
Experimental study of the effects of pirfenidone and nintedanib on joint inflammation and pulmonary fibrosis in a rheumatoid arthritis-associated interstitial lung disease mouse model
Highlight box
Key findings
• Pirfenidone and nintedanib can alleviate joint symptoms and pulmonary fibrosis through transforming growth factor-β (TGF-β) signaling pathway, janus kinase 2/signal transducer and activator of transcription 3 (Jak2/Stat3) signaling pathway, and polarization of macrophages to the M2 phenotype.
What is known and what is new?
• Pirfenidone and nintedanib are drugs for interstitial lung disease, especially idiopathic pulmonary fibrosis.
• This manuscript adds that pirfenidone and nintedanib could relieve pulmonary fibrosis and joint inflammation in rheumatoid arthritis-associated interstitial lung disease (RA-ILD) mouse through TGF-β, Jak2/Stat3 signaling pathway, and polarization of macrophages to the M2 phenotype.
What is the implication, and what should change now?
• This research implies that pirfenidone and nintedanib are potential drugs for treatment of RA-ILD, adding the potential indication of these two drugs. More evidence of clinical use of the drugs are needed for RA-ILD patients, further study of the mechanisms involved should be performed.
Introduction
Background
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease with an unknown etiology (1), which affecting 0.5–1.0% of the global population. Joint pain, deformity and chronic synovial inflammation [characterized by fibroblast-like synovial (FLS)] are the characters of RA. Other changes of joint include cartilage damage, joint swelling and bone malformation. RA is a multi-system disease. It is believed that lung involvement is the most common extra-articular manifestation affecting more than 50% of RA patients (approximately 14% clinically significant, 41% asymptomatic) (2), and approximately 20% of RA patients can develop RA-associated interstitial lung disease (RA-ILD) (3). The mortality of RA-ILD patients is approximately 3 times greater than that of RA-only patients (4) with median survival time about 3–10 years upon diagnosis (5). Most standard treatments for RA, such as glucocorticoids and disease-modifying antirheumatic drugs, exhibit pulmonary toxicity and may accelerate the development of interstitial lung disease (ILD) (6). Since the pathological changes in lung of RA-ILD patient are fibrosis and scarring caused by chronic inflammation of the interstitial space, controlling of fibrosis is important in the therapy of RA-ILD (7). Usual interstitial pneumonia (UIP) is the most common histological pattern of RA-ILD (8), which is considered the most analogous to idiopathic pulmonary fibrosis (IPF). RA-ILD and IPF are associated with pulmonary fibrosis and acute exacerbations. Up-regulation of some genetic markers [interleukin-8 (IL-8), matrix metalloproteinase-2, 7, 9 and FMS-related tyrosine kinase 3 ligand] and mutations [telomerase reverse transcriptase, regulator of telomere elongation helicase, poly (A)-specific ribonuclease and surfactant associated protein C] are observed in patients with RA-ILD and IPF (9,10). These similarities support the rationale for the implementing of medication for IPF in patients with RA-ILD (11). The drugs tested mostly for RA-ILD now is the first-line medicines for IPF, pirfenidone and nintedanib. Pirfenidone is an antifibrotic agent, modulating fibrogenic growth factors (FGFs), thereby attenuating fibroblast proliferation, myofibroblast differentiation, collagen and fibronectin synthesis, and in the end deposition of extracellular matrix (ECM). This effect is mediated by suppression of transforming growth factor-β1 (TGF-β1) and other growth factors (12). Nintedanib is an inhibitor of fibroblast growth factor receptor (FGFR)-1 and vascular endothelial growth factor (VEGFR)-2, both of which are pro-angiogenic receptor tyrosine kinases. Bioinformatic study finds that mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K)/Akt, Janus kinase-signal transducer and activator of transcription (Jak-Stat), TGF-β, VEGF and WNT/β-catenin signalings are the main molecular pathways modulated by nintedanib (13). Existing studies show that antifibrotic effects of these two drugs are related to Jak/Stat pathways (14-16). Jak/Stat pathway is activated by a broad number of profibrotic/pro-inflammatory cytokines, like IL-6, IL-11 and IL-13, among others, which are increased in ILDs (17). The polarization of macrophages also plays an important role in the development of RA-ILD. M0 macrophages polarize toward the M1 phenotype to release factors such as tumor necrosis factor alpha (TNF-α), IL-1β, 6 and 12 to promote inflammation. In contrast, M0 macrophages polarize toward the M2 phenotype in the presence of IL-4, IL-10, IL-13, and TGF-β to promote cell growth and tissue repair. M2a macrophages is a subtype of M2-type macrophages, which can be activated by IL-4 and IL-13 and promote cell growth and tissue repair through obviously increased expression of IL-10 and TGF-β (18-21). M2 macrophages are positively correlated with pulmonary fibrosis through the secretion of IL-10, TGF-β, arginase-1 (ARG-1), mannose receptor (CD206) and scavenger receptor (CD163) (22,23). Currently, relevant researches have shown that M2 macrophage polarization can be promoted through the Jak2/Stat3 signaling pathway (24,25).
