
Mortality-related risk factors of idiopathic pulmonary fibrosis: a systematic review and meta-analysis
Highlight box
Key findings
• Six mortality-related risk factors of idiopathic pulmonary fibrosis (IPF) including age, forced vital capacity (FVC), FVC to predicted value ratio, diffusing capacity of the lungs for carbon monoxide to predicted value ratio, gender-age-physiology index, and lung cancer were identified.
What is known and what is new?
• Recent years have witnessed a surfeit of studies aimed at investigating mortality-related risk factors in IPF patients, however, the findings from these studies remained incoincident.
• This study endeavored to comprehensively explore the association between risk factors and mortality in patients with IPF.
What is the implication, and what should change now?
• We summarized the risk factors associated with mortality in patients with IPF, however, there was heterogeneity among studies and more evidence is needed to support the conclusions.
Introduction
Idiopathic pulmonary fibrosis (IPF) is an obscure etiology chronic, progressive, fibrotic interstitial lung disease predominantly affecting middle-aged and elderly males. Its primary manifestations consist of dyspnea and a gradual decline in pulmonary function. The global prevalence of IPF ranges from 0.09 to 1.30 per 10,000 individuals, exhibiting an escalating trend over time. This ailment manifests rapidly, exhibiting high mortality rates, poor prognoses, and 5-year survival rates ranging only from 20% to 40%. The median survival period following diagnosis is merely 3 to 5 years. Consequently, this condition imposes huge burdens on both patients and society (1-3). As a result, early identification and assessment of mortality-associated risk factors in IPF patients, assume pivotal significance in guiding doctors to implement effective clinical interventions in time, diminishing mortality rates and enhancing long-term prognoses.
Recent years have witnessed a surfeit of studies aimed at investigating mortality-related risk factors in IPF patients, however, the findings from these studies remained incoincident. For instance, Suzuki et al. (4) asserted a higher mortality risk in male IPF patients relative to females, whereas Mochizuka et al. (5) observed no significant variance in mortality risk between genders in IPF patients. Furthermore, Ghang et al. (6) identified forced vital capacity to predicted value ratio (FVC% pred) and diffusing capacity of the lungs for carbon monoxide to predicted value ratio (DLCO% pred) as mortality-related risk factors in IPF; nevertheless, Lee et al. (7) posited the absence of a correlation between FVC% pred and DLCO% pred and mortality risk in IPF patients. Given the circumstances, this study included the Cohort study of IPF mortality, and conducted a meta-analysis to summarize mortality-related risk factors, hoping to provide references for the prevention and treatment of IPF. We present this article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1908/rc).
Methods
The protocol of this study has been registered in PROSPERO (CRD42023458513).
Search strategy
Two researchers independently conducted literature searches. PubMed, Cochrane Library, Embase, and Web of Science were searched from their inception to August 10, 2023. Medical Subject Headings (MeSH) terms and keywords used in the search were (“Idiopathic Pulmonary Fibrosis” OR “IPF”) AND (“mortality” OR “mors*” OR “mortality*” OR “died” OR “die” OR “dying” OR “death” OR “fatal*” OR “decease*”) AND (“Risk Factors” OR “risk factor*” OR “influencing factor*” OR “influence factor*” OR “affecting factor*” OR “relevant factor*” OR “correlative factor*” OR “associated factor*” OR “Predicting factor*” OR “related factor*”). Furthermore, the reference lists of the included studies were meticulously reviewed for relevant articles. The detailed search strategies can be found in Appendix 1.
Eligibility criteria
Inclusion criteria: (I) type of study: cohort studies; (II) participants: patients diagnosed with IPF; (III) outcomes: risk factors that may be associated with mortality in patients with IPF; and (IV) study data: analysis of hazard ratios (HRs) from multivariable analysis for each risk factor and corresponding 95% confidence intervals (CIs). Exclusion criteria: (I) duplicate publications; (II) reviews, conference papers, and other types of studies; and (III) literature for which full-text access was not available.
Data extraction and quality assessment
Literature screening and data extraction were carried out independently by two researchers and cross-checked. In the event of any disparities, consensus was reached in consultation with the third researcher. Following the exclusion of duplicate literature, an initial screening was carried out based on titles and abstracts, after which the full texts were read to make the final determination. The extracted information encompassed the first author, publication year, country of origin, sample size, age, gender, follow-up time, outcome indicators, etc.
The quality of the included literature was evaluated using the Newcastle-Ottawa Scale (NOS). The assessment included three sections, comprising a total of eight items, each of which corresponded to a specific score. Studies scoring 5 to 9 were classified as high-quality research, while studies scoring 0 to 4 were regarded as low-quality research (8).
