
Effect of exercise on physical fitness and quality of life in patients with pulmonary embolism: a systematic review and meta-analysis
Highlight box
Key findings
• This meta-analysis suggested that exercise programs may have some effect on patients with pulmonary embolism (PE), but the evidence remains inconclusive.
What is known and what is new?
• Exercise has a positive impact on the quality of life (QoL), physical fitness, and dyspnea in patients with heart disease and chronic obstructive pulmonary disease.
• This study aims to investigate the effects of exercise on patients with PE, in order to provide a rational, informed basis for decision-making.
What is the implication, and what should change now?
• The study evaluated the impact and safety of exercise on PE patients.
• Exercise may have a beneficial effect on physical fitness and QoL in patients with PE. Future scientific, personalized exercise protocols need to be developed and high-quality, large-sample randomized controlled trials need to be conducted to validate these findings.
Introduction
Pulmonary embolism (PE) is a potentially fatal condition caused by the obstruction of the pulmonary arterial system by endogenous or exogenous embolism (1), typically referring to acute PE (2), and rarely chronic pulmonary artery obstruction (3). PE is relatively common in clinical settings, with an annual global incidence of approximately 1 out of 1,000 people. The 30-day mortality for high-risk acute PE patients reaches 22% (4), highlighting its potential lethality. It ranks as the third most prevalent cause of cardiovascular death worldwide, trailing behind coronary artery disease and stroke (5,6). Survivors of PE may endure long-term damage to both physical and mental health (7-10), with symptoms such as persistent dyspnea, functional limitations, and reduced quality of life (QoL) (11,12).
Reviewing rehabilitation studies for heart disease or chronic obstructive pulmonary disease (COPD), strong evidence has suggested that rehabilitation exercise positively impacts patients’ QoL, physical fitness, fatigue, and dyspnea (13,14). Currently, evidence on the benefits of post-PE rehabilitation exercises is limited. Some studies suggested that cardiac rehabilitation after PE appears to be safe and may offset the long-term damage suffered by survivors (15-18), potentially improving the exercise capacity of PE patients (17,18). However, other studies have found no significant effects in PE patients following exercise (17).
In 2019, the European Society of Cardiology (ESC) updated its guidelines concerning the diagnosis and management of PE, in which, post-embolism management goals aim to provide appropriate care for patients with persistent symptoms, such as exercise, rehabilitation, treatment of complications, and behavioral education (19). However, due to the limited research on post-PE physical exercise and activities and the inconsistency of existing original research results (17,20), healthcare providers may find it challenging to implement the ESC’s recommendations (21). Therefore, it is necessary to provide valuable information based on evidence.
This study aimed to explore the impact of exercise on physical capacity and QoL in patients with a prior diagnosis of acute PE through a meta-analysis (22). This study is presented in accordance with the PRISMA reporting checklist (23) (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1017/rc).
Methods
Eligibility for included trials
Inclusion criteria in this study were defined as follows: (I) adult patients (>18 years old) with a prior diagnosis of acute PE; (II) studies involving exercise interventions for adult PE patients; (III) in the control group, patients received routine care. In cases where both intervention and control group patients receive general adjunctive therapy, consistency in the adjunctive therapy was ensured; (IV) studies that reported at least one of the listed outcomes: incremental shuttle walk test (ISWT), pulmonary embolism quality of life (PEmbQoL), and the EuroQol-5 Dimensions questionnaire (EQ-5D); (V) studies that adopted a randomized controlled design [i.e., randomized controlled trial (RCT)]; (VI) studies that reported in English.
Exclusion criteria in this study were defined as follows: (I) non-PE patients; (II) studies with unclear diagnostic and efficacy criteria; (III) cohort studies, case reports, review articles, opinion articles, descriptive studies, or abstracts; (IV) studies presenting incomplete or inaccurate data unsuitable for pooling.
