
Efficacy and safety of a whole lung lavage program for artificial stone silicosis
Highlight box
Key findings
• In an observational prospective study for patients with artificial stone silicosis, we found whole lung lavage (WLL) is safe when performed in an expert center, and may confer some benefit in carefully selected patients.
What is known and what is new?
• The incidence of acute and accelerated artificial stone associated silicosis has increased in many countries. WLL is postulated to reduce silica burden and inflammatory cells.
• This study demonstrates a small improvement in the ICOERD CT scores in selected patients at six months post lavage. There was no difference in forced vital capacity % predicted, forced expiratory volume in 1 second % predicted, diffusing capacity for carbon monoxide % predicted, cardiopulmonary exercise testing, or forced oscillation technique measurements.
What is the implication, and what should change now?
• Treatments for silicosis are urgently needed. This study shows that WLL is safe and may confer some benefit in carefully selected patients in the short term. Larger studies are required to support or refute this and to further investigate the impact on future disease progression, and to determine who will benefit most.
Introduction
Background
Silicosis is a parenchymal lung disease caused by the inhalation of respirable crystalline silica (RCS). Its incidence has increased in Australia and other countries due to silica exposure caused by fabrication and installation of artificial (engineered) stone benchtops (1-5). People with artificial stone associated silicosis develop disease over a much shorter timeframe due to their significantly high intensity of exposure (5). Those with silicosis experience a significantly reduced quality of life, reduced exercise tolerance, inability to continue in the workforce, financial stress, and a negative impact on family and caregivers (6,7). Whilst prevention of silicosis through effective dust control measures are essential, disease-modifying treatments for silicosis are thus far lacking.
Rationale and knowledge gap
Determination of the most suitable treatments requires an understanding of the pathogenesis of silicosis. Once inhaled, RCS particles are engulfed by alveolar macrophages, which leads to cell death, autophagy, and release of intracellular silica. This in turn attracts further macrophages and up-regulation of a pro-inflammatory response (8,9). Pro-fibrotic pathways are subsequently activated and lead to fibrosis. Acute, high intensity silica exposure leads to activation of type II pneumocytes, which produce excessive amounts of proteinaceous material and surfactant protein in the alveoli (10). This process leads to ongoing inflammation and scar tissue formation of the surrounding lung tissue, leading to permanent distortion and fibrosis and a reduction in gas exchange surfaces (11).
In the early stages of disease, radiological imaging demonstrates micronodularity and ground glass opacities (GGO), which later coalesce into larger silicotic nodules leading to progressive massive fibrosis (PMF). Histopathology of early silicosis demonstrates small nodules containing histiocytes and fibroblasts with abundant silica, with a predominantly macrophage-containing cellular infiltrate and some with proteinaceous material present within the alveolar spaces (12). Bronchoalveolar lavage fluid analysis demonstrates silica particles as well as an increase in macrophage and lymphocyte populations, with a greater cell count in those with earlier stage disease (13). In the later stages of the disease, silicotic nodules coalesce, followed by extensive parenchymal destruction and hyalinised fibrosis (12). Based on these findings, it is plausible that whole lung lavage (WLL) might reduce the silica load and inflammatory response in those with early-stage disease, potentially reducing the risk of progression to fibrosis.
WLL involves the instillation and drainage of large volumes of fluid into the lungs under general anaesthesia. Its most common indication is pulmonary alveolar phospholipoproteinosis (PAP) (14), but there is also limited evidence for its use in other diseases, including silicosis. In silicosis, there is some evidence that WLL in natural stone-associated silicosis improves dyspnoea and lung function in the short-term (six months), though longer term evidence is limited (15). There is limited evidence in artificial stone silicosis. Chambers et al. reported the application of WLL in five cases of artificial stone silicosis, and found radiological improvement in most cases (16), however the evidence of effect on clinical and physiological outcomes remains unknown.