Statistical analysis
Data were analyzed using STATA (version 17.0), with the HRs as the effect indicator and corresponding 95% CIs provided. I2 statistic was used for the heterogeneity analysis. Meta-analysis was performed using a fixed-effects model if P>0.1 and I2<50% and a random-effects model if P≤0.1 and I2≥50%. Sensitivity analysis was performed by transforming the different effect models and excluding articles at a time. The subgroup analysis was conducted according to geographical region. Publication bias of the results was examined using Begg’s and Egger’s tests.
Results
Literature screening process and results
A total of 3,127 articles were retrieved. After deleting 716 duplicate references, 2,336 were excluded by reading titles and abstracts, and 18 were finally included by reading the full texts (4-7,9-22). The flowchart of the literature selection process is shown in Figure 1.
Study characteristics
Among 18 included studies, six studies (4,5,11,12,18,19) were conducted in Japan, five studies (6,7,14,17,22) in South Korea, two studies (15,21) in China-Taiwan, three studies (9,10,20) in Italy, one studies (16) in Germany, one study (13) in Denmark. A total of 8,408 subjects were enrolled, among the included studies, the minimum sample size was 40 and the maximum one was 5,665. The basic characteristics of the included studies were shown in Table 1. Two studies (4,18) scored 8 points on the NOS scale, while 16 studies (5-7,9-17,19-22) scored 9 points, indicating high-quality research. The quality assessment of the included studies was shown in Appendix 2.
Table 1
| Inclusion of studies | Country/area | Number of patients [M/F] | Age (years) | Follow-up time (months) | Risk factors | Quality evaluation |
|---|---|---|---|---|---|---|
| Biondini et al., 2021 (9) | Italy | 88 [71/17] | 70.0 | 68 | GERD, respiratory failure, FVC, TLC | High |
| Caminati et al., 2009 (10) | Italy | 44 [23/21] | 61.9 | 60 | Age, gender, 6MWD, VC, FVC, DLCO, SaO2 | High |
| Furukawa et al., 2017 (11) | Japan | 182 [155/27] | 65.6 | 67 | FVC% pred, SGRQ | High |
| Ghang et al., 2019 (6) | South Korea | 512 [412/100] | NR | 86 | Age, malignancy, FVC% pred, DLCO% pred, 6MWD, CRP, WBC, SaO2 | High |
| Hachisu et al., 2019 (12) | Japan | 84 [59/25] | 78 | 60 | CRP, LDH, T-chol | High |
| Hyldgaard et al., 2020 (13) | Denmark | 260 [205/55] | 72.6 | 60 | Gender, age, smoking, FVC, DLCO | High |
| Kim et al., 2022 (14) | South Korea | 215 [175/40] | 71.8 | 60 | Age, FVC% pred, DLCO% pred, weight loss | High |
| Lai et al., 2019 (15) | China-Taiwan | 114 [99/15] | 77.8 | 132 | Pulmonary hypertension, heart disease, lung cancer, GERD, pulmonary fibrosis score, SpO2 | High |
| Lee et al., 2023 (7) | South Korea | 134 [124/10] | 67.3 | 44 | Albumin, heart disease, FVC% pred, DLCO% pred, 6MWD, weight loss | High |
| Loeh et al., 2019 (16) | German | 70 [48/22] | 66.4 | 108 | Gender | High |
| Mochizuka et al., 2023 (5) | Japan | 301 [247/54] | 72 | 164 | Age, gender, FVC% pred, GAP index | High |
| Moon et al., 2019 (17) | South Korea | 180 [143/37] | 69.1 | 72 | BMI, smoking, GAP index | High |
| Nakano et al., 2020 (18) | Japan | 119 [98/21] | 67.0 | 6 | BMI, FVC% pred | High |
| Oda et al., 2018 (19) | Japan | 5,665 [4,122/1,543] | 73.5 | 36 | Age, gender, bacterial pneumonia, pulmonary hypertension, lung cancer | High |
| Sonaglioni et al., 2023 (20) | Italy | 103 [82/21] | 70.7 | 36 | CCI, CRP, NT-proBNP, 6MWD, LVEF | High |
| Suzuki et al., 2021 (4) | Japan | 208 [176/32] | NR | NR | Age, gender, BMI, FVC% pred, DLCO% pred | High |
| Tseng et al., 2022 (21) | China-Taiwan | 40 [31/9] | 75.6 | 29 | Chest tightness, pestle finger, acute exacerbation, FVC% pred, FEV1% pred | High |
| Yoon et al., 2021 (22) | South Korea | 89 [84/5] | 68.1 | 144 | FVC, DLCO, 6MWD | High |
Data types: number of patients: number; age: mean or median; follow-up time: number. M, male; F, female; GERD, gastro-oesophageal reflux disease; FVC, forced vital capacity; TLC, total lung capacity; 6MWD, 6-minute walk distance; VC, vital capacity; DLCO, diffusing capacity of the lungs for carbon monoxide; SaO2, oxygen saturation; FVC% pred, forced vital capacity to predicted value ratio; SGRQ, Saint George Respiratory Questionnaire; DLCO% pred, diffusing capacity of the lungs for carbon monoxide to predicted value ratio; CRP, C-reactive protein; WBC, white blood cell; LDH, low-density lipoprotein; T-chol, total cholesterol; SpO2, peripheral oxygen saturation; GAP, gender-age-physiology; BMI, body mass index; CCI, Charlson comorbidity index; NT-proBNP, N-terminal pro-brain natriuretic peptide; LVEF, left ventricular ejection fraction; FEV1% pred, forced expiratory volume at 1 second to predicted value ratio; NR, not reported.