Search strategy
The study was registered in PROSPERO with the registration number CRD 42023490505 in which could be assessed the study protocol. Two researchers (M.C. and Y.W.) independently conducted a comprehensive search on PubMed, Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica Database (EMBASE), Scopus, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science (WOS) from data available up until May 21, 2024, with no restrictions regarding document type, date/time, or publication status. The literature search was conducted using all known spellings of Medical Subject Headings (MeSH) and free-text keywords, including “Pulmonary Embolism”, “Lung Embolism”, “Pulmonary Thromboembolism”, “Physical Activity”, “Exercise”, “Sport”, “Physical Exercise”, “Acute Exercise”, “Isometric Exercise”, “Aerobic Exercise”, and “Exercise Training”. Details of the search strategy are outlined in Appendix 1.
Literature selection
In accordance with our pre-established eligibility criteria, two researchers (M.C. and Y.W.) independently selected the studies. All pertinent studies were imported into EndNote v20 to remove duplicates. Then, titles and abstracts were scrutinized to exclude studies not meeting the criteria, followed by a thorough examination of full texts. Any discrepancies were resolved by discussion or consultation with the third researcher (P.H.).
Data extraction and quality assessment
The data and information were meticulously gathered in alignment with the Cochrane data extraction form, including: (I) basic information, such as title, name of the first author, year of publication, and country; (II) principal characteristics of the study population, covering age, gender, number of participants in each group along with dropouts, diagnostic criteria; (III) detailed descriptions of the intervention, specifying the measures, as well as timing, frequency, and intensity; (IV) ISWT, PEmbQoL, and EQ-5D at baseline and post-exercise; (V) critical elements for assessing the bias risk. The extraction of data was initially conducted by one researcher (M.C.) and subsequently verified for accuracy by another researcher (Y.W.).
The methodological quality of the studies included was assessed by the Cochrane Collaboration’s tool for the risk of bias (24). Two researchers (M.C. and Y.W.) independently evaluated the following items: random sequence generation, allocation concealment, blinding of participants and personnel involved in the study, blinding of outcome assessment, incomplete outcome data, selective reporting, and the presence of other bias sources. Each domain’s bias risk was independently judged by the two researchers to be either low (green), unclear (yellow), or high (red). Discrepancies were consulted with the corresponding author.
Data synthesis and statistical analysis
When studies examined the same intervention and outcomes in similar populations using comparable methods, we used meta-analyses to estimate the combined intervention effects in combination with more than one trial (25). Outcomes for ISWT, PEmbQoL, and EQ-5D are continuous variables, and thus the effect size was summarized using mean difference (MD) and 95% confidence interval (CI). I2 was used to assess the statistical heterogeneity of included studies. I2 values of 0% to 40%, 41% to 74%, and 75% to 100% respectively indicate low, moderate, and considerable heterogeneity (25). When statistical heterogeneity was low, a fixed-effects model was adopted to combine data; when P<0.1 or I2>50%, a random-effects model was used to provide more conservative effect estimates (26). This study used Review Manager (v5.4) for statistical analysis. Details of the original data for this study are outlined in Appendix 2.
Sensitivity analysis and publication bias
To explore the sources of heterogeneity, a sensitivity analysis was conducted. Additionally, a funnel plot was employed to visually assess publication bias (27). STATA (v16.0; College Station, TX, USA) was utilized for sensitivity analysis and testing for publication bias.
Results
Search results
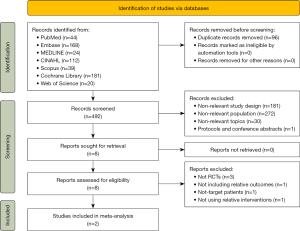
The database search yielded a total of 588 records. After removing duplicates, 492 records were obtained. After the initial screening of titles and abstracts, 181 records were excluded due to incompatible study types, 272 records were excluded due to incompatible study populations, and 30 records were excluded as unrelated to the study topic, leaving 8 records. After reading the full texts of the remaining 8 records, 3 were excluded for not being RCTs, 1 for having incompatible outcome measures, 1 for including the incompatible target population, and 1 for having incompatible intervention content. Finally, 2 articles were finally included in this study. The process of literature screening is presented in Figure 1.