In summary, the information mentioned above seems to reveal the relationships between the interactive factors, pirfenidone and nintedanib might affect fibrosis via TGF-β-Smad-Jak/Stat-ECM molecules pathway, in which M2 macrophages modulate TGF-β expression in a by-pass way. The exact mechanism of the antifibrotic effect of pirfenidone and nintedanib in RA-ILD are still unclear. So, we designed and performed this study to further explore the mechanisms and molecular signal pathway involved.
Rationale and knowledge gap
As is mentioned above that Jak/Stat and TGF-β signalings are related to antifibrotic effects of pirfenidone and nintedanib. Meanwhile, Jak/Stat signaling pathway and macrophage polarization also play important roles in the pathogenesis of RA-ILD and FLS (1,17,26-28). Jak/Stat signaling pathway, predominantly Jak2/Stat3, is upregulated in ILD patients and animal models (17). Jak2 is not only a downstream target of TGF-β1 in fibroblasts, but also performs positive feedback to amplify TGF-β1 signaling by stimulating more TGF-β1 expression (17). M2 macrophage polarization is highly expressed in ILD, too, which is promoted by TGF-β (29). In pathological procedure of FLS, Jak2 expression is significantly increased, which activates downstream Stat3 to increase production of inflammatory cytokines and chemokines (26,30). Since pirfenidone and nintedanib were approved for the treatment of IPF and progressive fibrotic ILD, including patients with RA-ILD (31-33), The effects and mechanisms are needed to be verified by experimental studies and real world data of effectiveness and safety is required to use pirfenidone and nintedanib properly for patients with RA-ILD. It is reasonable to speculate that pirfenidone and nintedanib could attenuate pulmonary fibrosis and RA-related synovitis via TGF-β1-Jak2-macrophage polarization axis.
Objective
The aim of this study was to explore the ameliorating effects of pirfenidone and nintedanib on the pulmonary fibrosis in mice with collagen-induced RA-ILD. The effect on symptoms of RA-related arthritis were also explored. We present this article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-882/rc).
Methods
Twenty-five 8-week-old male specific pathogen-free DBA/1 mice weighing approximately 18–22 g were purchased from Beijing Charles River Co., Ltd. [Animal License No. SCXK (Beijing) 2021-0006]. Experiments were performed under a project license (No. IAC-B2019011-P-01) granted by ethics board of Greentech committee, in compliance with institutional guidelines for the care and use of animals. The mice were housed under a 12 h light/dark cycle and at a constant temperature of 22±2 ℃ with food and water available ad libitum. The mice were allowed to acclimate to these conditions for 7 days prior to initiation of the experiment. The mouse macrophage line RAW264.7 (Cat No. CL-0190) and mouse primary FLS (Cat No.CP-M323) cells were purchased from Wuhan Procell Biotechnology Co., Ltd. (Wuhan, China). Pirfenidone was nintedanib were purchased from MedChemExpress (Cat No. HY-B0673; Shanghai, China) and nintedanib was purchased from Adooq (Cat No. A10137; Nanjing, China).
Treatments of animals
Mice were randomly divided into a normal control group (control group, n=3) and a model group (n=22). Mice in the model group were injected with emulsified bovine type II collagen (bCII, 2 mg/mL) at the tail root to induce the RA-ILD model. On day 0 (0 weeks), these mice were injected with 0.1 mL of complete Freund’s adjuvant (CFA) emulsified bCII, and on day 21 (3 weeks), with 0.1 mL of incomplete Freund’s adjuvant (IFA) emulsified bCII. Two mice died during injection, 3 mice showed no joint changes, and 17 mice showed joint swelling. The 17 mice with joint swelling were divided into 3 groups based on the arthritis scores, with 5 mice in the RA-ILD group (0.1 mL CMCNa), 6 mice in the pirfenidone group (20 mg/kg), and 6 mice in the nintedanib group (60 mg/kg). The mice in the control group received 0.1 mL CMCNa. Then each group of mice was administered by gavage every other day for 5 weeks, they were anesthetized and sacrificed to collect bronchoalveolar lavage fluid (BALF), lung tissue, right knee joints (see gross flow chart in Figure 1). One mouse in the RA-ILD group died during gavage, and another mouse did not show interstitial changes in the lungs and were not included in the analysis. Between collagen induction and sacrifice, the body weights and arthritis scores of all the mice were measured (34). The arthritis scores are shown in Table 1. For a single animal, the maximum score for a single limb was 4 points, and the total score for the four limbs was 16 points.