Objectives
Based on this literature, we aimed to determine the feasibility (probability of successful application), safety (potential for adverse events), and possible benefit of WLL in patients with artificial stone silicosis. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1050/rc).
Methods
Participants
In this prospective observational study, participants were recruited from the Alfred Occupational Respiratory Clinic, which manages a large proportion of silicosis patients in Victoria, Australia. Participants were offered WLL if they had simple silicosis [i.e., small (<1 cm) rounded micronodular GGO with the absence of PMF on high resolution computed tomography (HRCT)], and a decline in lung function and/or progression of symptoms despite cessation of exposure, as determined by a multi-disciplinary team meeting [including at least five respiratory physicians specialising in occupational lung disease, WLL proceduralist, chest radiologist, and an interstitial lung disease (ILD) nurse specialist]. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Alfred Health (No. 347/19), exposure history was collected through the Monash Silica Associated Disease Registry (Monash University No. 18476). Informed consent was prospectively obtained from all individual participants.
Procedure
WLL was performed under general anaesthesia in the Intensive Care Unit. Patients were intubated with a dual-lumen endotracheal tube. While one lung was ventilated, 15 to 25 L 0.9% normal saline was instilled passively (1 L at a time) into the other lung under gravity. Drainage was aided by Trendelenburg positioning and chest percussion. The following day the contralateral lung was lavaged. An 50 mL aliquot was sampled from the first and last bag, and the number of pigment laden macrophages per high-powered field was quantified visually.
Outcome assessments
Occupational and exposure histories were collected using a previously described exposure assessment instrument (17). All patients underwent lung function tests using standardised criteria (18) [including forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and diffusion capacity for carbon monoxide (DLCO)], assessment of small airway dysfunction using forced oscillation technique (FOT) (19) [respiratory resistance (Rrs 5–19% predicted), area of reactance (AX5% predicted), respiratory reactance (Xrs5% predicted)], cardiopulmonary exercise tests (CPET) [including assessment of maximal oxygen consumption (VO2 max) and ventilatory response (VR), HRCT], and X-ray velocimetry (XV) [including Specific Ventilation (defined as the change in volume of a lung region since the start of inspiration, divided by the volume of the same region at end-expiration) (20) and low ventilation region (defined as the proportion of lung with specific ventilation values of less than 0.1) (21)]. For this study, HRCT scans were scored using a modified International Classification of High Resolution Computed Tomography for Occupational and Environmental Respiratory Diseases (ICOERD) criteria (22) by two independent radiologists blinded to clinical data (M.S., J.P.). ICOERD uses a 4-point grade scale [0–3] to quantify the extent (profusion) of the following parenchymal abnormalities: well-defined rounded opacities (RO), irregular and/or linear opacities (IR), emphysema (EM), GGO and honeycombing (HC). A grade is assigned for each of the upper, middle, and lower zones of both lungs. For the purpose of this study, the domain “round opacities” was divided into ROG (round opacities, ground glass density) and ROS (round opacities, soft tissue density) given the presumption that GGO nodularity may reflect reversible disease and soft tissue density nodularity is presumably irreversible. The summed score for this study includes the domains: ROG, ROS, regular and linear opacities, and GGO. Where there was a >5-point difference in scores, radiologists discussed the case to achieve a consensus score. Symptom change was measured subjectively and included breathlessness, cough, and exercise tolerance. Lung function tests, CPET, FOT, HRCT, and XV were measured one week prior and six months following the procedure. Lung function tests were collected 12 months prior to and 12 months following the procedure.
Statistical analyses
Sample size was determined using a pre-post design (23) based on the expected change in lung function over time (2,24). Continuous variables (e.g., FEV1% predicted, FVC% predicted, DLCO% predicted) were analysed using a paired t-test (dependent t-test) where data were parametric or a Wilcoxon rank test where data were non-parametric. Categorical data were analysed using McNemar’s test. The mean average change scores are presented. To determine if WLL changed the rate of progression in lung function, the annualised rate of change was determined based on 12 months of data preceding WLL and compared to the annualised rate of change 12 months following WLL.
