on (18)F-FDG PET/CT imaging for therapy monitoring of immune checkpoint inhibitors: inter-observer variability, prognostic value and evolution during the treatment course of melanoma patients. Eur J Nucl Med Mol Imaging 2021;48:2573-85. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. [Crossref] [PubMed]
- Shen DH, Chan HP, Tsai FR, et al. Prognostic Value of (18)F-FDG PET/CT Volume-Based Metabolic Parameters in Patients with Node-Negative Stage II Esophageal Squamous Cell Carcinoma. Metabolites 2021;12:7. [Crossref] [PubMed]
- Ha LN, Chau ND, Bieu BQ, et al. The Prognostic Value of Sequential (18) F-FDG PET/CT Metabolic Parameters in Outcomes of Upper-Third Esophageal Squamous Cell Carcinoma Patients Treated with Definitive Chemoradiotherapy. World J Nucl Med 2023;22:226-33. [Crossref] [PubMed]
- Wan J, Guo Y, Chen H, et al. Application and development of Deuterium Metabolic Imaging in tumor glucose metabolism: visualization of different metabolic pathways. Front Oncol 2023;13:1285209. [Crossref] [PubMed]
- Olaechea S, Gannavarapu BS, Alvarez C, et al. Primary Tumor Fluorine-18 Fluorodeoxydglucose ((18)F-FDG) Is Associated With Cancer-Associated Weight Loss in Non-Small Cell Lung Cancer (NSCLC) and Portends Worse Survival. Front Oncol 2022;12:900712. [Crossref] [PubMed]
- Yamaguchi O, Kaira K, Hashimoto K, et al. Tumor metabolic volume by (18)F-FDG-PET as a prognostic predictor of first-line pembrolizumab for NSCLC patients with PD-L1 ≥ 50. Sci Rep 2020;10:14990. [Crossref] [PubMed]
- Chiu CH, Chen WH, Wen YW, et al. Association between the thoroughness of the histopathological examination and survival in patients with esophageal squamous cell carcinoma who achieve pathological complete response after chemoradiotherapy. Dis Esophagus 2016;29:634-41. [Crossref] [PubMed]
- Zhang C, Xu F, Qiang Y, et al. Prognostic significance of tumor regression grade in esophageal squamous cell carcinoma after neoadjuvant chemoradiation. Front Surg 2022;9:1029575. [Crossref] [PubMed]
- Chen X, Yu Y, Wu H, et al. A Novel Model Combining Tumor Length, Tumor Thickness, TNM_Stage, Nutritional Index, and Inflammatory Index Might Be Superior to the 8th TNM Staging Criteria in Predicting the Prognosis of Esophageal Squamous Cell Carcinoma Patients Treated With Definitive Chemoradiotherapy. Front Oncol 2022;12:896788. [Crossref] [PubMed]
- Yang J, Liu Y, Li B, et al. Prognostic significance of tumor length in patients with esophageal cancer undergoing radical resection: A PRISMA-compliant meta-analysis. Medicine (Baltimore) 2019;98:e15029. [Crossref] [PubMed]
- Mehta S, Yang C, Jadvar H, et al. Advancements and future directions in positron emission tomography-guided radiotherapy: a narrative review. Chin Clin Oncol 2024;13:24. [Crossref] [PubMed]
(English Language Editor: J. Gray)