Characteristics of the included studies
The characteristics and details of each study included in our meta-analysis are delineated in Tables 1,2. Among the studies, one (17) was conducted in Denmark and the other (20) was in Norway. Altogether, 348 patients with PE were enrolled across the 2 RCTs, with the study sample size varying between 137 to 211 participants and the median age ranging from 55 to 61.8 years; among the subjects, there were 207 males. The duration of exercise interventions in both studies was 8 weeks, with the intervention being conducted at home in one study (17), where patients completed the exercise scheme under the guidance of physiotherapists and nurses. In the other study (20), patients were asked to perform exercise training both in outpatient and at home. In terms of study design, all included studies (17,20) were RCTs and also were two-group studies.
Table 1
| Studies | Year | Country | Population | Setting | Participant inclusion criteria | Male I/C, % | Age I/C, years† |
Attrition rate | Safety |
|---|---|---|---|---|---|---|---|---|---|
| Rolving et al. (17) | 2020 | Denmark | PE | Home | Presented with an objectively verified first-time acute PE, received anticoagulant drugs, were aged 18 to 80 years, and were competent in the Danish language | 42/48 | 60.3 (12.2)/ 61.8 (10.5) |
15 participants drop out (2 lack of energy, 2 diagnosed with cancer, 7 did not come to follow‐up appointment, 2 withdrew consent, 1 had personal reasons, 1 not mentioned) | No adverse event |
| Jervan et al. (20) | 2023 | Norway | PE | Outpatient, home | PE greater than isolated subsegmental emboli diagnosed with CT pulmonary angiography 6 to 72 months prior to study inclusion; age 18 to 75 years; and persistent self-reported dyspnea | 63/54 | 55 [48–66]/ 60 [52–67] |
14 drop out in intervention group (3 did not respond to telephone/mail and never started the rehabilitation program, 7 were unwilling to continue or did not complete the required number of exercise sessions, 1 withdrew because of pregnancy, and 3 withdrew because of orthopedic injury or other health issues) | No adverse event |
†, data are presented as mean (SD) or median [25th to 75th percentile]. I, intervention group; C, control group; PE, pulmonary embolism; CT, computed tomography; SD, standard deviation.
Table 2
| Studies | Year | Intervention | Control | Frequency | Total exercise time (minutes) | Intensity | Instruments | ||
|---|---|---|---|---|---|---|---|---|---|
| Minutes | Time (weeks) | Total (weeks) | |||||||
| Rolving et al. (17) | 2020 | NC, physiotherapist-guided home-based exercise program (an exercise modality patients enjoyed or were familiar) (n=69) | UC, NC (n=68) | 30–60 | 3 | 8 | 720–1,440 | Moderate intensity to vigorous intensity | ISWT, PembQoL, EQ-5D, SLD, UPD |
| Jervan et al. (20) | 2023 | Supervised outpatient exercise and home-based exercise (based on existing pulmonary rehabilitation programs and international guidelines) (n=108) | UC (n=103) | 60 | 2 | 8 | 960 | NR | ISWT, ESWT, EQ-5D, PEmb-QoL, SOBQ |
NC, nurse consultations; UC, usual care; ISWT, incremental shuttle walk test; PembQoL, pulmonary embolism quality of life; EQ-5D, EuroQol-5 Dimensions questionnaire; SLD, sick-leave day (self-reported); UPD, use of psychotropic drugs (self-reported); NR, not reported; ESWT, Endurance Shuttle Walk Test; SOBQ, Shortness of Breath Questionnaire.
Quality assessment
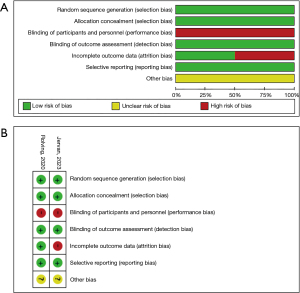
The quality of the included studies was assessed by the Cochrane Collaboration’s tool for the risk of bias. Both studies used random sequence generation and allocation concealment for subjects and study staff. Neither of the studies was able to blind study participants or intervention implementers, but blinding was applied to outcome assessment. Jervan et al. (20) reported missing data in their study. None of the studies had issues with selective reporting or other biases. The results of the quality assessment are shown in Figure 2.
