
A systematic review and meta-analysis of intraoperative neuromonitoring (IONM) of the recurrent laryngeal nerve during minimally invasive esophagectomy
Highlight box
Key findings
• Intraoperative neuromonitoring (IONM) in minimally invasive esophagectomy (MIE) has been shown to significantly reduce the risk of surgical complications, enhancing patient outcomes and overall safety.
What is known and what is new?
• IONM has been widely adopted in thyroid surgery, where it has demonstrated its value in preventing nerve damage, thereby improving patient outcomes.
• This manuscript adds recent evidence suggesting that IONM in esophagectomy can similarly reduce the incidence of recurrent laryngeal nerve paralysis, a serious complication associated with this procedure. Additionally, its application in MIE has been linked to a decrease in other postoperative complications and a shorter hospital stay, further underscoring its potential benefits.
What is the implication, and what should change now?
• The successful application of IONM in MIE highlights its feasibility and effectiveness in this context. Given these positive outcomes, it is recommended that the use of IONM be expanded and routinely applied in MIE procedures to enhance patient safety and surgical success.
Introduction
Esophageal cancer (EC) exhibits a notably high incidence in certain regions of China. Endoscopy and neoadjuvant chemoradiotherapy have made significant advances, but EC patients still have an unfavorable prognosis. Currently, surgical resection remains the primary treatment modality for EC patients (1).
EC is characterized by its high malignancy and lethality. In Western countries, EC is primarily caused by adenocarcinoma. In contrast, EC is mostly caused by squamous cell carcinoma in China (2). A critical aspect of treatment for EC is the dissection of the mediastinal lymph nodes; this process is conducive to the evaluation of both clinical stage and prognosis. The recurrent laryngeal nerve (RLN) lymph node is the most common site of lymph node metastasis in EC, and RLN dissection is necessary to improve the prognosis. In contrast, RLN damage can occur after lymphadenectomy in the area of the RLN during esophagectomy. There is an incidence of RLN injury ranging from 1% to 45.3%, depending on the extent of lymphadenectomy performed (3). Recurrent laryngeal nerve paralysis (RLNP) can also occur; this is due to the anatomical location of the RLN and indirect damage caused by intraoperative compression, traction, thermal damage or ischemia (4).
The RLN is responsible for coordinating the movement of the vocal cords by innervating the laryngeal muscles (excluding the cricothyroid muscle). The RLN is usually damaged when the vocal cords are paralyzed, manifesting as hoarseness or aphasia, or experience severe respiratory problems, and can even cause serious complications such as aspiration and pneumonia via the pulmonary reflex. Previous research has shown that RLN palsy is associated with a higher incidence of pneumonia [odds ratio (OR): 6.210; 95% confidence interval (CI): 2.728 to 14.480; P<0.0001] (5). Damage to these critical nerves is notably frequent when complete lymphadenectomy is performed in close proximity to the RLN. A range of 14.0–45.3% of patients suffer from RLNP following esophagectomy, according to the degree of lymph node dissection (6). While RLNP can sometimes resolve temporarily, there is evidence that it is a risk factor for pulmonary complications (4). Further research is necessary to develop strategies for protecting the RLN during esophageal surgery.
Intraoperative neuromonitoring (IONM), a method used for nerve recognition in thyroid surgery, has been shown to be an effective strategy to protect the RLN. IONM displays changes in the electromyographic activity of the vocal cord muscles via both visual and auditory signals (7). The IONM system can detect pharyngeal muscle contractions using a specialized tube equipped with electrodes. When the stimulation probe contacts the RLN, electrical stimulation causes the vocal cord muscle to contract; the IONM system identifies this event through electromyogram (EMG) signals and also monitors vocal fold activity using auditory signals (8,9).
When the RLN is affected during lymph node dissection, the function of the vocal cord muscle may be compromised, resulting in its unresponsiveness to electrical stimulation. This loss of response (LOR) occurs due to the absence of action potentials during IONM stimulation. Following lymph node dissection around the RLN, a signal reduction of >50% (L2/L1 <50% or R2/R1) is considered as a reduction of signal (ROS). An EMG waveform amplitude of 0 [loss of signal (LOS)] indicates the inability to detect R2 and L2. Both ROS and LOS are known as key abnormalities on the IONM system (10).
There have been many reports of IONM being used in esophagectomy in recent years, and the feasibility and effectiveness of IONM to protect the RLN has been investigated over time. The purpose of this systematic review and meta-analysis was to determine the extent to which IONM protects the RLN during esophagectomy in the presence of EC. We present this article in accordance with the MOOSE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1024/rc).
Methods
Literature search and selection
We searched the EBSCO, PubMed, EMBASE, CNKI, and Cochrane libraries for all relevant literature up to the 1st of November 2022. Search terms included ((esophageal cancer [MeSH Terms]) OR (esophageal cancer [Title/Abstract])) AND (((Recurrent Laryngeal Nerve [MeSH Terms]) OR (Recurrent Laryngeal Nerve [Title/Abstract])) OR (nerve monitoring [Title/Abstract])). Patients diagnosed with EC who underwent esophagectomy were included in the study to compare IONM and non-IONM outcomes.