Meta-analysis results
The results of meta-analysis were shown in Table 2.
Table 2
| Risk factors | Number of studies included | Heterogeneity test | Effect model | Meta-analysis results | ||
|---|---|---|---|---|---|---|
| I2 (%) | P value | HR (95% CI) | P value | |||
| Age | 7 (4-6,10,13,14,19) | 59.3 | 0.02 | Random-effects model | 1.03 (1.01, 1.04) | <0.001 |
| Gender | 6 (4,5,10,13,16,19) | 63.8 | 0.02 | Random-effects model | 1.21 (0.85, 1.71) | 0.29 |
| Smoking | 2 (13,17) | 0.0 | 0.99 | Fixed-effects model | 0.99 (0.98, 1.01) | 0.39 |
| BMI | 3 (4,17,18) | 83.0 | 0.003 | Random-effects model | 0.95 (0.86, 1.05) | 0.33 |
| FVC | 3 (10,13,22) | 54.7 | 0.11 | Random-effects model | 0.97 (0.96, 0.99) | 0.005 |
| FVC% pred | 7 (4-7,11,14,21) | 61.0 | 0.02 | Random-effects model | 0.98 (0.97, 0.99) | <0.001 |
| DLCO | 3 (10,13,22) | 82.7 | 0.003 | Random-effects model | 0.96 (0.93, 1.00) | 0.03 |
| DLCO% pred | 4 (4,6,7,14) | 0.0 | 0.96 | Fixed-effects model | 0.98 (0.97, 0.99) | <0.001 |
| 6MWD | 5 (6,7,10,20,22) | 74.8 | 0.003 | Random-effects model | 1.00 (0.99, 1.00) | 0.003 |
| SaO2 | 2 (6,10) | 68.4 | 0.08 | Random-effects model | 1.04 (0.87, 1.25) | 0.64 |
| GAP index | 2 (5,17) | 72.6 | 0.06 | Random-effects model | 1.70 (1.20, 2.40) | 0.003 |
| CRP | 3 (6,12,20) | 84.5 | 0.002 | Random-effects model | 1.02 (0.90, 1.15) | 0.77 |
| Lung cancer | 2 (15,19) | 69.6 | 0.07 | Random-effects model | 2.75 (1.23, 6.15) | 0.01 |
| Pulmonary hypertension | 2 (15,19) | 43.0 | 0.19 | Fixed-effects model | 1.06 (0.75, 1.51) | 0.73 |
| GERD | 2 (9,15) | 89.6 | 0.002 | Random-effects model | 0.49 (0.03, 8.81) | 0.63 |
| Heart disease | 2 (7,15) | 0.0 | 0.85 | Fixed-effects model | 1.28 (0.69, 2.37) | 0.43 |
IPF, idiopathic pulmonary fibrosis; HR, hazard ratio; CI, confidence interval; BMI, body mass index; FVC, forced vital capacity; FVC% pred, forced vital capacity to predicted value ratio; DLCO, diffusing capacity of the lungs for carbon monoxide; DLCO% pred, diffusing capacity of the lungs for carbon monoxide to predicted value ratio; 6MWD, 6-minute walk distance; SaO2, oxygen saturation; GAP, gender-age-physiology; CRP, C-reactive protein; GERD, gastro-oesophageal reflux disease.