Results of meta-analysis
ISWT
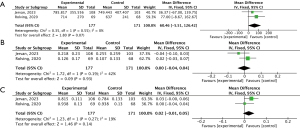
Overall, the two studies assessed the impact of exercise training on ISWT in patients with acute PE. There was no heterogeneity between the studies (I2=0%, P=0.55), and a fixed-effects model was therefore used for the meta-analysis. The results indicated that exercise training did not significantly improve ISWT in patients (MD =60.46; 95% CI: −5.51, 126.42; P=0.07), compared to the control group (Figure 3A).
PEmbQoL
The two studies assessed the impact of exercise training on PEmbQoL in patients with acute PE. A low heterogeneity between the studies was noted (I2=42%, P=0.19), and a fixed-effects model was therefore used for the meta-analysis. The findings indicated that compared to the control group, exercise training did not significantly reduce PEmbQoL in patients (MD =0.00; 95% CI: −0.04, 0.04; P=0.93) (Figure 3B).
EQ-5D
The two studies assessed the impact of exercise training on EQ-5D in patients with acute PE. No heterogeneity between the studies (I2=19%, P=0.27) was noted, and a fixed-effects model was therefore used for the meta-analysis. The results suggested that compared to the control group, exercise training did not significantly improve EQ-5D in patients (MD =0.02; 95% CI: −0.01, 0.05; P=0.14) (Figure 3C).
Consistency and publication bias assessment
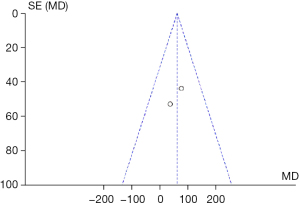
Since only two studies were included in this study, performing a sensitivity analysis through the sequential exclusion of studies was not feasible. The heterogeneity range for the meta-analysis outcome measures was from 0% to 42%, indicating low heterogeneity and relatively stable results. Furthermore, Begg’s test revealed no publication bias (P=0.32), and the funnel plot for ISWT was almost symmetrical, providing no evidence of publication bias (Figure 4).
Discussion
This meta-analysis, based on the current data, found no significant differences in ISWT, PEmbQoL, or EQ-5D after 8 weeks of exercise in patients with a prior history of acute PE (17,20). However, this does not imply that there will be no statistically significant effects in future practical applications, nor does it suggest that exercise is unnecessary for PE patients in the future. The study by Jervan et al. (20) revealed statistically significant differences in ISWT and PEmbQoL after exercise among these patients. In contrast, in the study by Rolving et al. (17), although the differences in ISWT between the two groups were not significant initially, the exercise group demonstrated significantly better ISWT results compared to the control group at the 2- and 6-month follow-ups. Both groups also exhibited improvements in PEmbQoL and EQ-5D. Therefore, we cannot categorically deny the value of exercise training. Based on the results of the meta-analysis and systematic review, the two original studies included in this study were discussed.
The two studies (17,20) displayed slight discrepancies in baseline characteristics. In the study by Rolving et al. (17), demographic and baseline outcomes were balanced between the exercise group and the control group. In the study by Jervan et al. (20), there was a slight age difference between the groups (median: 55 vs. 60 years), and the exercise group exhibited slightly poorer baseline ISWT performance (median: 680 vs. 730 m). However, apart from these variances, the groups were balanced in terms of baseline characteristics. The study by Rolving et al. (17) included PE patients who began exercising 2–3 weeks post-discharge. Unfortunately, the authors did not provide detailed information on the time interval between acute PE diagnosis and the start of exercise. The study by Jervan et al. (20) included patients who were diagnosed with acute PE 6–72 months prior, with 56.9% of the patients diagnosed 6–12 months prior. These patients were randomly assigned to two groups. Rolving et al. (17) assessed participants using the Simplified PE Severity Index (sPESI) (28) before the intervention, showing that the sPESI scores were balanced between the groups, with 71.3% of participants having an sPESI score of 0, indicating low-risk PE. Jervan et al. (20) recorded the PE severity index (PESI) (29) scores at the time of PE diagnosis, with the intervention group having a median score of 64 (25th to 75th percentile, 52–77) and the control group having a median score of 67 (25th to 75th percentile, 58–75). Typically, a PESI score below 65 indicates Class I, signifying an extremely low risk of poor prognosis, while a score of 66–85 indicates Class II, signifying a low risk of poor prognosis. This suggested that the participants in Jervan et al.’s (20) study were also low-risk PE patients. Additionally, the study by Jervan et al. (20) reported that all participants self-reported symptoms of dyspnoea. In Rolving et al.’s (17) study, the Borg Category-Ratio 10 scale (30) was used to assess dyspnoea levels before and after the intervention. Although Rolving et al. (17) did not provide specific results for the Borg scale scores, we cannot rule out that their (17) participants experienced dyspnoea symptoms. Therefore, despite minor differences in the baseline characteristics of the two studies (17,20), it is still justified to conduct a meta-analysis.