The specific inclusion criteria were as follows: (I) comparative studies between IONM and non-IONM groups; (II) randomized controlled trials or observational studies (cohort and case-control); and (III) it was required that studies report at least one relevant outcome. Non-comparative studies, review articles, abstracts, case reports, editorials, expert opinions, studies in simulated settings, and robotic surgery studies were excluded in order to control for variables and other factors.
Data extraction and quality assessment
Data were retrieved independently from the identified studies by two authors (J.J. and Z.X.) and any differences were resolved by discussion. Each study provided data on the following: author, year of publication, study design, number of patients, sex, smoking status, alcohol consumption, tumor location, pathological type, and neoadjuvant therapy, American Joint Committee on Cancer (AJCC) staging, incidence of RLNP, number of dissected lymph nodes, operative blood loss, operation time, postoperative pneumonia, aspiration, and length of stay in the hospital.
Using the Newcastle-Ottawa Scale, high-quality studies were defined as those having a score greater than 6. Two authors conducted quality assessments independently (Z.Z. and W.W.). Additionally, a third author (L.Z.) was consulted when discrepancies arose.
Statistical analysis
Heterogeneity among studies was assessed using the Higgins I2 statistic using Review Manager software (version 5.4) (11). If high levels of heterogeneity were evident, then we used the random-effects model for estimation. Whenever there was a low level of heterogeneity, the fixed effects model was used. In high heterogeneity conditions, sensitivity analysis was performed. For dichotomous outcomes, combined ORs and their corresponding 95% CIs were estimated. Analyzing dichotomous data was conducted using Mantel-Haenszel methods, and analysis of continuous data was conducted using inverse variance methods. We assessed publication bias using funnel plots qualitatively. Sample size, median, range, and/or interquartile range were utilized to estimate the sample mean and standard deviation, following the methodology described by Wan et al. (12).
The research program has been registered with PROSPERO (registration number: CRD42022338327).
Results
Literature search
Initially, 1,362 studies met the inclusion criteria based on a literature search across five electronic databases. Four studies did not compare IONM with non-IONM after screening, but IONM was not compared with non-IONM in the remaining 15 studies assessed for eligibility. And one case report on IONM along with four conference abstracts were inaccessible in full text. Ultimately, ten retrospective cohorts (10,13-21) were included. The studies included 1,227 patients with EC who underwent thoracoscopic esophagectomy. A total of 672 and 545 patients underwent IONM and non-IONM procedures, respectively. The PRISMA flowchart of the patients identified in the trial is shown in Figure 1.

Patient characteristics
Table 1 presents a comprehensive overview of baseline patient characteristics. In brief, the mean age of patients in the IONM group ranged from 59.94 to 72 years. The average age of patients in the non-IONM group was 56.16 to 72 years. Tumor location was consistently reported across all trials, with a predominant localization in the middle and lower esophagus. Nearly all studies provided information on pathological tumor types, predominantly involving squamous cell carcinoma, except for five trials.
Table 1
| Group | Fujimoto 2021 (13) | Hikage 2017 (14) | Huang 2022 (15) | Kobayashi 2018 (16) | Komatsu 2022 (17) | Takeda 2021 (18) | Wong 2021 (19) | Yuda 2022 (20) | Zhao 2022 (10) | Zhong 2014 (21) |
|---|---|---|---|---|---|---|---|---|---|---|
| Study design | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort |
| Patients | ||||||||||