Results
Between June 2021 and November 2022, eight patients underwent WLL; 7% of our silicosis cohort (5). All patients were male, mean age of 37 years old, with an average of 12 years exposure to dry cutting of artificial stone, and an average of 22 months since last exposure (Table 1). All participants had a predominance of GGO on computed tomography (CT); three had additional nodular coalescence and three had fibrotic bands that did not meet criteria for PMF.
Table 1
| Variable | Mean (SD) or number (%) |
|---|---|
| Age (years; at time of WLL procedure) | 37.75 (2.92) |
| Months since diagnosis | 39.13 (9.66) |
| Duration in stone benchtop industry (years) | 12.38 (5.66) |
| Duration since last exposure (months) | 22 (5.63) |
| Ever smoker | 5 (63%) |
| FEV1 at baseline, L | 3.51 (0.84) |
| FEV1% predicted baseline | 88.5 (15.27) |
| FVC baseline, L | 4.62 (0.92) |
| FVC% predicted baseline | 94.88 (10.76) |
| DLCO absolute baseline | 27.83 (6.147) |
| DLCO% predicted baseline | 92.38 (14.46) |
| VO2 max (%pred) | 71.25 (14.84) |
DLCO, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SD, standard deviation; VO2, maximal oxygen consumption; WLL, whole lung lavage.
Participants received a mean of 20.8 L lavage to the left lung [standard deviation (SD) 3.11] with a residual volume of 1.08 L (SD 0.26) and a mean of 19.2 L lavage to the right lung (SD 3.56), residual volume 0.23 L (SD 0.46). The median duration of intubation was two days (IQR 0) and the median total length of stay was three days [interquartile range (IQR) 1]. There were no significant adverse safety events. One patient had a fever following the first lung lavage, and so the lavage of the contralateral lung was delayed for two weeks. This and one other patient had a course of oral antibiotics for cough following the procedure.
Pigment-laden macrophages were quantified at the first and last aliquot. There was a reduction in pigment-laden macrophages following lavage (1st aliquot: >50 pigment-laden macrophages per high-powered field, last aliquot mean 14, SD 6; Figure 1).

People with silicosis tend to have a progressive decline in lung function over time. To determine whether WLL attenuates lung function decline, we measured FEV1, FVC, and DLCO% predicted in the 12 months preceding and 12 months post-WLL. There was no significant difference in FVC% predicted [annualised rate of change mean difference (MD) 1.81; 95% CI: −1.53 to 5.15; P=0.27], FEV1% predicted (MD −1.13; 95% CI: −5.08 to 2.83; P=0.55), or DLCO% predicted (MD −2.62; 95% CI: −10.04 to 4.80; P=0.46) (Table 2; Figures S1-S3).