The exercise modalities in the two studies (17,20) differed to some extent. In Rolving et al.’s (17) study, patients in the exercise group began a home-based exercise program under the guidance of a physiotherapist. Throughout the exercise period, the physiotherapist monitored the patients via follow-up phone calls and provided exercise guidance. Additionally, patients in the exercise group in the study by Rolving et al. (17) were required to keep exercise diaries, recording the type, frequency, and intensity of their training sessions. In contrast, patients in Jervan et al.’s (20) study participated in supervised outpatient exercise sessions for 2 hours per week. They were also required to attend a 90-minute educational session covering topics such as the diagnosis, treatment, and prognosis of PE, the benefits of exercise and physical activity, and the management of dyspnea. Both studies’ interventions lasted for 8 weeks. Rolving et al. (17) defined exercise compliance as completing at least 75% of the prescribed exercise program and returning exercise diaries. Jervan et al. (20) considered compliance as attending more than 80% of the exercise sessions. The results showed that 42 patients in Rolving et al.’s (17) study completed the exercise program, with a compliance of 60.9%, while 9 patients were considered non-compliant due to not returning their exercise diaries. In Jervan et al.’s (20) study, 14 patients did not complete the program, resulting in a compliance of 87.0%. Additionally, 89.1% of the baseline population in Rolving et al.’s (17) study participated in follow-up visits, compared to 91.0% in Jervan et al.’s (20) study. These data suggested that patients in Jervan et al.’s (20) exercise group demonstrated a higher level of self-management compared to those in Rolving et al.’s (17) study group. This may be partly attributed to the educational session provided in Jervan et al.’s (20) study, which may have improved patients’ self-management skills. Kim et al. (31) compared hospital-supervised exercise training with home-based exercise training in heart failure patients and found that the hospital group showed significant improvements in respiratory function and QoL compared to the home-based group. However, there was no statistically significant difference in cardiac events between the two groups during the one-year follow-up period. Similarly, Vallier et al. (32) compared the effects of home-based rehabilitation and inpatient rehabilitation in patients affected by coronavirus disease 2019 (COVID-19). The study found significant improvements in the 6-minute walking test (6MWT) distance for both groups, but no statistically significant difference between them. Therefore, whether outpatient exercise for PE patients is more effective than home-based exercise still requires validation through scientifically rigorous RCTs.
In Jervan et al.’s (20) study, participants were assessed using the modified Medical Research Council (mMRC) dyspnea scale prior to the exercise intervention, with approximately 70.1% of patients reporting mild dyspnoea. The results showed that after 8 weeks of exercise, there was no significant difference in Shortness of Breath Questionnaire (SOBQ) scores between the two groups. SOBQ (33) is utilized to evaluate the severity of dyspnea and comprises 24 self-reported items/daily activities. Each item is rated from 0 (no shortness of breath) to 5 (maximum shortness of breath or inability to perform activity due to breathlessness), resulting in a total score ranging from 0 to 120. Subjects must complete over 80% of the 24 items to obtain a valid score. Due to missing data, 18% of the patients in the exercise group and 14% in the control group in Jervan et al.’s (20) study did not have their SOBQ scores included in the analysis. This suggested that future intervention studies PE patients should carefully select outcome measurement tools to avoid data loss caused by an excessive number of scales, which may overwhelm patients and result in incomplete responses.