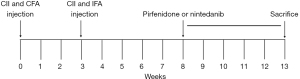
Table 1
| Score | Single limb |
|---|---|
| 0 | No redness or swelling |
| 1 | The small joint of the toe is red but not swollen |
| 2 | The toe joint is slightly red and swollen |
| 3 | The joint below the ankle joint is swollen |
| 4 | The ankle joint and feet are swollen with slight movement disorders |
Cells and treatments
RAW264.7 cells and primary mouse FLS cells were cultured in a cell culture incubator at 37 ℃ and 5% CO2, and experiments were conducted when the cells were 80–90% confluent. RAW264.7 cells were divided into 6 groups: the RAW264.7 control (normal culture), RAW + IL-4/IL-13 (60 ng/mL IL-4/IL-13; 12 h), RAW + IL-4/IL-13 + pirfenidone (0.5 or 1 mmol/L pirfenidone + 60 ng/mL IL-4/IL-13; 12 h), and RAW + IL-4/IL-13 + nintedanib (0.1 or 0.5 µmol/L nintedanib + 60 ng/mL IL-4/IL-13; 12 h) groups. FLS cells were divided into 6 groups: the FLS control (normal culture), FLS + TGF-β1 (10 µg/L TGF-β1), pirfenidone intervention (after 4 h of 10 µg/L TGF-β1 treatment, cells were treated with 0.5 and 1 mmol/L pirfenidone for 48 h), and nintedanib intervention (after 4 h of treatment with 10 µg/L TGF-β1, cells were treated with 0.5 and 1 µmol/L nintedanib for 48 h) groups.
Flow cytometric assessment
Phosphate buffer solution (PBS) was used to lavage the alveoli 3 times, the BALF was harvested, and the proportions of M1 macrophages and M2 macrophages were determined using flow cytometry. Antibodies for flow cytometric assessment are listed in Table S1.
Pathological assessment and immunofluorescence analysis of lung and joints
Left lung tissue was fixed in 4% paraformaldehyde, embedded in paraffin, dewaxed, dehydrated, cleared, and mounted for hematoxylin-eosin (H&E) staining and Masson staining and immunofluorescence. Pulmonary inflammation was evaluated by the Szapiel score (35) after H&E staining (Table 2), and the pulmonary fibrosis score was calculated based on Masson staining (36). Right knee joints muscle tissue were removed, fixed in 0.4% paraformaldehyde for 24 hours, decalcified with decalcification solution, and decalcified for pathologic HE staining and immunofluorescence. H&E staining analysis of the right knee joint and immunofluorescence analysis of p-Jak2 and p-Stat3 protein expression in the left lung and right knee joint. Three images were randomly selected using CaseViewer software, and the fluorescence intensity was quantified using ImageJ-win64.
Table 2
| Degree of alveolitis | Histopathologic features |
|---|---|
| 0 | No alveolitis |
| 1 | Thickening of the alveolar septum by a mononuclear cell infiltrate, with involvement limited to focal, pleural-based lesions occupying less than 20% of the lung and with good preservation of the alveolar architecture |
| 2 | A more widespread alveolitis involving 20–50% of the lung, although still predominantly pleural based |
| 3 | A diffuse alveolitis involving >50% of the lung, with occasional consolidation of air spaces by the intra-alveolar mononuclear cells and some hemorrhagic areas within the interstitium and/or alveolus |
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the right lung tissue of mice. The purity of the RNA was determined with a ultraviolet (UV) spectrophotometer. Next, complementary DNA (cDNA) was synthesized according to the instructions of the reverse transcription kit. Then, synergetic binding reagent (SYBR) fluorescent dye was added to the PCR mixture. The PCR conditions were as follows: 95 ℃ for 30 s and 40 cycles of 95 ℃ for 10 s and 60 ℃ for 30 s. Then, the PCR products were monitored in real time. The mRNA levels of Jak2, Stat3 and collagen-IV (Col-IV) was calculated by the 2−ΔΔCt method, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serving as an internal control. The sequences of the primers used were as follows: GAPDH, F: 5'-AGGTCGGTGTGAACGGATTTG-3'; R: 3'-ACAACGGTAGTTGCTGGGG-5'; Jak2, F: 5'-AAGATGCTTTCTGGGTTGG-3'; R: 3'-GACGAGGGAGAATCTGTTACA-5'; Stat3, F: 5'-ACCTCCAGGACGACTTTGAT-3'; R: 3'-ACCTCATGCACGTTTCTGT-5'; Collagen IV, F: 5'-ATGCCCTTTCTCTTCTGCAA-3'; R: 3'-ACACCTAGCCGATAAGGAAG-5'.