| IONM | 17 | 54 | 38 | 31 | 25 | 84 | 157 | 142 | 70 | 54 |
| Non-IONM | 15 | 54 | 37 | 56 | 16 | 83 | 98 | 45 | 80 | 61 |
| Age (years) | ||||||||||
| IONM | 66.33 | 68 [41–84] | 60.18±8.99 | 67 [53–77] | 72 [53–81] | 68 [45–86] | 66 [61–72.5] | 65.9±8.10 | 62.99±8.81 | 59.94±8.966 |
| Non-IONM | 69.47 | 65 [49–80] | 56.16±9.78 | 67.5 [53–80] | 72 [50–85] | 66 [47–81] | 64 [58–72.3] | 67.6±6.60 | 61.96±7.12 | 58.93±9.593 |
| Male | ||||||||||
| IONM | 13 | 49 | 34 | 25 | 19 | 70 | 122 | 118 | 59 | 45 |
| Non-IONM | 15 | 41 | 34 | 44 | 10 | 72 | 82 | 77 | 70 | 49 |
| Smoking history | ||||||||||
| IONM | 12 | 44 | 25 | 28 | ||||||
| Non-IONM | 11 | 45 | 44 | 40 | ||||||
| Alcohol history | ||||||||||
| IONM | 12 | 27 | ||||||||
| Non-IONM | 11 | 40 | ||||||||
| Location of tumor | ||||||||||
| IONM | ||||||||||
| Ut | 4 | 8 | 27 | 5 | 3 | 21 | 13 | 16 | 5 | |
| Mt | 8 | 25 | 10 | 13 | 38 | 85 | 76 | 45 | 42 | |
| Lt | 4 | 13 | 11 | 16 | 9 | 25 | 49 | 50 | 20 | 12 |
| Ae | 1 | 8 | 0 | |||||||
| Non-IONM | ||||||||||
| Ut | 1 | 3 | 24 | 6 | 3 | 16 | 17 | 7 | 6 | |
| Mt | 11 | 29 | 23 | 7 | 41 | 54 | 25 | 49 | 46 | |
| Lt | 3 | 16 | 13 | 25 | 6 | 26 | 19 | 13 | 25 | 15 |
| Ae | 0 | 6 | 2 | |||||||
| Pathological type | ||||||||||
| IONM | ||||||||||
| SCC | 16 | 46 | 33 | 26 | 133 | |||||
| AC | 1 | 5 | 4 | 3 | 5 | |||||
| Others | 0 | 3 | 1 | 2 | 4 | |||||
| Non-IONM | ||||||||||
| SCC | 15 | 48 | 34 | 51 | 39 | |||||
| AC | 0 | 4 | 2 | 2 | 3 | |||||
| Others | 0 | 2 | 1 | 3 | 3 | |||||
| NAC | ||||||||||
| IONM | 10 | 19 | 23 | 14 | 19 | 64 | 90 | 25 | 45 | |
| Non-IONM | 6 | 6 | 17 | 25 | 11 | 34 | 24 | 23 | 49 | |
| AJCC stage | ||||||||||
| IONM | ||||||||||
| I | 4 | 17 | 11 | 8 | 36 | 25 | 10 | |||
| II | 4 | 13 | 10 | 6 | 44 | 18 | 12 | |||
| III | 8 | 22 | 9 | 10 | 53 | 22 | 32 | |||
| IV | 1 | 2 | 1 | 1 | 9 | 5 | ||||
| Non-IONM | ||||||||||
| I | 5 | 21 | 28 | 8 | 12 | 27 | 18 | |||
| II | 2 | 15 | 18 | 2 | 15 | 16 | 19 | |||
| III | 7 | 17 | 9 | 3 | 16 | 34 | 24 | |||
| IV | 1 | 1 | 1 | 3 | 2 | 3 | ||||
“Age” is presented as mean, or mean ± SD, or median [range]. AC, adenocarcinoma; Lt, lower thoracic esophagus; Mt, middle thoracic esophagus; Ut, upper thoracic esophagus; SCC, squamous cell carcinoma; IONM, intraoperative neuromonitoring; NAC, neoadjuvant chemotherapy; AJCC, American Joint Committee on Cancer; AE, abdominal esophagus; SD, standard deviation.
According to the AJCC, all studies included in the analysis reported cancer stage. There were no statistically significant differences in cancer stage observed between the IONM and non-IONM groups.
RLNP analyses
In this analysis, RLNP was reported as a surgical outcome in all studies, including Zhong et al.’s (22), which reported occurrence in IONMs as well as non-IONMs. Table 2 presents detailed IONM results. Non-IONM group incidence ranged from 9.8% to 53.3%, while IONM group incidence ranged from 0% to 46.3%. IONM significantly reduced the incidence of RLNP after thoracoscopic esophagectomy, according to a pooled analysis (Figure 2). Among these, the IONM group was found to be correlated with signal loss during surgery and subsequent RLNP in eight studies.
Table 2
| Group | Fujimoto 2021 (13) | Hikage 2017 (14) | Huang 2022 (15) | Kobayashi 2018 (16) | Komatsu 2022 (17) | Takeda 2021 (18) | Wong 2021 (19) | Yuda 2022 (20) | Zhao 2022 (10) | Zhong 2014 (21) |
|---|---|---|---|---|---|---|---|---|---|---|
| RLNP | ||||||||||
| IONM | ||||||||||
| Right | 0 | 2 | 2 | 0 | 1 | 1 | 10 | 32 | 6 | 0 |
| Left | 1 | 21 | 2 | 3 | 16 | 17 | ||||
| Both | 1 | 2 | 0 | 0 | 1 | 1 | ||||
| No | 15 | 29 | 34 | 28 | 13 | 66 | 129 | 110 | 64 | 54 |
| Non-IONM | ||||||||||
| Right | 0 | 3 | 3 | 1 | 5 | 1 | 8 | 20 | 17 | 6 |
| Left | 8 | 19 | 10 | 11 | 18 | 18 | ||||
| Both | 0 | 1 | 1 | 6 | 3 | 6 | ||||
| No | 7 | 31 | 23 | 38 | 11 | 61 | 66 | 25 | 63 | 55 |
IONM, intraoperative neuromonitoring; RLNP, recurrent laryngeal nerve paralysis.