The study by Rolving et al. (17) demonstrated that exercise counseling and fitness testing may reassure patients that physical activity is safe after a PE event, thereby reducing the risk of exercise-related fear.
The psychological changes of PE survivors throughout the exercise process cannot be overlooked (34). Some respondents viewed PE as a traumatic, life-threatening event, with fear of recurrence being a significant source of psychological distress (8). Early psychological interventions for PE patients can reduce the occurrence of long-term sequelae (21). Appropriate emotional support from family or friends can enhance patients’ sense of security, alleviate or release negative emotions, increase self-efficacy in disease management, and may be a key factor in enhancing PE management and rehabilitation (21). Popoola et al. (35) explored patients’ preferences for information on venous thromboembolism prevention education, finding that patients preferred education in the context of clinical encounters over videos or paper materials. Therefore, it is necessary to incorporate psychological interventions and informational support throughout the entire exercise process for patients with PE.
The baseline average physical fitness (measured by ISWT) of the exercise group in the study by Rolving et al. (17) was below the age-specific reference values reported (36), but it improved to comparable levels at the 6-month follow-up. This observation suggested that the confounding effect of time might influence the exercise outcomes in patients with PE. Jervan et al. (20) noted that the collection of follow-up data was notably affected by a ceiling effect, which may have led to an underestimation of the ISWT effect size. Similarly, Rolving et al.’s (17) team encountered a similar ceiling effect when using the ISWT to evaluate the effectiveness of home-based exercise in PE patients. This suggested that ISWT may not be the ideal indicator for assessing physical fitness in this population. Future studies should consider using alternative exercise assessment measures. According to Andersen (37), patients’ cardiopulmonary function can be evaluated using an incremental maximum work test on a bicycle ergometer, known as the Watt max test. Peak oxygen consumption (VO2peak) measured during cardiopulmonary exercise testing (CPET) provides insight into the symptom burden, right ventricular structure and function, and exercise capacity in patients with both massive and submassive PE. CPET (38) is a safe and effective tool capable of quantifying patients’ excitatory response to exercise and distinguishing among different potential sources of exercise limitation. In the management standards for many cardiovascular diseases including coronary artery disease (39,40), congestive heart failure (41), and pulmonary arterial hypertension (42), quantifying patients’ functional capacity through peak oxygen consumption during CPET is recognized as an independent predictor of long-term outcomes (43). CPET holds the promise to be employed for risk stratification of PE patients, enabling the development of tailored exercise prescriptions based on CPET results.
Currently, exercise interventions have shown promising results in patients with various pulmonary diseases. For instance, patients with COPD have improved lung function, exercise capacity, and QoL through high-intensity interval training (HIIT) (44). Although exercise was once considered contraindicated for patients with asthma, multiple studies (45,46) have demonstrated that well-designed and appropriate exercise training can effectively improve respiratory function and exercise capacity in this population. From the evidence, implementing exercise programs for PE patients may also be feasible. However, further high-quality, multicenter RCTs with long-term follow-up periods are needed to determine the optimal patient selection, timing of exercise initiation, type of exercise, and duration of the intervention.
Despite the negative results of this meta-analysis, the researchers adhered to strict quality control protocols. All data were cross-entered by two researchers, while a third researcher handled data management and analysis. Additionally, data distribution and the selection of analysis approach were further assessed and approved by an independent statistician not involved in the study. The methods used to obtain all outcome indicators were scientifically rigorous and accurate, and were not the cause of the negative results observed in this meta-analysis.
There are certain limitations in this study. First, baseline imbalances between groups and slight differences in interventions in the included studies may lead to heterogeneity. Second, conducting RCTs on exercise interventions in patients with acute PE is challenging, resulting in fewer subjects, which is one of the reasons for the limited number of relevant original studies. The limited number of eligible RCTs may introduce bias into the study’s conclusions. Third, due to the scarcity of studies, subgroup analysis could not be performed. Finally, our study was confined to English-language publications, which may introduce selection bias. Given the constraints of current clinical studies and evidence, future studies should prioritize larger sample sizes, employ more stringent quality control measures, and implement stricter study designs to authenticate these findings.
