Protective role of the matricellular protein CCN3 in abdominal aortic aneurysm
Abdominal aortic aneurysms (AAA) are a serious medical condition occurring in up to 9% of humans older than 65 years (1). It can end up deadly due to aortic rupture (2), accounting in the United States for about 15,000 deaths annually (3). Understanding the mechanism of AAA formation and progression, and elaboration of clinical strategies to effectively slow down the progression of AAA, are of utmost importance (4,5).
In the recent work published in Journal of Clinical Investigation (6) Zhang et al. report on a protective role of the protein CCN3, a member of the CCN family (Cyr61, Ctgf, Nov) proteins, in the development and progression of AAA. CCN3, a matricellular protein recognized by several integrins (7), was originally identified, as an in virus-induced nephroblastomas overexpressed protein, and designated as Nov (8). In the vasculature it is mainly expressed in endothelial cells of arteries, small resistance vessels, and veins (9) but also in smooth muscle cells (10,11). CCN3 was demonstrated to induce angiogenesis (12) and upon overexpression in ApoE-deficient mice shown to inhibit inflammation and progression of atherosclerosis (13). It was also reported to inhibit neointimal hyperplasia in femoral arteries through inhibition of smooth muscle proliferation and migration (11,14), a finding that might have inspired the authors of the current study to investigate a possible protective role of CCN3 in AAA.
In this extensive study Zhang et al. showed that loss of CCN3 markedly increased the susceptibility for development and progression of AAA, while overexpression of CCN3 had a protective effect against aneurysm formation. The authors analyzed CCN3 expression in human samples with AAA, but primarily made use of animal models of AAA and previously generated CCN3-deficient mice. In these mice that develop normally when unchallenged (11), they employed two established murine in vivo models of aortic aneurism—elastase perfusion (15) and angiotensin II (Ang II) infusion (16-18). Transient elastase perfusion of the mouse aorta results in partial degradation of the aortic elastin and an associated transmural mononuclear inflammatory response, which leads to a feed-forward cycle increasing local production of several elastolytic MMPs, thereby resulting in destruction of the elastic lamellae, and subsequent aneurysm development (15). The high levels of Ang II found in human AAA lesions (19) form a conceptual base of the Ang II infusion model used in this study. This model is known to elicit, in mice, AAAs mimicking features of human AAA, including dramatic aortic dilation, aortic media destruction, and inflammatory cell infiltration as well as ROS and MMP expression (18).
Here it was shown, that prolonged infusion of Ang II resulted in the development of AAA and concomitantly markedly reduced CCN3 levels in the afflicted abdominal aorta of wild-type mice. Correspondingly, the progression of AAA in CCN3-deficient mice as compared to the age matched wild-type controls was exacerbated in both experimental models of AAA, as seen by an higher increase in infra-renal abdominal aortic diameter and by death of several CCN3-deficient mice due to the aortic rupture. In agreement with these murine data, the levels of CCN3 in the aortic wall in affected areas of human AAA samples were also much lower when compared to healthy aortic regions.
Thanks to previous reports over the last decades, it has become clear that AAA are not simply passively enlarging vascular structures, but exhibit signs of overt inflammation, increased oxidative stress, matrix degradation, and apoptosis of smooth-muscle cells (20,21), features found also in many other pathologies. These features were in this study further analyzed, mainly using histological and biochemical analyses, and found exacerbated by deficiency in CCN3. The authors detected, upon inducing AAA by Ang II infusion, and by performing immuno-staining for MAC3 and CD3, highly increased recruitment and accumulation of inflammatory cells into the aortic walls of CCN3-deficient mice, as compared to wild-type mice. In CCN3-deficient animals, they furthermore found increased production of reactive oxygen species (ROS) and increased levels and activity of matrix degrading enzymes MMP2 and MMP9, as well as increased levels of apoptosis of SMCs upon such treatment. Also in the plasma MMP2 and MMP9 levels were significantly increased in CCN3-deficient mice in response to Ang II infusion.