Western blot
Right lung tissue, RAW264.7 cells and FLS cells were collected. Total protein was extracted and loaded onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels for electrophoresis. The proteins on the gel were transferred to a polyvinylidene difluoride membrane was incubated with primary antibodies at 4 ℃ overnight, after which the membrane was washed and incubated with secondary antibodies at 37 ℃ for 1 h. The membrane was washed again and the bands were visualized. GAPDH or β-actin was used as an internal control. ImageJ software (Image J-win64) was used to determine the grayscale value of the bands, where relative protein expression level = gray value of target protein/gray value of GAPDH or β-actin. Antibodies for western blotting are listed in Table S1.
Statistical analysis
Analyses were performed using GraphPad Prism 10.0.2 software, conforming to normal distribution. The data were expressed as the mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used to compare multiple groups, and intergroup comparisons were performed using a group t-test; P<0.05 was considered to indicate statistical significance.
Results
Animal model of RA-ILD and the alleviating effect of pirfenidone and nintedanib
Body weight and arthritis scores were measured in all mice between weeks 0–13 (Figure 2). The body weights of the mice in the pirfenidone and nintedanib groups were higher than those of the mice in the RA-ILD group and lower than those in the control group. The arthritis scores were lower in both the pirfenidone and nintedanib intervention groups compared with those in the RA-ILD group. There were significant differences in the visual arthritis score between the pirfenidone group and the nintedanib group before 13 weeks. Significant differences began to appear for the pirfenidone and nintedanib groups after 4 and 2 weeks of treatment, respectively, indicating that the effects of nintedanib may occur faster than those of pirfenidone (Figure 2B). In the RA-ILD group, obvious swelling, stiffness and deformation of the ankle joint and toe joint were observed, while the joint inflammation in the intervention group (pirfenidone and nintedanib) relieved (Figure 2C).

To evaluate the ameliorative effects of pirfenidone and nintedanib on pulmonary fibrosis and joint inflammation in mice, pathomorphologic analyses of the lungs and knee joints were performed. H&E staining revealed thickening of the alveolar septa and inflammatory cell infiltration in the lungs of the mice in the RA-ILD group, while the lungs in the pirfenidone and nintedanib groups exhibited reduced inflammatory cell infiltration and improved alveolar structure. Mouse pulmonary inflammation scores were lower in the pirfenidone and nintedanib groups (Figure 3A,3B). Masson staining revealed a large area of blue staining in the lung tissue and alveolar septa of the mice in the RA-ILD group, indicating substantial deposition of ECM and reduced ECM deposition and fibrosis in the pirfenidone and nintedanib groups. Mouse pulmonary fibrosis scores were lower in the pirfenidone and nintedanib groups (Figure 3A,3C).

H&E staining showed significant arthritis in the knee joints of the mice in RA-ILD group. The inflammatory reactions of arthritis were alleviated by pirfenidone and nintedanib (Figure 4A). The synovitis scores (Table 3) were lower in the pirfenidone and nintedanib groups (Figure 4B), showed that pirfenidone and nintedanib improved joint inflammation in RA-ILD mice.

Table 3
| Score | Synovial inflammation |
|---|---|
| 0 | Healthy, 1–2 cell layers of synovial membrane, no inflammatory infiltrates |
| 1 | 3–5 layered synovial membranes, mild cellular infiltrates into synovium and exudate in joint cavity, low cell density |
| 2 | Multi-layered synovial cell membranes, enhanced cellular infiltrates and increased cell density throughout the joints |
| 3 | Maximal expanded inflammation filling all joint cavities, hyperplastic synovial tissue, high cell density |
Changes of M1 and M2 macrophages
The pathogenesis of RA is related to macrophage polarization, and to clarify this mechanism in this experiment, we used flow cytometry to detect macrophage polarization status in BALF. Flow cytometry of BALF was performed to determine the percentages of M1 and M2 macrophages. F4/80 was used as a marker of total macrophages, CD86 was used as a marker of M1 macrophages, and CD163 was used as a marker of M2 macrophages (Figure 5). Proportion of alveolar M1 macrophages (F4/80+CD86+) in the pirfenidone and nintedanib intervention groups showed the downward tendency without significant difference (Figure 5A,5B). The percentage of M2 macrophages was lower in the nintedanib intervention groups than in the RA-ILD group.

Pirfenidone and nintedanib inhibited Jak2 expression
The protein expression of p-Jak2/p-Stat3 in the lungs and knee joints was assessed using immunofluorescence, the expression of p-Jak2 and p-Stat3 increased in the lungs and knee joints of the mice in the RA-ILD group. After the intervention of pirfenidone and nintedanib, the fluorescence signals of p-Jak2 and p-Stat3 decreased significantly (Figures 6,7).