Conclusions
To our knowledge, our study is the first meta-analysis comparing the effects of exercise on the physical fitness and QoL of patients with PE. This study elucidates the possible reasons for the negative results of the meta-analysis from the perspectives of the inclusion criteria, intervention measures, outcome assessment tools of the original literature and the time factor. It also provides detailed recommendations for future exercise intervention studies in patients with PE, offering valuable insights for the design of future original research.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1017/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1017/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1017/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 2020;4:4693-738. [Crossref] [PubMed]
- Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010;363:266-74. [Crossref] [PubMed]
- Nishiyama KH, Saboo SS, Tanabe Y, et al. Chronic pulmonary embolism: diagnosis. Cardiovasc Diagn Ther 2018;8:253-71. [Crossref] [PubMed]
- Becattini C, Agnelli G. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J 2017;49:1601732. [Crossref] [PubMed]
- Giri J, Sista AK, Weinberg I, et al. Interventional Therapies for Acute Pulmonary Embolism: Current Status and Principles for the Development of Novel Evidence: A Scientific Statement From the American Heart Association. Circulation 2019;140:e774-801. [Crossref] [PubMed]
- Essien EO, Rali P, Mathai SC. Pulmonary Embolism. Med Clin North Am 2019;103:549-64. [Crossref] [PubMed]
- Klok FA, van Kralingen KW, van Dijk AP, et al. Quality of life in long-term survivors of acute pulmonary embolism. Chest 2010;138:1432-40. [Crossref] [PubMed]
- Rolving N, Brocki BC, Andreasen J. Coping with everyday life and physical activity in the aftermath of an acute pulmonary embolism: A qualitative study exploring patients' perceptions and coping strategies. Thromb Res 2019;182:185-91. [Crossref] [PubMed]
- Kahn SR, Akaberi A, Granton JT, et al. Quality of Life, Dyspnea, and Functional Exercise Capacity Following a First Episode of Pulmonary Embolism: Results of the ELOPE Cohort Study. Am J Med 2017;130:990.e9-990.e21. [Crossref] [PubMed]
- Danielsbacka JS, Olsén MF, Hansson PO, et al. Lung function, functional capacity, and respiratory symptoms at discharge from hospital in patients with acute pulmonary embolism: A cross-sectional study. Physiother Theory Pract 2018;34:194-201. [Crossref] [PubMed]
- Kahn SR, Hirsch AM, Akaberi A, et al. Functional and Exercise Limitations After a First Episode of Pulmonary Embolism: Results of the ELOPE Prospective Cohort Study. Chest 2017;151:1058-68. [Crossref] [PubMed]
- Albaghdadi MS, Dudzinski DM, Giordano N, et al. Cardiopulmonary Exercise Testing in Patients Following Massive and Submassive Pulmonary Embolism. J Am Heart Assoc 2018;7:e006841. [Crossref] [PubMed]
- Anderson L, Taylor RS. Cardiac rehabilitation for people with heart disease: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2014;2014:CD011273. [Crossref] [PubMed]
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;2015:CD003793. [Crossref] [PubMed]
- Ghram A, Shafiee A, Soori R, et al. Safety and efficacy of high intensity interval training in a patient with acute pulmonary embolism. Prog Cardiovasc Dis 2020;63:393-4. [Crossref] [PubMed]
- Højen AA, Nielsen PB, Overvad TF, et al. Long-Term Management of Pulmonary Embolism: A Review of Consequences, Treatment, and Rehabilitation. J Clin Med 2022;11:5970. [Crossref] [PubMed]
- Rolving N, Brocki BC, Bloch-Nielsen JR, et al. Effect of a Physiotherapist-Guided Home-Based Exercise Intervention on Physical Capacity and Patient-Reported Outcomes Among Patients With Acute Pulmonary Embolism: A Randomized Clinical Trial. JAMA Netw Open 2020;3:e200064. [Crossref] [PubMed]
- Lakoski SG, Savage PD, Berkman AM, et al. The safety and efficacy of early-initiation exercise training after acute venous thromboembolism: a randomized clinical trial. J Thromb Haemost 2015;13:1238-44.