Lymph node dissection and surgical outcome
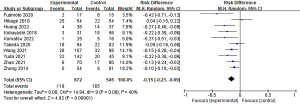
Table 3 provides details about the lymph node dissection and surgical outcomes of all studies that dissected mediastinal lymph nodes. In the non-IONM group, participants were dissected between 15.95 and 28.9 lymph nodes, whereas those in the IONM group were dissected between 20.6 and 30.75 nodes. The IONM group dissected significantly more mediastinal lymph nodes than the non-IONM group, according to a pooled analysis (Figure 3). In three studies, the total number of lymph nodes removed (mediastinal and abdominal) was also reported, showing a higher count of anatomic lymph nodes in the IONM group compared to the non-IONM group in pooled analysis (mean difference: 0.82; 95% CI: 0.20 to 1.44; P<0.05; Figure 4).
Table 3
| Group | Fujimoto 2021 (13) | Hikage 2017 (14) | Huang 2022 (15) | Kobayashi 2018 (16) | Komatsu 2022 (17) | Takeda 2021 (18) | Wong 2021 (19) | Yuda 2022 (20) | Zhao 2022 (10) | Zhong 2014 (21) |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | ||||||||||
| IONM | 17 | 54 | 38 | 31 | 25 | 84 | 157 | 142 | 70 | 54 |
| Non-IONM | 15 | 54 | 37 | 56 | 16 | 83 | 98 | 45 | 80 | 61 |
| RLNP | ||||||||||
| IONM | ||||||||||
| Right | 0 | 2 | 2 | 0 | 1 | 1 | 10 | 32 | 6 | 0 |
| Left | 1 | 21 | 2 | 3 | 16 | 17 | ||||
| Both | 1 | 2 | 0 | 0 | 1 | 1 | ||||
| No | 15 | 29 | 34 | 28 | 13 | 66 | 129 | 110 | 64 | 54 |
| Non-IONM | ||||||||||
| Right | 0 | 3 | 3 | 1 | 5 | 1 | 8 | 20 | 17 | 6 |
| Left | 8 | 19 | 10 | 11 | 18 | 18 | ||||
| Both | 0 | 1 | 1 | 6 | 3 | 6 | ||||
| No | 7 | 31 | 23 | 38 | 11 | 61 | 66 | 25 | 63 | 55 |
| Bleeding (mL) | ||||||||||
| IONM | 351.12±214.06 | 100±23.41 | 271.5±144.61 | 182±101.82 | 140.75±25.24 | 379±625 | 125±21.10 | |||
| Non-IONM | 348.69±251.53 | 150±23.53 | 558.5±381.36 | 865±839.54 | 535±328.95 | 512±363 | 125±20.67 | |||
| Operative time (minutes) | ||||||||||
| IONM | 552.87±82.95 | 670±116.16 | 579.25±25.98 | 264±51.61 | 438.5±51.42 | 565.75±92.15 | 535.27±81.19 | 566±75 | 314.36±70.10 | 237.78±29.49 |
| Non-IONM | 590.82±82.50 | 595.5±65.12 | 635±24.00 | 245±39.80 | 435.25±111.09 | 588.5±90.05 | 487.12±89.11 | 612±253 | 309.06±66.49 | 257.70 ±21.79 |
| No. of TLN | ||||||||||
| IONM | 39±16.06 | 41.9±13.9 | 42.5±10.69 | 69±23 | 28.69±9.23 | 30.39±8.41 | ||||
| Non-IONM | 40.25±15.18 | 41.5±11.7 | 28.75±13.29 | 57±27 | 25.03±10.32 | 24.37±6.82 | ||||
| No. of positive TLN | ||||||||||
| IONM | 6.25±5.06 | 1.4±1.8 | 2.82±3.03 | |||||||
| Non-IONM | 2.75±2.42 | 1.3±1.7 | 1.66±2.14 | |||||||
| No. of MLN | ||||||||||
| IONM | 20.6 | 27.75±12.10 | 30.0±12.9 | 23.75±9.01 | 21±10.18 | 30.75±10.06 | 28.69±9.23 | 22.06±6.50 | ||
| Non-IONM | 18.4 | 27.75±14.30 | 28.9±8.7 | 23.75±8.97 | 17.5±8.48 | 26.75±9.05 | 25.03±10.32 | 15.95±5.94 | ||
| No. of positive MLN | ||||||||||
| IONM | 3±2.64 | 1.0±1.6 | 2.47±2.60 | |||||||
| Non-IONM | 1.75±1.54 | 0.7±1.0 | 1.40±1.84 | |||||||
| Pneumonia | ||||||||||
| IONM | 4 | 10 | 5 | 5 | 1 | 21 | 28 | 5 | 7 | |
| Non-IONM | 6 | 4 | 14 | 11 | 3 | 19 | 13 | 15 | 21 | |
| Aspiration | ||||||||||
| IONM | 2 | 24 | 0? | 2 | ||||||
| Non-IONM | 7 | 17 | 1? | 16 | ||||||
| Hospital stay (days) | ||||||||||
| IONM | 21.1±9.7 | 35.25±17.77 | 19.5±7.64 | 62±34.89 | 31.6±25 | 8±0.42 | 12.51±2.99 | |||
| Non-IONM | 19.8±7.7 | 106±68.66 | 49.75±25.16 | 81.75±49.55 | 40.6±40 | 12.5±0.83 | 16.06±13.69 | |||
Data are presented as mean ± SD. IONM, intraoperative neuromonitoring; RLNP, recurrent laryngeal nerve paralysis; TLN, total lymph nodes; MLN, mediastinal lymph nodes; SD, standard deviation.