Gene expression and protein of Jak2/Stat3 and Col-IV in lung tissue were assessed by qRT-PCR and western blot. The mRNA expression of Jak2, Stat and Col-IV in the lungs in the RA-ILD group was high, and the expression levels of these genes were lower in the pirfenidone and nintedanib groups (Figure 8). Western blot analysis was performed to investigate the effects of pirfenidone and nintedanib on the expression of ECM-related proteins (Col-IV, fibronectin) and the Jak2/Stat3 and TGF-β1/Smad3 signaling pathways in the lungs. Western blot analysis revealed that the expression levels of Col-IV, fibronectin, p-Jak2/Jak2, p-Stat3/Stat3, p-Smad3/Smad3, and TGF-βR2 were greater in the RA-ILD group than in the pirfenidone and nintedanib groups (Figure 9).


Cell experiments further explained the mechanisms involved in therapy of RA-ILD by pirfenidone and nintedanib
In order to investigate the correlation between M2 polarization and Jak2/Stat3 signaling pathway, we induced M2 polarization of RAW264.7 cells by IL-4/IL-13, and then detected the expression of related proteins by western blot after the intervention of pirfenidone and nintedanib. The levels of the M2 macrophage markers ARG-1 and CD206 in the RAW + IL-4/IL-13 model group were high, indicating the successful induction of M2 polarization (Figure 10A-10C), as well as the high expression of Jak2/Stat3 and TGF-β signaling pathway components; the expression of the above proteins was reduced in the pirfenidone and nintedanib intervention groups with different concentrations. Western blotting of animal experiment confirmed high p-Jak2, Jak2, p-Stat3, Stat3, and TGF-βR2 expression in the FLS + TGF-β1 model group, and the Jak2/Stat3 signaling pathway was inhibited after intervention with different concentrations of pirfenidone and nintedanib (Figure 11).


Discussion
In our study, we established a bCII-induced RA-ILD mouse model and investigated the ameliorative effects of pirfenidone and nintedanib on joint inflammation and pulmonary fibrosis, and explored the possible mechanisms. We found that pirfenidone and nintedanib blocked TGF-βR2 and the activation of Smad3, then the Jak2/Stat3 pathway. The M2 polarization of macrophages was also blocked by these drugs (significantly by nintedanib, insignificantly by pirfenidone). We speculate that pirfenidone and nintedanib insert their therapeutic effect via TGF-βR2-Smad3-Jak2/Stat3 axis, meanwhile, increased M2 macrophages amplify the inflammation and fibrosis.
Extra-articular manifestation of RA increases the mortality of RA patients. Since there are little clinical symptoms, most RA-ILD patients are diagnosed after severe impairment of lung function. Early recognition and slowing of disease progression are important (5). In our study, in the lung of the 13-week mice with RA-ILD, alveolar wall thickening, inflammatory cell infiltration and collagen deposition were reduced by pirfenidone and nintedanib. Therapeutic effects were also observed on the joint inflammation like ankle swelling, stiffness and deformation. Several clinical trials have approved the therapeutic effects of these two drugs on RA-ILD (3,32,37-40). The INBUILD trial was a phase 3 clinical trial, in which nintedanib demonstrated an improvement in patients with RA-ILD by significantly lowering the average rate of forced vital capacity (FVC) decline by 59% and reducing the risk of acute exacerbation or death (32). Patients treated with nintedanib since their initial ILD diagnosis exhibited significant improvement of lung function within 24 months of treatment (37). Nintedanib affects arthritis in murine model, reverses joint swelling and decreases the arthritis score (3). These effects of nintedanib are the same with the therapeutic effects observed in our experiments. Multiple clinical trials also demonstrated the promising potential of pirfenidone for the treatment of RA-ILD. Pirfenidone lowered the rate of FVC decline (38,40). The decrease of diffusing capacity of the lungs for carbon monoxide (DLCO%), which is a sign of falling of gas exchange, was also enhanced (39).
We reported the relief of lung fibrosis and joint injury by pirfenidone and nindatenib was via TGF-β-Jak2/Stat3 signaling pathways in this experiment. Some studies have shown that p-Jak is highly expressed in the lung of RA-ILD patients (41-43). Inhibition of the Jak2/Stat3 signaling pathway alleviated the synovitis in RA mouse (30). As a result, many Jak2 inhibitors are currently used for the treatment of RA (44). Pirfenidone is now widely used in ILD, mechanisms involved were mainly through the inhibition of Jak2/Stat3 signaling. In short, pirfenidone inhibited the expression of TGF-β1 to block Jak2 and downstream signaling, while Jak2 is not only a downstream target of TGF-β1, but also stimulates the expression of the TGF-β1 gene dynamically (45,46). These signalings are also related tightly to nintedanib. Nintedanib inhibits the progression of pulmonary fibrosis by blocking TGF-β and the activation of Smad3 in the lung of mice with RA-ILD (47). Pirfenidone and nintedanib intervention reversed the increase of the ECM components, TGF-β1/Smad3 and Jak2/Stat3 signaling pathways significantly, which was consistent with the former studies (45-47). The same phenomenon was also observed in FLS cells, further confirmed that relieving effect of pirfenidone and nintedanib was via TGF-βR2-Jak2/Stat3.