Age
Seven studies reported association between age and mortality in patients with IPF (4-6,10,13,14,19). There was significant heterogeneity among the included studies (P=0.02, I2=59.3%), and the random-effects model was used for meta-analysis. The results suggested that older age was associated with a higher risk of mortality in IPF patients (HR =1.03; 95% CI: 1.01, 1.04; P<0.001).
Gender
Six studies reported association between gender and mortality in patients with IPF (4,5,10,13,16,19). There was significant heterogeneity among the included studies (P=0.02, I2=63.8%), and the random-effects model was used for meta-analysis. The results suggested that gender was not statistically associated with mortality in IPF patients (HR =1.21; 95% CI: 0.85, 1.71; P=0.29).
Smoking
Two studies reported association between smoking and mortality in patients with IPF (13,17). There was no heterogeneity among the included studies (P=0.99, I2=0.0%), and the fixed-effects model was used for meta-analysis. The results suggested that smoking was not statistically associated with mortality in IPF patients (HR =0.99; 95% CI: 0.98, 1.01; P=0.39).
Body mass index (BMI)
Three studies reported association between BMI and mortality in patients with IPF (4,17,18). There was significant heterogeneity among the included studies (P=0.003, I2=83.0%), and the random-effects model was used for meta-analysis. The results suggested that BMI was not statistically associated with mortality in IPF patients (HR =0.95; 95% CI: 0.86, 1.05; P=0.33).
Forced vital capacity (FVC)
Four studies reported association between FVC and mortality in patients with IPF (9,10,13,22). As one study (9) did not use unified methods to analyze the indicators, it assessed the risk of death in patients with FVC <2.60 at the start of treatment, and FVC <2.56 after antifibrotic therapy, so we performed a meta-analysis of the remaining three studies (10,13,22). There was significant heterogeneity among the included studies (P=0.11, I2=54.7%), and the random-effects model was used for meta-analysis. The results suggested that lower FVC was associated with a higher risk of mortality in IPF patients (HR =0.97; 95% CI: 0.96, 0.99; P=0.005).
FVC% pred
Seven studies reported association between FVC% pred and mortality in patients with IPF (4-7,11,14,21). There was significant heterogeneity among the included studies (P=0.02, I2=61.0%), and the random-effects model was used for meta-analysis. The results suggested that the decrease of FVC% pred increased the risk of mortality in IPF patients (HR =0.98; 95% CI: 0.97, 0.99; P<0.001).
Diffusing capacity of the lungs for carbon monoxide (DLCO)
Three studies reported association between DLCO and mortality in patients with IPF (10,13,22). There was significant heterogeneity among the included studies (P=0.003, I2=82.7%), and the random-effects model was used for meta-analysis. The results suggested that DLCO was not statistically associated with mortality in IPF patients (HR =0.96; 95% CI: 0.93, 1.00; P=0.03).
DLCO% pred
Four studies reported association between DLCO% pred and mortality in patients with IPF (4,6,7,14). There was no heterogeneity among the included studies (P=0.96, I2=0.0%), and the fixed-effects model was used for meta-analysis. The results suggested that the decrease in DLCO% pred was a risk factor for mortality in patients with IPF (HR =0.98; 95% CI: 0.97, 0.99; P<0.001).
6-minute walk distance (6MWD)
Five studies reported association between 6MWD and mortality in patients with IPF (6,7,10,20,22). There was significant heterogeneity among the included studies (P=0.003, I2=74.8%), and the random-effects model was used for meta-analysis. The results suggested that 6MWD was not statistically associated with mortality in IPF patients (HR =1.00; 95% CI: 0.99, 1.00; P=0.003).
Oxygen saturation (SaO2)
Two studies reported association between SaO2 and mortality in patients with IPF (6,10). There was significant heterogeneity among the included studies (P=0.08, I2=68.4%), and the random-effects model was used for meta-analysis. The results suggested that SaO2 was not associated with an increased risk of mortality in IPF patients (HR =1.04; 95% CI: 0.87, 1.25; P=0.64).
Gender-age-physiology (GAP) index
Two studies reported association between GAP index and mortality in patients with IPF (5,17). There was significant heterogeneity among the included studies (P=0.06, I2=72.6%), and the random-effects model was used for meta-analysis. The results suggested that higher score of the GAP index was associated with an increased risk of mortality in IPF patients (HR =1.70; 95% CI: 1.20, 2.40; P=0.003).