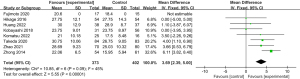

The average duration of surgery varied between 237.8 and 670 minutes for the IONM group, and between 245 and 635 minutes for the non-IONM group. Pooled analysis found no significant difference in operative time when compared between the IONM and non-IONM groups (mean difference: −1.35; P>0.05) (Figure 5). Seven studies reported the average duration of hospitalization; As a result of IONM, this ranged from 8 to 35.25 days and as a result of non-IONM, it ranged from 12.5 to 106 days. Pooled analysis showed that the mean length of hospital stay in the IONM group was significantly shorter than that in the non-IONM group (mean difference: −13.40; 95% CI: −19.97 to −6.83; P<0.001; Figure 6).

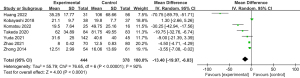
Seven studies reported the volume of bleeding during surgery. The mean bleeding volume ranged from 100 to 507.5 mL in the IONM group and 125 to 865 mL in the non-IONM group. Comparing the IONM group to the non-IONM group, combined analysis showed a significantly lower intraoperative blood loss (Figure 7).
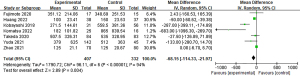
Postoperative pneumonia has been documented in nine studies, with rates ranging between 4% and 25% in the IONM group and 7.4% to 40% in the non-IONM group. Pooled analysis demonstrated a statistically significant lower incidence of pneumonia in the IONM group compared to the non-IONM group (Figure 8).
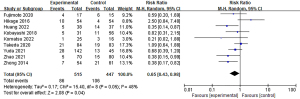
We observed significant heterogeneity in our dataset in terms of operation time, blood loss, and length of hospital stay (I2 was 93%, 94%, and 92%, respectively). Therefore, we conducted a sensitivity analysis by systematically excluding every study and recalculating the overall estimates or 95% CIs. Despite variations in study inclusion, no significant differences were observed in operative time, mean hospital stay, or surgical blood loss between the IONM and non-IONM groups, although these parameters generally favored lower values in the IONM group. However, significant heterogeneity remained; this may have been caused by patient selection bias.
Publication bias
Figure 9 illustrates the funnel plot of RLNP, which shows nearly symmetrical sides; therefore, there is no indication of publication bias.

Quality assessment
The quality evaluation of this study is shown in Table 4. The Newcastle-Ottawa scale score for all studies was 9 points, thus indicating good methodological quality. Thus, none of the studies were excluded from analysis due to low quality.
Table 4
| Study | Publication year | Selection | Comparability (based on design and analysis | Outcome | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed | Selection of non-exposed | Ascertainment of exposed | Outcome of interest absent at | Assessment outcome | Follow-up long enough for outcomes to occur | Adequacy of follow-up | ||||
| Fujimoto (13) | 2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Hikage (14) | 2017 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Huang (15) | 2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Kobayashi (16) | 2018 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | |
| Komatsu (17) | 2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Takeda (18) | 2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Wong (19) | 2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Yuda (20) | 2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Zhao (10) | 2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Zhong (21) | 2014 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
Discussion
Previous research has shown that IONM provides a number of protective effects during esophagectomy (14,18,21). In the reviewed literature, only a single researcher employed discontinuous combined IONM, while the remaining studies utilized discontinuous IONM exclusively. We posit that IONM is particularly advantageous at this stage of surgical procedures, especially when applied during critical moments. The purpose of this study was to determine the impact of IONM upon RLNP incidence following thoracoscopic esophagectomy for EC through a systematic review and meta-analysis. There was a significant reduction in RLNP occurrence in these patients with IONM. In addition, IONM increased the effect of lymph node dissection, and had significant benefits in reducing postoperative pneumonia, surgical bleeding and reducing the length of hospital stay. However, no significant differences in operation time were observed between patients evaluated with IONM and those without IONM evaluation in our study. Upon thorough examination of the included literature, it was determined that the duration of IONM encompasses both the standard operative time and the additional time required for IONM procedures. Despite the advanced state of IONM technology, it was observed that thoracic surgeons generally exhibit limited proficiency in its application. Nevertheless, the ultimate findings did not demonstrate a statistically significant increase in operative time for the IONM group compared to the non-IONM group.