The results of flow cytometry revealed high numbers of M1 (F4/80+CD86+) and M2 (F4/80+CD163+) macrophages in BALF of the RA-ILD group. The proportions of M1 (F4/80+CD86+) and M2 (F4/80+CD163+) macrophages decreased after pirfenidone and nintedanib intervention, suggesting that these drugs have anti-inflammatory and antifibrotic effects, a finding that is consistent with the results of the former researches (24,25). RAW264.7 cells polarized towards the M2 phenotype after IL-4 and IL-13 induction, expressing highly expressed Jak2/Stat3 and TGF-β signaling pathway-related proteins. These elevated proteins were then reduced by pirfenidone and nintedanib intervention with different concentrations, suggesting that pirfenidone and nintedanib intervention may result in the inhibition of M2 macrophage polarization through TGF-β signaling pathway and the suppression of the Jak2/Stat3 signaling pathway. Former research on effect of nintedanib in RA-ILD mouse revealed that nintedanib reduced fibrosis, suppressed M2 macrophage polarization and hyperplasia of type 2 alveolar epithelial cells, which supported our results (48). In contrast to the results of our experiment, some researches got the different findings. In mice silicosis fibrosis model, bosutinib increased the number of alveolar M2 macrophages and had antifibrotic effects (49). In a hyperoxic lung injury mouse model, the number of alveolar M2 macrophages increased, but the progression of pulmonary fibrosis decreased (50). In our study, the effect of pirfenidone and nintedanib occurred 5 weeks after collagen injection, at comparably late stage. One of the explanations is that pulmonary fibrosis represents the middle and late stages of RA-ILD and the M2 hyperpolarization exacerbates the progression of pulmonary fibrosis in the late stage. Effects of M2-type polarization inhibitor drugs become evident at the late stage, too.
The proportions of M2 macrophages in the BALF of the mice in the pirfenidone and RA-ILD groups were not significantly different, while there was a significant difference between the proportions of M2 macrophages in the BALF of the mice in the nintedanib and RA-ILD groups. Based on the studies by Flaherty et al. and Noble et al. (32,51), we speculated that nintedanib may have a stronger anti-inflammatory or antifibrotic effect than pirfenidone, while pirfenidone has a slower onset of effects. Further verification through future experiments is needed. According to the label information of pirfenidone, the pharmacokinetic characters might explain this partially (see Table S2). We postulate that because of longer half-life time and less disturbance of food intake, nintedanib exists in the body longer than pirfenidone, its effect on reduction of M2 polarization is stronger at the early stage. As a result, it might take a long time to observe the obvious effect on M2 polarization of pirfenidone. A comparative study on the different effect of the drugs should be designed to further explore the mechanisms, thus provides supportive information for the therapy on RA-ILD.
Limitations of our study are: (I) the observation on effects of signaling pathway inhibitors was not performed; (II) some other key factors in the signaling pathway should be observed later in PCR and western blot; (III) samples of clinical patients should be collected and tested to further support the results in animals.
Conclusions
In summary, pirfenidone and nintedanib can relieve symptoms of arthritis and the degree of pulmonary fibrosis in a bCII-induced RA-ILD mouse model. The mechanism may be related to the inhibition of the TGF-β signaling pathway, the Jak2/Stat3 signaling pathway, and M2 polarization, thereby reducing the symptoms of arthritis and the deposition of collagen in the lungs and attenuating the extent of pulmonary fibrosis. This study provides new insight into the clinical treatment of RA and RA-ILD using pirfenidone and nintedanib. We found that the effects of pirfenidone and nintedanib on M2 macrophages polarization were different.
Acknowledgments
Thanks to Yang Yan from Chongqing Medical University for her help in the experiments.
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-882/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-882/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-882/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-882/coif). X.G. is an employee of West China-Frontier PharmaTech Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. IAC-B2019011-P-01) granted by the ethics board of Greentech committee, in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205-19. [Crossref] [PubMed]
- Gabbay E, Tarala R, Will R, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 1997;156:528-35. [Crossref] [PubMed]
- Redente EF, Aguilar MA, Black BP, et al. Nintedanib reduces pulmonary fibrosis in a model of rheumatoid arthritis-associated interstitial lung disease. Am J Physiol Lung Cell Mol Physiol 2018;314:L998-1009. [Crossref] [PubMed]
- Albrecht K, Strangfeld A, Marschall U, et al. Interstitial lung disease in rheumatoid arthritis: incidence, prevalence and related drug prescriptions between 2007 and 2020. RMD Open 2023;9:e002777. [Crossref] [PubMed]
- Liang M, Matteson EL, Abril A, et al. The role of antifibrotics in the treatment of rheumatoid arthritis-associated interstitial lung disease. Ther Adv Musculoskelet Dis 2022;14:1759720X221074457.