Table 2
| Parameter | Pre-WLL [mean (SD)] | Post-WLL [mean (SD)] | Difference | |
|---|---|---|---|---|
| MD (95% CI) | P value | |||
| FEV1% change over 12 months | −0.13 (3.94) | −1.25 (3.41) | −1.13 (−5.08, 2.83) | 0.55 |
| FVC% change over 12 months | −2.19 (3.20) | −0.38 (3.03) | 1.81 (−1.53, 5.15) | 0.27 |
| DLCO% change over 12 months | −0.62 (4.98) | 2 (8.43) | −2.62 (−10.04, 4.80) | 0.46 |
| VO2 max (% pred) | 71 (15) | 70 (17) | −1 (−20, 18) | 0.91 |
| CPET load (% pred) | 82 (20) | 81 (24) | −1 (−27, 25) | 0.99 |
| VR | 26 (18) | 34 (25) | 8 (−17, 33) | 0.51 |
| VE/CO2 nadir | 27 (3) | 26 (3) | −1 (−4, 2) | 0.55 |
| Rrs 5–19 %pred | 479 (1,012) | 151 (169) | −328 (−1,172, 516) | 0.41 |
| AX5 %pred | 239 (200) | 225 (225) | −14 (−261, 224) | 0.90 |
| Xrs5 %pred | 99 (41) | 86 (29) | −13 (−54, 28) | 0.51 |
| ICOERD ROG score | 10 (3.24) | 9 (2.99) | −0.88 (−2.32, 0.57) | 0.20 |
| ICOERD ROS score | 7 (2.73) | 6 (2.73) | −1.125 (−2.09, −0.16) | 0.03 |
| ICOERD IR score | 4 (3.07) | 4 (2.30) | −0.35 (−0.78, 0.08) | 0.10 |
| ICOERD ground glass score | 4 (4.17) | 3 (4.70) | −1.13 (−3.29, 1.04) | 0.26 |
| ICOERD total summed score | 24 (6.98) | 21 (8.04) | −2.39 (−5.29, 0.16) | 0.09 |
| XV low ventilation region | 35 (19.59) | 30 (14.02) | −4.88 (−8.05, 17.81) | 0.41 |
WLL, whole lung lavage; SD, standard deviation; MD, mean difference; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; DLCO, diffusing capacity for carbon monoxide; VO2, maximal oxygen consumption; CPET, cardiopulmonary exercise test; VR, ventilatory response; VE/CO2, ventilatory equivalent of carbon dioxide; Rrs, respiratory resistance; AX5, area of reactance; Xrs5, respiratory reactance; ICOERD, International Classification of High Resolution Computed Tomography for Occupational and Environmental Respiratory Diseases; ROG, round opacities ground glass; ROS, round opacities soft tissue; IR, regular and linear reticulation; XV, X-ray velocimetry.
We postulated that the greatest degree of difference in lung function would be at the small airway level, therefore we utilised FOT measurements. There was a trend towards a reduction in peripheral airway dysfunction measured with FOT at baseline and six months post-WLL, but the overall effect was not significant (Table 2). We aimed to assess the effect of WLL on exercise capacity using CPET. Four of seven participants had a stabilisation or improvement in VO2 max, but the degree of change was small (Table 2; Figure S4). One patient did not complete their post-WLL CPET, this was omitted from the analysis, otherwise there was no missing data.
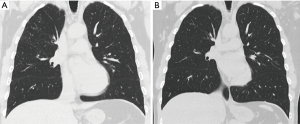
We utilized several measurements for radiological change pre and post-WLL. The ICOERD scoring system, although devised for diagnosis rather than assessment of progression of silicosis, aims to quantify the profusion of GGO and development of fibrosis. Five of eight participants had a reduction in the summed ICOERD scores pre- and post-WLL, although the overall effect was not significant (MD −2.39; 95% CI: −5.29, 0.16; P=0.09; Figures 2,3, Table 2). There was no difference in the change in ICOERD scores between those who had nodular coalescence or fibrotic bands or absence of these features (Figure S5). We also utilized XV low ventilation region (LVR), a novel artificial intelligence-based imaging technique that aims to assess ventilation, particularly at the small airway level. Six participants demonstrated improvement in XV LVR with a median change of 32% (range, 8% to 60%) (Figure 4A). With XV, changes in regional ventilation (Figure 4B), at an individual level, were found to geospatially diverse, with the largest improvements in regional ventilation found predominantly in the upper and dorsal zones, in the absence of gross structural lung changes (Figure 4B; Table S1).


Subjectively, all patients reported improved symptoms, including improvement in breathlessness (8/8), an increase in exercise tolerance (3/8) and a reduction in cough (2/8). The most common adverse event was throat discomfort, which occurred in all patients.
Discussion
Key findings
This case series assessed the safety and potential benefit of WLL in progressive artificial stone-associated silicosis. This study shows that whilst WLL may be safe when performed at an expert centre, its benefit may only be limited to a select group of patients, and the degree of short-term improvement is limited.