In spite of a significant degree of heterogeneity, the IONM group experienced relatively less blood loss and spent less time in the hospital than the non-IONM group. This observation held true even after excluding the study by Komatsu et al. (17), the IONM group showed clear benefit in terms of blood loss and length of hospital stay, with no heterogeneity among the studies; IONM might have a lower sensitivity, thus explaining this phenomenon. And we attribute this persistent heterogeneity to the varying surgical habits and experience levels among different surgeons, which likely contributed to the substantial differences in operation times. The surgeons were unfamiliar with manipulating the neurosurveillance system in the initial 10 cases. In addition, nearly 30% of the IONM group discontinued treatment due to a variety of reasons. The observed heterogeneity was also related to the lack of studies describing blood loss and hospital stay. We replicated the prior procedure and obtained consistent results. Our analysis suggests that IONM may not substantially decrease intraoperative bleeding. However, it appears to effectively mitigate certain avoidable injuries, thereby reducing hemorrhage specifically during the lymph node dissection phase. The results showed that the length of hospital stay in the IONM group was smaller than that in the non-IONM group. However, the results remained consistent, and substantial heterogeneity persisted when each article was excluded individually. Nevertheless, all included studies indicated that the length of hospital stay was shorter in the IONM group compared to the non-IONM group. The observed heterogeneity may be attributed to variations in physicians’ experience and practices, as well as differing criteria for assessing a patient’s readiness for discharge.
A significant reduction in postoperative pneumonia can also be achieved with IONM, according to our analysis; this may also be correlated with the reduction of RLNP, at least in part. In recent years, the prognosis of patients with EC has improved significantly, largely owing to advancements in surgical techniques, perioperative care, and multidisciplinary treatment approaches (22-25). However, postoperative morbidity and mortality have increased over recent years (26-28). Postoperative pneumonia is one of the most common complications and can occasionally lead to death. Therefore, reducing the incidence of postoperative complications may help to improve the short-term and long-term outcomes of patients after esophagectomy. Postoperative pneumonia in EC is recognized as an independent risk factor affecting the prognosis of patients with this condition (29). A previous study reported that RLN palsy was associated with an increased incidence of McKeown pulmonary complications (30). However, other studies have reported that RLNP becomes a risk factor for significant morbidity only via pulmonary complications such as aspiration pneumonia (30,31).
The RLN is mainly responsible for regulating the production of sound and protection of the airway. By regulating the tension and position of the vocal cords, the RLN enables the opening and closing of the glottis to achieve the production of sound and the regulation of airflow to protect the airway from inhaled foreign bodies. The RLN causes the lungs and airways to remove foreign bodies and secretions through the cough reflex. Scttic insufficiency caused by RLN may lead to reduced cough intensity and weakened airway protection during swallowing. Therefore, patients with RLNP are at higher risk of serious complications such as residual lung infection from aspiration pneumonia or postoperative pneumonia.
The incidence of vocal cord palsy (VCP) following esophagectomy depends on several factors. In the left RLN, the aortic arch extends from a confined space adjacent to the bronchus. In addition to the left main bronchus, aortic arch, main pulmonary artery, and thoracic duct, the left RLN is surrounded by intricate anatomical structures. Therefore, the left RLN is particularly vulnerable to injury (32). Furthermore, in a previous multivariate analysis, prolonged surgery duration and advanced age were independently associated with the development of VCP during esophagectomy. Various risk factors contribute to postoperative pneumonia. Study has demonstrated that an Enhanced Recovery After Surgery (ERAS) protocol is effective in reducing the incidence of postoperative pneumonia following esophagectomy (33). Preoperative care kits have also been reported for the effective management of pneumonia (34). IONM can help surgeons to protect the RLN, thus creating a more secure surgical environment. Following chemotherapy or chemotherapy/radiotherapy. In cases where tumor tissue blurs the surrounding structures, a positive response to IONM can promptly confirm the accurate positioning of the RLN. Second, IONM can be used to verify the surgical procedure leading to RLN damage by providing key information and permitting surgical video review during lymph node dissection.
In our study, we found that the number of removed lymph nodes was significantly increased and the RLNP rate was significantly decreased in patients receiving IONM. Comprehensive lymphadenectomy has demonstrated considerable value due to its association with significantly higher 5-year survival rates (35). In a study of esophagectomy for cancer, Malassagne et al. reported that recurrent paralaryngeal lymph node metastasis plays an independent role in poor prognoses (36). Compared with previous studies, our study found that IONM has a certain effect on reducing the incidence of pneumonia and the length of hospital stay, and we believe that the occurrence of pneumonia and the length of hospital stay may be proportional. In order to improve the prognosis of patients with metastatic lymph nodes, it is crucial to remove them completely, although the special dissection location involved can lead to RLNP during lymph node dissection. According to most western studies, the incidence of RLNP after esophagectomy ranges from 14.0% to 45.3%. However, RLNP has become prevalent in Japan, reaching an incidence of 80%. Law and Wong previously suggested that EC can frequently invade the lymphatic chains of bilateral RLNs, with reported metastasis rates ranging from 18% to 43.4%. The superior mediastinal lymph node adjacent to the RLN is a common site for the metastasis of thoracic EC. The human chest is narrowed above this region, especially at the cervicothoracic junction; the confined space between the trachea and the left RLN, coupled with the immobility of the upper esophagus, renders lymphadenectomy along the RLN a notable risk factor for RLN damage. In the surgeries of some patients undergoing IONM, minute branches of the RLN can be identified and preserved. In the absence of RLNP, it is possible that minor branches can be damaged even without the observation of severe RLM damage; the overall contribution of such minor damage to the development of postoperative RLNP remains unclear.