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J 2019;54:1901647. [Crossref] [PubMed]
- Jervan Ø, Haukeland-Parker S, Gleditsch J, et al. The Effects of Exercise Training in Patients With Persistent Dyspnea Following Pulmonary Embolism: A Randomized Controlled Trial. Chest 2023;164:981-91. [Crossref] [PubMed]
- Rolving N, Bloch-Nielsen JR, Brocki BC, et al. Perspectives of patients and health professionals on important factors influencing rehabilitation following acute pulmonary embolism: A multi-method study. Thromb Res 2020;196:283-90. [Crossref] [PubMed]
- Glass GV. Primary, Secondary, and Meta-Analysis of Research. Educational Researcher 1976;5:3-8.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2008.
- Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97-111. [Crossref] [PubMed]
- Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ 1997;315:1533-7. [Crossref] [PubMed]
- Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010;170:1383-9. [Crossref] [PubMed]
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041-6. [Crossref] [PubMed]
- Borg G. Borg’s perceived exertion and pain scales. Champaign, IL, USA: Human Kinetics; 1998:104-viii.
- Kim M, Kim MS, Lim SJ, et al. Comparison of Supervised Hospital-based versus Educated Home-based Exercise Training in Korean Heart Failure Patients. Korean Circ J 2017;47:742-51. [Crossref] [PubMed]
- Vallier JM, Simon C, Bronstein A, et al. Randomized controlled trial of home-based vs. hospital-based pulmonary rehabilitation in post COVID-19 patients. Eur J Phys Rehabil Med 2023;59:103-10. [Crossref] [PubMed]
- Eakin EG, Resnikoff PM, Prewitt LM, et al. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 1998;113:619-24. [Crossref] [PubMed]
- Noble S, Lewis R, Whithers J, et al. Long-term psychological consequences of symptomatic pulmonary embolism: a qualitative study. BMJ Open 2014;4:e004561. [Crossref] [PubMed]
- Popoola VO, Lau BD, Shihab HM, et al. Patient Preferences for Receiving Education on Venous Thromboembolism Prevention - A Survey of Stakeholder Organizations. PLoS One 2016;11:e0152084. [Crossref] [PubMed]
- Probst VS, Hernandes NA, Teixeira DC, et al. Reference values for the incremental shuttle walking test. Respir Med 2012;106:243-8. [Crossref] [PubMed]
- Andersen LB. A maximal cycle exercise protocol to predict maximal oxygen uptake. Scand J Med Sci Sports 1995;5:143-6. [Crossref] [PubMed]
- McKelvie RS, Jones NL. Cardiopulmonary exercise testing. Clin Chest Med 1989;10:277-91.
- Damluji AA, Forman DE, Wang TY, et al. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement From the American Heart Association. Circulation 2023;147:e32-62. [Crossref] [PubMed]
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289-367. [Crossref] [PubMed]
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:1757-80. [Crossref] [PubMed]
- Sherman AE, Saggar R. Cardiopulmonary Exercise Testing in Pulmonary Arterial Hypertension. Heart Fail Clin 2023;19:35-43. [Crossref] [PubMed]
- Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793-801. [Crossref] [PubMed]
- Gao M, Huang Y, Wang Q, et al. Effects of High-Intensity Interval Training on Pulmonary Function and Exercise Capacity in Individuals with Chronic Obstructive Pulmonary Disease: A Meta-Analysis and Systematic Review. Adv Ther 2022;39:94-116. [Crossref] [PubMed]
- Xing S, Feng S, Zeng D. Effect of exercise intervention on lung function in asthmatic adults: a network meta-analysis. Ann Med 2023;55:2237031. [Crossref] [PubMed]
- Hansen ESH, Pitzner-Fabricius A, Toennesen LL, et al. Effect of aerobic exercise training on asthma in adults: a systematic review and meta-analysis. Eur Respir J 2020;56:2000146. [Crossref] [PubMed]