The importance of ROS production, and its interrelatedness with other features in the induction of AAA, was demonstrated by oral feeding of mice with apocynin, an antioxidant that has been previously utilized in a number of experimental in vivo studies and inhibits the assembly of the ROS-producing NADPH-oxidase (22). Its utilization reduced the incidence of AAA by 25% and was accompanied by reduced infiltration of leukocytes, as revealed by immuno-staining for MAC3 and CD3, and by diminished MMP levels in aortic wall.
As previously CCN3 has been suggested to regulate hematopoietic stem or progenitors cells (23), the authors ruled out any significant contribution of leukocyte-provided CCN3 to the process of experimentally induced AAA, by employing a reciprocal bone marrow transplantation approach. Thereby they clarified that the crucial beneficial contribution of CCN3 originates from CCN3 provided by cells of the vascular wall. In accordance with these findings they furthermore established that freshly isolated SMCs derived from CCN3-deficient mice produced, upon Ang II treatment, higher levels of ROS and exhibited higher levels of apoptosis than SMCs derived from wild-type animals. This way, they pointed to the protective effect of CCN3 expression in SMCs.
Several signaling pathways are implicated in the development of AAA. By analyzing how the signaling response to Ang II infusion in CCN3-deficient mice differs from the response in wild-type mice, the authors deduced that CCN3-deficiency primarily affects MEK1/2 and ERK1/2 activation. These results were further augmented by in vivo experiments employing pharmacological inhibition of MEK and by use of ERK1/2-deficient mice. The compounded evidence indicates that it is ERK1/2 signaling that mediates the effects of CCN3-deficiency in Ang II–induced AAA formation in mice.
Last, but not least, the authors showed in proof of concept experiments, that sustained overexpression of CCN3—achieved by lentiviral transduction—was sufficient to substantially hamper AAA development in the murine elastase model. Such overexpression-treatment significantly reduced the dilation of aortas and was accompanied by less pronounced elastin degradation and leukocyte infiltration as well as by reduced ROS production and MMP levels. Additionally, it resulted also in diminished ERK1/2 phosphorylation. Overexpression of CCN3 achieved by an adenoviral construct also resulted in reduced dilation of aortas upon Ang II infusion, further compounding the notion that overexpression of CCN3 is effective in hampering progression of AAA.
In the current study demonstrating the protective role of CCN3 in the pathogenesis of AAA, the authors focused primarily on effects of CCN3 expression in SMCs. In light of previous studies showing the anti-inflammatory effect of CCN3 in endothelial cells (9), we presume that part of the protective effects of CCN3 during initiation and progression of AAA might be additionally caused by the presence of CCN3 in the endothelial layer of the aortas. Its presence here might, through the inhibition of NF-κB translocation (9), exert an anti-inflammatory effect. It might inhibit the expression of inflammatory adhesion molecules, thus hampering leukocyte recruitment to the aortic wall. This would be expected to reduce generation of ROS levels and diminish MMP-dependent matrix degradation in the aorta, and consequently hamper progression of AAA development, as outlined on Figure 1.
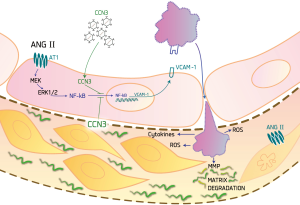
In summary, this study revealed an important protective effect of CCN3 against the development and progression of AAA. It also provided additional evidence for the promoting role of inflammation, oxidative stress, and ERK1/2 signaling in the pathology of AAA. Moreover, it created a base for the design of novel therapeutic approaches utilizing CCN3 to curb the progression of AAA.