The primary objective of this review and meta-analysis is to determine whether IONM directly impacts RLN protection during thoracoscopic esophagectomy in patients with EC. It is important to recognize, however, that our study suffers from several limitations. First and foremost, the small number of studies and patients included in our meta-analysis may have limited the precision of our assessment regarding the actual effect of IONM on reducing RLNP. Second, we only included studies that had been published in English; relevant studies published in other languages were omitted. Third, although we found significant differences in the number of dissected RLN nodes, we did not perform survival analysis to evaluate the superiority of IONM. Additionally, the findings of this study exhibited significant heterogeneity and should be interpreted cautiously. In our literature review, the application of IONM to minimally invasive esophagectomy (MIE) was identified in a limited number of cases, with our selection confined to English-language publications. This choice was made for the following reasons: Firstly, English is the predominant academic language in the medical field, often encompassing the most widely cited and discussed research findings. Secondly, due to constraints in resources and time, a comprehensive search across all languages was not feasible. Additionally, RLNP may also be influenced by ethnicity, prior treatments, and surgical techniques. While this study offers valuable insights into the short-term effects and immediate complications associated with IONM, the absence of survival analysis may constrain our comprehensive understanding of the long-term benefits of IONM. Survival analysis is a crucial methodological approach for assessing long-term patient outcomes, as it elucidates the impact of IONM on extended survival rates and quality of life. However, given that this study was principally designed to investigate short-term outcomes, long-term follow-up data were not incorporated. Future research should consider incorporating survival analysis as a key component to better assess the potential benefits and risks of IONM over long-term follow-up.
Conclusions
The findings of this systematic review and meta-analysis suggest that IONM is associated with a lower incidence of RLNP and postoperative pneumonia. Furthermore, lymphadenectomy is more effective and reduces both hospital stay and blood loss in patients with EC. However, IONM has no significant benefit for reducing operative time.
Acknowledgments
Funding: This study was supported by a grant from the Special Project for Health Research Talents of Jilin Province [construction of 3D model for precise navigation of thoracoscopic lung segmental (subsegmental) resection], funded by
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1024/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1024/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1024/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deboever N, Jones CM, Yamashita K, et al. Advances in diagnosis and management of cancer of the esophagus. BMJ 2024;385:e074962. [Crossref] [PubMed]
- Zhu H, Ma X, Ye T, et al. Esophageal cancer in China: Practice and research in the new era. Int J Cancer 2023;152:1741-51. [Crossref] [PubMed]
- Koyanagi K, Igaki H, Iwabu J, et al. Recurrent Laryngeal Nerve Paralysis after Esophagectomy: Respiratory Complications and Role of Nerve Reconstruction. Tohoku J Exp Med 2015;237:1-8. [Crossref] [PubMed]
- Gockel I, Kneist W, Keilmann A, et al. Recurrent laryngeal nerve paralysis (RLNP) following esophagectomy for carcinoma. Eur J Surg Oncol 2005;31:277-81. [Crossref] [PubMed]
- Oshikiri T, Takiguchi G, Hasegawa H, et al. Postoperative recurrent laryngeal nerve palsy is associated with pneumonia in minimally invasive esophagectomy for esophageal cancer. Surg Endosc 2021;35:837-44. [Crossref] [PubMed]
- Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg 2001;72:1918-24; discussion 1924-5. [Crossref] [PubMed]
- Yuda M, Nishikawa K. Intraoperative Nerve Monitoring System during Esophagectomy to Prevent Recurrent Laryngeal Nerve Palsy. Kyobu Geka 2018;71:886-9.