Explanations of findings
The incidence and prevalence of silicosis is increasing in Australia, particularly accelerated silicosis with a younger age onset in relation to the use of artificial stone (5). At present, there are no treatments for silicosis. WLL is postulated to reduce silica burden and inflammatory cell infiltrates relating to silicosis. Whilst there have been previous studies examining the use of WLL in silicosis and pneumoconiosis relating to miners and other occupations (15,25-27), there is limited information on the use of WLL in artificial-stone associated silicosis. In addition, previous studies have demonstrated a significant risk of complications peri-procedure [including pneumothorax, pulmonary haemorrhage, prolonged intubation, requirement for extracorporeal membrane oxygenation (ECMO)], confounded by the fact that some of these patients presented with hypoxemic respiratory failure prior to the procedure (25-28). This case series demonstrates this procedure is safe and well tolerated when performed in a centre with experience in the selection of such patients and experience in performing this procedure.
Strengths and limitations
Patients with silicosis present with a spectrum of disease severity. We selected patients with a high profusion of ground glass nodules, with limited or no larger silicotic nodules or fibrosis, as we postulated that these patients are most likely to benefit. However, we also selected patients who demonstrated some degree of clinical, physiologic, or radiologic progression following cessation of exposure given that some patients will stabilise post exposure cessation. Therefore, some of our patients demonstrated nodular coalescence and fibrotic bands at baseline, and these patients may be less likely to benefit from WLL. Although we did not see any great difference between those with and without nodular coalescence or fibrotic bands, it may be that we would have seen a greater degree of improvement prior to progression overall. Our patient cohort differed slightly to that of Chambers et al., who did not select patients with fibrotic bands at baseline. It is possible this explains why our radiological improvement was not to the same degree as previously described by Chambers et al., who reported an almost complete resolution of semi-solid centrilobular nodules in 4/5 patients (16). Further research is required to carefully select patients who are most likely to benefit.
We utilised a range of clinical endpoints to assess outcomes. We assessed CT imaging using a modified ICOERD scoring system, though it is recognised that the ICOERD scoring system has been designed to diagnose and classify disease severity, and each domain has not been validated to detect differences in prognosis or treatment response. We did, however, find that there was the greatest degree of change in ground glass and soft tissue opacities following WLL, which may reflect improvement in areas of alveolar proteinosis, and this and other domains may be useful to select patients most likely to benefit and assess treatment response in the future.
Novel imaging methods including assessment of regional ventilation may be a better marker to assess improvement than conventional tests, such as spirometry and CT scanning (20,21). We found in this series that XV has the potential to quantify and visualise the regional ventilation changes associated with WLL in silicosis, with most participants found to have an improvement in LVR. The ability of XV to measure functional lung changes at a regional level is promising, especially in early silicosis where structural changes are lagging; however, the method warrants further investigation for more conclusive findings. Given there was no control group in this study, further studies are required to assess the changes of XV in the natural course of silicosis disease are required to fully interpret the meaning of these changes post-WLL.
We postulated that we might have seen an improvement in DLCO due to improved gas transfer from washing out micronodules present in the alveolar space (supported by the radiological findings), however, as we excluded those with fibrosis, we did not expect to see a significant change in volume loss. In addition, lung function tests may not be the most accurate marker of impairment in patients with silicosis given that many patients exhibit normal lung function at diagnosis despite marked radiological disease, and that there appears to be a large variation in lung function over time (5). In addition, we utilised alternative measures of physiologic impairment, including FOT and CPET. FOT demonstrated a non-significant reduction in peripheral airway dysfunction, and may be useful in measuring treatment response in a larger cohort of patients. We assessed some outcomes out to 12 months however longer follow up is required to determine if there are further effects on measured parameters and if WLL reduces progression of disease. It is important to note that given there was no control group in this study, the conclusions that can be made regarding the efficacy of WLL is limited. Furthermore, the decline in lung function in patients with silicosis may not be linear (2), therefore any effect of WLL described in this study should be interpreted with caution.