- Cassone G, Manfredi A, Vacchi C, et al. Treatment of Rheumatoid Arthritis-Associated Interstitial Lung Disease: Lights and Shadows. J Clin Med 2020;9:1082. [Crossref] [PubMed]
- Huang S, Kronzer VL, Dellaripa PF, et al. Rheumatoid arthritis-associated interstitial lung disease: Current update on prevalence, risk factors, and pharmacologic treatment. Curr Treatm Opt Rheumatol 2020;6:337-53. [Crossref] [PubMed]
- Farquhar H, Vassallo R, Edwards AL, et al. Pulmonary Complications of Rheumatoid Arthritis. Semin Respir Crit Care Med 2019;40:194-207. [Crossref] [PubMed]
- Matson S, Lee J, Eickelberg O. Two sides of the same coin? A review of the similarities and differences between idiopathic pulmonary fibrosis and rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2021;57:2002533. [Crossref] [PubMed]
- Kass DJ, Nouraie M, Glassberg MK, et al. Comparative Profiling of Serum Protein Biomarkers in Rheumatoid Arthritis-Associated Interstitial Lung Disease and Idiopathic Pulmonary Fibrosis. Arthritis Rheumatol 2020;72:409-19. [Crossref] [PubMed]
- Jeong E, Hong H, Lee YA, et al. Potential Rheumatoid Arthritis-Associated Interstitial Lung Disease Treatment and Computational Approach for Future Drug Development. Int J Mol Sci 2024;25:2682. [Crossref] [PubMed]
- Ruwanpura SM, Thomas BJ, Bardin PG. Pirfenidone: Molecular Mechanisms and Potential Clinical Applications in Lung Disease. Am J Respir Cell Mol Biol 2020;62:413-22. [Crossref] [PubMed]
- Landi C, Carleo A, Vantaggiato L, et al. Common molecular pathways targeted by nintedanib in cancer and IPF: A bioinformatic study. Pulm Pharmacol Ther 2020;64:101941. [Crossref] [PubMed]
- Yang Y, Wang X, Zhang J. Pirfenidone and nintedanib attenuate pulmonary fibrosis in mice by inhibiting the expression of JAK2. J Thorac Dis 2024;16:1128-40. [Crossref] [PubMed]
- Cao ZJ, Liu Y, Zhang Z, et al. Pirfenidone ameliorates silica-induced lung inflammation and fibrosis in mice by inhibiting the secretion of interleukin-17A. Acta Pharmacol Sin 2022;43:908-18. [Crossref] [PubMed]
- Chen Z, Zhou H, Huang X, et al. Pirfenidone attenuates cardiac hypertrophy against isoproterenol by inhibiting activation of the janus tyrosine kinase-2/signal transducer and activator of transcription 3 (JAK-2/STAT3) signaling pathway. Bioengineered 2022;13:12772-82. [Crossref] [PubMed]
- Montero P, Milara J, Roger I, et al. Role of JAK/STAT in Interstitial Lung Diseases; Molecular and Cellular Mechanisms. Int J Mol Sci 2021;22:6211. [Crossref] [PubMed]
- Vasse GF, Nizamoglu M, Heijink IH, et al. Macrophage-stroma interactions in fibrosis: biochemical, biophysical, and cellular perspectives. J Pathol 2021;254:344-57. [Crossref] [PubMed]
- Bosco MC. Macrophage polarization: Reaching across the aisle? J Allergy Clin Immunol 2019;143:1348-50. [Crossref] [PubMed]
- Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016;44:450-62. [Crossref] [PubMed]
- Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol 2020;15:123-47. [Crossref] [PubMed]
- Zhang L, Qu S, Wang L, et al. Tianlongkechuanling Inhibits Pulmonary Fibrosis Through Down-Regulation of Arginase-Ornithine Pathway. Front Pharmacol 2021;12:661129. [Crossref] [PubMed]
- He Q, Zhang W, Zhang J, et al. Cannabinoid Analogue WIN 55212-2 Protects Paraquat-Induced Lung Injury and Enhances Macrophage M2 Polarization. Inflammation 2022;45:2256-67. [Crossref] [PubMed]
- Zeng H, Zhao B, Zhang D, et al. Viola yedoensis Makino formula alleviates DNCB-induced atopic dermatitis by activating JAK2/STAT3 signaling pathway and promoting M2 macrophages polarization. Phytomedicine 2022;103:154228. [Crossref] [PubMed]
- Kong LN, Lin X, Huang C, et al. Hesperetin derivative-12 (HDND-12) regulates macrophage polarization by modulating JAK2/STAT3 signaling pathway. Chin J Nat Med 2019;17:122-30. [Crossref] [PubMed]
- Ding Q, Hu W, Wang R, et al. Signaling pathways in rheumatoid arthritis: implications for targeted therapy. Signal Transduct Target Ther 2023;8:68. [Crossref] [PubMed]
- Wang S, Liu M, Li X, et al. Canonical and noncanonical regulatory roles for JAK2 in the pathogenesis of rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis. FASEB J 2022;36:e22336. [Crossref] [PubMed]