- Hsieh CY, Tan H, Huang HF, et al. Optimization of Intraoperative Neural Monitoring of the Recurrent Laryngeal Nerve in Thyroid Surgery. Medicina (Kaunas) 2022;58:495. [Crossref] [PubMed]
- Ikeda Y, Inoue T, Ogawa E, et al. Recurrent laryngeal nerve monitoring during thoracoscopic esophagectomy. World J Surg 2014;38:897-901. [Crossref] [PubMed]
- Zhao L, He J, Qin Y, et al. Application of intraoperative nerve monitoring for recurrent laryngeal nerves in minimally invasive McKeown esophagectomy. Dis Esophagus 2022;35:doab080. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Fujimoto D, Taniguchi K, Kobayashi H. Intraoperative neuromonitoring during prone thoracoscopic esophagectomy for esophageal cancer reduces the incidence of recurrent laryngeal nerve palsy: a single-center study. Updates Surg 2021;73:587-95. [Crossref] [PubMed]
- Hikage M, Kamei T, Nakano T, et al. Impact of routine recurrent laryngeal nerve monitoring in prone esophagectomy with mediastinal lymph node dissection. Surg Endosc 2017;31:2986-96. [Crossref] [PubMed]
- Huang CL, Chen CM, Hung WH, et al. Clinical Outcome of Intraoperative Recurrent Laryngeal Nerve Monitoring during Thoracoscopic Esophagectomy and Mediastinal Lymph Node Dissection for Esophageal Cancer. J Clin Med 2022;11:4949. [Crossref] [PubMed]
- Kobayashi H, Kondo M, Mizumoto M, et al. Technique and surgical outcomes of mesenterization and intra-operative neural monitoring to reduce recurrent laryngeal nerve paralysis after thoracoscopic esophagectomy: A cohort study. Int J Surg 2018;56:301-6. [Crossref] [PubMed]
- Komatsu S, Konishi T, Matsubara D, et al. Continuous Recurrent Laryngeal Nerve Monitoring During Single-Port Mediastinoscopic Radical Esophagectomy for Esophageal Cancer. J Gastrointest Surg 2022;26:2444-50. [Crossref] [PubMed]
- Takeda S, Iida M, Kanekiyo S, et al. Efficacy of intraoperative recurrent laryngeal neuromonitoring during surgery for esophageal cancer. Ann Gastroenterol Surg 2021;5:83-92. [Crossref] [PubMed]
- Wong IYH, Zhang RQ, Tsang RKY, et al. Improving Outcome of Superior Mediastinal Lymph Node Dissection During Esophagectomy: A Novel Approach Combining Continuous and Intermittent Recurrent Laryngeal Nerve Monitoring. Ann Surg 2021;274:736-42. [Crossref] [PubMed]
- Yuda M, Nishikawa K, Ishikawa Y, et al. Intraoperative nerve monitoring during esophagectomy reduces the risk of recurrent laryngeal nerve palsy. Surg Endosc 2022;36:3957-64. [Crossref] [PubMed]
- Zhong D, Zhou Y, Li Y, et al. Intraoperative recurrent laryngeal nerve monitoring: a useful method for patients with esophageal cancer. Dis Esophagus 2014;27:444-51. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Till BM, Grenda TR, Okusanya OT, et al. Robotic Minimally Invasive Esophagectomy. Thorac Surg Clin 2023;33:81-8. [Crossref] [PubMed]
- Shemmeri E, Wee JO. Minimally Invasive Modified McKeown Esophagectomy. Surg Oncol Clin N Am 2024;33:509-17. [Crossref] [PubMed]
- Dyas AR, Stuart CM, Bronsert MR, et al. Minimally invasive surgery is associated with decreased postoperative complications after esophagectomy. J Thorac Cardiovasc Surg 2023;166:268-78. [Crossref] [PubMed]
- Murad H, Huang B, Ndegwa N, et al. Postoperative hiatal herniation after open vs. minimally invasive esophagectomy; a systematic review and meta-analysis. Int J Surg 2021;93:106046. [Crossref] [PubMed]
- Yip HC, Shirakawa Y, Cheng CY, et al. Recent advances in minimally invasive esophagectomy for squamous esophageal cancer. Ann N Y Acad Sci 2020;1482:113-20. [Crossref] [PubMed]
- Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014;260:259-66. [Crossref] [PubMed]
- Oshikiri T, Nakamura T, Miura Y, et al. A new method (the "Pincers maneuver") for lymphadenectomy along the right recurrent laryngeal nerve during thoracoscopic esophagectomy in the prone position for esophageal cancer. Surg Endosc 2017;31:1496-504. [Crossref] [PubMed]
- Scholtemeijer MG, Seesing MFJ, Brenkman HJF, et al. Recurrent laryngeal nerve injury after esophagectomy for esophageal cancer: incidence, management, and impact on short- and long-term outcomes. J Thorac Dis 2017;9:S868-78. [Crossref] [PubMed]
- Söderström H, Moons J, Nafteux P, et al. Major Intraoperative Complications During Minimally Invasive Esophagectomy. Ann Surg Oncol 2023;30:8244-50. [Crossref] [PubMed]
- Zhang Y, Dong D, Cao Y, et al. Robotic Versus Conventional Minimally Invasive Esophagectomy for Esophageal Cancer: A Meta-analysis. Ann Surg 2023;278:39-50. [Crossref] [PubMed]
- Findlay JM, Gillies RS, Millo J, et al. Enhanced recovery for esophagectomy: a systematic review and evidence-based guidelines. Ann Surg 2014;259:413-31. [Crossref] [PubMed]
- Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today 2020;50:12-20. [Crossref] [PubMed]
- Gottlieb-Vedi E, Kauppila JH, Mattsson F, et al. Extent of Lymphadenectomy and Long-term Survival in Esophageal Cancer. Ann Surg 2023;277:429-36. [Crossref] [PubMed]
- Malassagne B, Tiret E, Duprez D, et al. Prognostic value of thoracic recurrent nerve nodal involvement in esophageal squamous cell carcinoma. J Am Coll Surg 1997;185:244-9. [Crossref] [PubMed]