Whilst we demonstrated there was a reduction in pigment-laden macrophages at the end of the procedure compared to its commencement, we did not formally assess silica particles, as has subsequently been described in the literature (29), nor assess any changes in the immunological profile pre- and post-WLL. This would be useful to understand the treatment effects on a cellular level, and may aid in determining who are most likely to benefit, and is the subject of further study (30).
Implications and actions needed
Much of the current research in silicosis still centres around diagnosis, and to some extent measures of prognosis. Given that an increasing number of patients with silicosis are developing rapidly progressive disease, particularly related to exposure to artificial stone, treatments for silicosis are urgently needed. This study shows that whilst WLL may be safe and some trends towards improvement were observed, we were unable to confirm clinical benefit beyond 12 months. Larger scale studies with control groups are required to support or refute this and to further investigate the impact on future disease progression, and to determine who will benefit most. As future studies examine WLL and other possible treatments for silicosis, it will be important to determine which clinical trial end-points are the most useful in detecting change. There is a need for a more objective and measurable assessment of impact on symptoms, and for longer term outcome measures to determine whether WLL or any other treatments result in a reduction to progression of irreversible fibrosis.
Conclusions
WLL for artificial stone silicosis is safe in an expert centre and there may be limited benefit in selected patients. Further research is required to select those who will derive the most benefit.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1050/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1050/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1050/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1050/coif). Janu Pirakalathanan is a current employee of I-Med Radiology. Piraveen Pirakalathanan and Nina Eikelis are employees of 4DMedical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Alfred Health (No. 347/19), exposure history was collected through the Monash Silica Associated Disease Registry (Monash University No. 18476). Informed consent was prospectively obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kramer MR, Blanc PD, Fireman E, et al. Artificial stone silicosis [corrected]: disease resurgence among artificial stone workers. Chest 2012;142:419-24. Erratum in: Chest 2012;142:1080.
- León-Jiménez A, Hidalgo-Molina A, Conde-Sánchez MÁ, et al. Artificial Stone Silicosis: Rapid Progression Following Exposure Cessation. Chest 2020;158:1060-8. [Crossref] [PubMed]
- Shi P, Xing X, Xi S, et al. Trends in global, regional and national incidence of pneumoconiosis caused by different aetiologies: an analysis from the Global Burden of Disease Study 2017. Occup Environ Med 2020;77:407-14. [Crossref] [PubMed]
- Nandi SS, Dhatrak SV, Sarkar K. Silicosis, progressive massive fibrosis and silico-tuberculosis among workers with occupational exposure to silica dusts in sandstone mines of Rajasthan state: An urgent need for initiating national silicosis control programme in India. J Family Med Prim Care 2021;10:686-91. [Crossref] [PubMed]
- Hoy RF, Dimitriadis C, Abramson M, et al. Prevalence and risk factors for silicosis among a large cohort of stone benchtop industry workers. Occup Environ Med 2023;80:439-46. [Crossref] [PubMed]
- Hoy RF, Chambers DC. Silica-related diseases in the modern world. Allergy 2020;75:2805-17. [Crossref] [PubMed]
- The National Dust Disease Taskforce. Final Report to Minister for Health and Aged Care. Department of Health and Aged Care. 2021. https://www.health.gov.au/resources/publications/national-dust-disease-taskforce-final-report?language=en
- Hernández-Gea V, Ghiassi-Nejad Z, Rozenfeld R, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 2012;142:938-46. [Crossref] [PubMed]
- Song L, Weng D, Dai W, et al. Th17 can regulate silica-induced lung inflammation through an IL-1β-dependent mechanism. J Cell Mol Med 2014;18:1773-84. [Crossref] [PubMed]
- Greenberg MI, Waksman J, Curtis J. Silicosis: a review. Dis Mon 2007;53:394-416. [Crossref] [PubMed]
- Fujimura N. Pathology and pathophysiology of pneumoconiosis. Curr Opin Pulm Med 2000;6:140-4. [Crossref] [PubMed]
- Levin K, McLean C, Hoy R. Artificial stone-associated silicosis: clinical-pathological-radiological correlates of disease. Respirol Case Rep 2019;7:e00470. [Crossref] [PubMed]
- Moreira VB, Ferreira AS, Soares PJ, et al. Relevância do lavado broncoalveolar na quantificação de partículas inaladas nas diversas formas de silicose. Revista Portuguesa de Pneumologia 2005;11:457-75. [Crossref] [PubMed]
- ROSEN SH. CASTLEMAN B, LIEBOW AA. Pulmonary alveolar proteinosis. N Engl J Med 1958;258:1123-42. [Crossref] [PubMed]
- Zhang YM, Zhang HT, Wang CY, et al. Long-term therapeutic effects of whole lung lavage in the management of silicosis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2012;30:690-3.