Acknowledgements
We thank Andre Oszwald for his critical manuscript review.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Lei Zhang, MD (Department of Vascular Surgery, Changhai Hospital, Second Military Medical University, Shanghai, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thompson RW. Detection and management of small aortic aneurysms. N Engl J Med 2002;346:1484-6. [Crossref] [PubMed]
- Lederle FA, Johnson GR, Wilson SE, et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA 2002;287:2968-72. [Crossref] [PubMed]
- Aortic Aneurysm Fact Sheet. Available online: http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_aortic_aneurysm.htm
- Davis FM, Rateri DL, Daugherty A. Mechanisms of aortic aneurysm formation: translating preclinical studies into clinical therapies. Heart 2014;100:1498-505. [Crossref] [PubMed]
- Piechota-Polanczyk A, Jozkowicz A, Nowak W, et al. The Abdominal Aortic Aneurysm and Intraluminal Thrombus: Current Concepts of Development and Treatment. Front Cardiovasc Med 2015;2:19. [Crossref] [PubMed]
- Zhang C, van der Voort D, Shi H, et al. Matricellular protein CCN3 mitigates abdominal aortic aneurysm. J Clin Invest 2016;126:1282-99. [Crossref] [PubMed]
- Lin CG, Chen CC, Leu SJ, et al. Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J Biol Chem 2005;280:8229-37. [Crossref] [PubMed]
- Joliot V, Martinerie C, Dambrine G, et al. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol 1992;12:10-21. [Crossref] [PubMed]
- Lin Z, Natesan V, Shi H, et al. A novel role of CCN3 in regulating endothelial inflammation. J Cell Commun Signal 2010;4:141-53. [Crossref] [PubMed]
- Ellis PD, Chen Q, Barker PJ, et al. Nov gene encodes adhesion factor for vascular smooth muscle cells and is dynamically regulated in response to vascular injury. Arterioscler Thromb Vasc Biol 2000;20:1912-9. [Crossref] [PubMed]
- Shimoyama T, Hiraoka S, Takemoto M, et al. CCN3 inhibits neointimal hyperplasia through modulation of smooth muscle cell growth and migration. Arterioscler Thromb Vasc Biol 2010;30:675-82. [Crossref] [PubMed]
- Lin CG, Leu SJ, Chen N, et al. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem 2003;278:24200-8. [Crossref] [PubMed]
- Liu J, Ren Y, Kang L, et al. Overexpression of CCN3 inhibits inflammation and progression of atherosclerosis in apolipoprotein E-deficient mice. PLoS One 2014;9:e94912. [Crossref] [PubMed]
- Abe J, Yan C. CCN notch signaling in vascular smooth muscle cells: good or bad? Arterioscler Thromb Vasc Biol 2010;30:667-8. [Crossref] [PubMed]
- Pyo R, Lee JK, Shipley JM, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest 2000;105:1641-9. [Crossref] [PubMed]
- Rush C, Nyara M, Moxon JV, et al. Whole genome expression analysis within the angiotensin II-apolipoprotein E deficient mouse model of abdominal aortic aneurysm. BMC Genomics 2009;10:298. [Crossref] [PubMed]
- Rateri DL, Howatt DA, Moorleghen JJ, et al. Prolonged infusion of angiotensin II in apoE(-/-) mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. Am J Pathol 2011;179:1542-8. [Crossref] [PubMed]
- Moxon JV, Liu D, Moran CS, et al. Proteomic and genomic analyses suggest the association of apolipoprotein C1 with abdominal aortic aneurysm. Proteomics Clin Appl 2014;8:762-72. [Crossref] [PubMed]
- Nishimoto M, Takai S, Fukumoto H, et al. Increased local angiotensin II formation in aneurysmal aorta. Life Sci 2002;71:2195-205. [Crossref] [PubMed]
- McCormick ML, Gavrila D, Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2007;27:461-9. [Crossref] [PubMed]
- Nordon IM, Hinchliffe RJ, Holt PJ, et al. Review of current theories for abdominal aortic aneurysm pathogenesis. Vascular 2009;17:253-63. [Crossref] [PubMed]
- Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm 2008;2008:106507.
- Gupta R, Hong D, Iborra F, et al. NOV (CCN3) functions as a regulator of human hematopoietic stem or progenitor cells. Science 2007;316:590-3. [Crossref] [PubMed]
- Pueyo ME, Michel JB. Angiotensin II receptors in endothelial cells. Gen Pharmacol 1997;29:691-6. [Crossref] [PubMed]
- Zhang X, Wu M, Jiang H, et al. Angiotensin II upregulates endothelial lipase expression via the NF-kappa B and MAPK signaling pathways. PLoS One 2014;9:e107634. [Crossref] [PubMed]