- Sato T, Satooka H, Ichioka S, et al. Citrullinated fibrinogen is a target of auto-antibodies in interstitial lung disease in mice with collagen-induced arthritis. Int Immunol 2020;32:533-45. [Crossref] [PubMed]
- Li S, Lu Z, Wu S, et al. The dynamic role of platelets in cancer progression and their therapeutic implications. Nat Rev Cancer 2024;24:72-87. [Crossref] [PubMed]
- Wang Q, Zhou X, Yang L, et al. The Natural Compound Notopterol Binds and Targets JAK2/3 to Ameliorate Inflammation and Arthritis. Cell Rep 2020;32:108158. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2022;205:e18-47. [Crossref] [PubMed]
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med 2019;381:1718-27. [Crossref] [PubMed]
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive interstitial lung diseases: data from the whole INBUILD trial. Eur Respir J 2022;59:2004538. [Crossref] [PubMed]
- Cao G, Li J, Mao Z, et al. Oxymatrine Alleviates Collagen-Induced Arthritis in Mice by Regulating the Immune Balance of T Cells. Molecules 2023;28:5879. [Crossref] [PubMed]
- Szapiel SV, Elson NA, Fulmer JD, et al. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis 1979;120:893-9. [Crossref]
- Hübner RH, Gitter W, El Mokhtari NE, et al. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 2008;44:507-11, 514-7. [Crossref] [PubMed]
- Serra Gaspar Silva AJ, Martins A, Campinho Ferreira C, et al. POS1307 nintedanib in rheumatic disease-associated interstitial lung disease – a multicentre nationwide cohort study. Annals of the Rheumatic Diseases 2023;82:1001-2.
- Behr J, Prasse A, Kreuter M, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med 2021;9:476-86. [Crossref] [PubMed]
- Wang J, Wang X, Qi X, et al. The Efficacy and Safety of Pirfenidone Combined With Immunosuppressant Therapy in Connective Tissue Disease-Associated Interstitial Lung Disease: A 24-Week Prospective Controlled Cohort Study. Front Med (Lausanne) 2022;9:871861. [Crossref] [PubMed]
- Solomon JJ, Danoff SK, Woodhead FA, et al. Safety, tolerability, and efficacy of pirfenidone in patients with rheumatoid arthritis-associated interstitial lung disease: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Respir Med 2023;11:87-96. [Crossref] [PubMed]
- Liu H, Yang Y, Zhang J, et al. Baricitinib improves pulmonary fibrosis in mice with rheumatoid arthritis-associated interstitial lung disease by inhibiting the Jak2/Stat3 signaling pathway. Adv Rheumatol 2023;63:45. [Crossref] [PubMed]
- Qi QR, Yang ZM. Regulation and function of signal transducer and activator of transcription 3. World J Biol Chem 2014;5:231-9. [Crossref] [PubMed]
- Kiu H, Nicholson SE. Biology and significance of the JAK/STAT signalling pathways. Growth Factors 2012;30:88-106. [Crossref] [PubMed]
- Fitton J, Melville AR, Emery P, et al. Real-world single centre use of JAK inhibitors across the rheumatoid arthritis pathway. Rheumatology (Oxford) 2021;60:4048-54. [Crossref] [PubMed]
- Saito S, Alkhatib A, Kolls JK, et al. Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J Thorac Dis 2019;11:S1740-54. [Crossref] [PubMed]
- Mukhi D, Kolligundla LP, Maruvada S, et al. Growth hormone induces transforming growth factor-β1 in podocytes: Implications in podocytopathy and proteinuria. Biochim Biophys Acta Mol Cell Res 2023;1870:119391. [Crossref] [PubMed]
- Rangarajan S, Kurundkar A, Kurundkar D, et al. Novel Mechanisms for the Antifibrotic Action of Nintedanib. Am J Respir Cell Mol Biol 2016;54:51-9. [Crossref] [PubMed]
- Miura Y, Ohkubo H, Niimi A, et al. Suppression of epithelial abnormalities by nintedanib in induced-rheumatoid arthritis-associated interstitial lung disease mouse model. ERJ Open Res 2021;7:00345-2021. [Crossref] [PubMed]
- Carneiro PJ, Clevelario AL, Padilha GA, et al. Bosutinib Therapy Ameliorates Lung Inflammation and Fibrosis in Experimental Silicosis. Front Physiol 2017;8:159. [Crossref] [PubMed]
- Gharib SA, Johnston LK, Huizar I, et al. MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis. J Leukoc Biol 2014;95:9-18. [Crossref] [PubMed]
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760-9. [Crossref] [PubMed]