- Chambers DC, Apte SH, Deller D, et al. Radiological outcomes of whole lung lavage for artificial stone-associated silicosis. Respirology 2021;26:501-3. [Crossref] [PubMed]
- Hoy RF, Glass DC, Dimitriadis C, et al. Identification of early-stage silicosis through health screening of stone benchtop industry workers in Victoria, Australia. Occup Environ Med 2021;78:296-302. [Crossref] [PubMed]
- Culver BH, Graham BL, Coates AL, et al. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med 2017;196:1463-72. [Crossref] [PubMed]
- Oostveen E, MacLeod D, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J 2003;22:1026-41. [Crossref] [PubMed]
- Kirkness JP, Dusting J, Eikelis N, et al. Association of x-ray velocimetry (XV) ventilation analysis compared to spirometry. Front Med Technol 2023;5:1148310. [Crossref] [PubMed]
- Karmali D, Sowho M, Bose S, et al. Functional imaging for assessing regional lung ventilation in preclinical and clinical research. Front Med (Lausanne) 2023;10:1160292. [Crossref] [PubMed]
- Tamura T, Suganuma N, Hering KG, et al. Relationships (I) of International Classification of High-resolution Computed Tomography for Occupational and Environmental Respiratory Diseases with the ILO International Classification of Radiographs of Pneumoconioses for parenchymal abnormalities. Ind Health 2015;53:260-70. [Crossref] [PubMed]
- Rosner B. Fundamentals of biostatistics. Seventh edition. Boston: Brooks/Cole, Cengage Learning; 2011.
- Quan H, Wu W, Yang G, et al. Risk Factors of Silicosis Progression: A Retrospective Cohort Study in China. Front Med (Lausanne) 2022;9:832052. [Crossref] [PubMed]
- Lyons JJ, Sime PJ, Ward D, et al. Case presentation: a breathless builder. Breathe 2007;3:386-90.
- Moreira JP, Ferraz S, Freitas C, et al. Whole-lung lavage for severe pulmonary alveolar proteinosis assisted by veno-venous extracorporeal membrane oxygenation: a case report. Can J Respir Ther 2018;55:9-12. [Crossref] [PubMed]
- Huang JH, Chen G, Ma GX. Observation on efficacy of large volume whole lung lavage in treatment of pneumoconiosis. Chinese Journal of Industrial Hygiene and Occupational Diseases 2008;26:428-30.
- Xuan YQ, Shang MW, Zhou JY. Clinical research on whole lung lavage for treatment of pneumoconiosis in early stage. Chinese Journal of Industrial Hygiene and Occupational Diseases 2009;27:623-5.
- Apte SH, Tan ME, Lutzky VP, et al. Alveolar crystal burden in stone workers with artificial stone silicosis. Respirology 2022;27:437-46. [Crossref] [PubMed]
- Chambers DC. The SHIELD study. NCT05546944 clinicaltrials.gov. 2022. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05546944