C-reactive protein (CRP)
Three studies reported association between CRP and mortality in patients with IPF (6,12,20). There was significant heterogeneity among the included studies (P=0.002, I2=84.5%), and the random-effects model was used for meta-analysis. The results suggested that CRP was not statistically associated with mortality in IPF patients (HR =1.02; 95% CI: 0.90, 1.15; P=0.77).
Lung cancer
Two studies reported association between lung cancer and mortality in patients with IPF (15,19). There was significant heterogeneity among the included studies (P=0.07, I2=69.6%), and the random-effects model was used for meta-analysis. The results suggested that IPF patients with lung cancer had a 2.75-fold increased risk of mortality compared with those with IPF alone (HR =2.75; 95% CI: 1.23, 6.15; P=0.01).
Pulmonary hypertension
Two studies reported association between pulmonary hypertension and mortality in patients with IPF (15,19). There was no heterogeneity among the included studies (P=0.19, I2=43.0%), and the fixed-effects model was used for meta-analysis. The results suggested that pulmonary hypertension was not statistically associated with mortality in IPF patients (HR =1.06; 95% CI: 0.75, 1.51; P=0.73).
Gastro-oesophageal reflux disease (GERD)
Two studies reported association between GERD and mortality in patients with IPF (9,15). There was significant heterogeneity among the included studies (P=0.002, I2=89.6%), and the random-effects model was used for meta-analysis. The results suggested that GERD was not statistically associated with mortality in IPF patients (HR =0.49; 95% CI: 0.03, 8.81; P=0.63).
Heart disease
Two studies reported association between heart disease and mortality in patients with IPF (7,15). There was no heterogeneity among the included studies (P=0.85, I2=0.0%), and the fixed-effects model was used for meta-analysis. The results suggested that heart disease was not statistically associated with mortality in IPF patients (HR =1.28; 95% CI: 0.69, 2.37; P=0.43).
Other risk factors
The Saint George Respiratory Questionnaire (SGRQ), smoking, forced expiratory volume at 1 second to predicted value ratio (FEV1% pred), bacterial pneumonia, acute exacerbations, Charlson comorbidity index (CCI), malignant tumors, respiratory failure, peripheral oxygen saturation (SpO2), N-terminal pro-brain natriuretic peptide (NT-proBNP), chest tightness, clubbing, and pulmonary fibrosis score were referenced solely in individual studies, without undergoing meta-analysis.
Sensitivity analysis, subgroup analyses, and publication bias
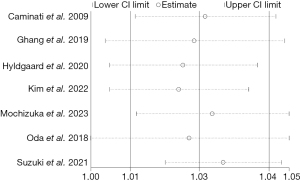
HRs and 95% CIs were calculated using both fixed-effects and random-effects models respectively. The results indicated that, except for DLCO, minimal changes were observed in the meta-analysis results of other risk factors, suggesting relatively robust outcomes. We performed sensitivity analysis for DLCO by excluding articles at a time, and the results were robust (Figure 2). Among the risk factors related to mortality in IPF patients, the heterogeneity of age and FVC% pred was high, sensitivity analysis by excluding one study at a time suggested that the results were stable (Figures 3,4).


We also performed subgroup analyses for age, gender, and 6MWD based on geographic region (Europe and Asia). The results showed that older age was a risk factor for mortality in patients with IPF in Asia (HR =1.02; 95% CI: 1.01, 1.04; P=0.002); while the European results showed no association (HR =1.03; 95% CI: 0.97, 1.09; P=0.35), considering that there were only two European studies, the results need to be further analyzed in future studies. Subgroup analysis of gender and 6MWD did not change the overall results.
Additionally, Begg’s and Egger’s tests were conducted on risk factors included in more than two studies. Among them, gender and BMI had potential publication bias, and Egger’s test showed the P values were 0.007 and 0.02 (Table 3).
Table 3
| Risk factors | Effect model and HR (95% CI) | P value (Begg’s test) |
P value (Egger’s test) |
|
|---|---|---|---|---|
| Fixed-effects model | Random-effects model | |||
| Age | 1.03 (1.02, 1.04) | 1.03 (1.01, 1.04) | 0.37 | 0.53 |
| Gender | 0.94 (0.81, 1.10) | 1.21 (0.85, 1.71) | 0.13 | 0.007 |
| Smoking | 0.99 (0.98, 1.01) | 0.99 (0.98, 1.01) | – | – |
| BMI | 0.98 (0.95, 1.02) | 0.95 (0.86, 1.05) | 0.30 | 0.02 |
| FVC | 0.97 (0.96, 0.98) | 0.97 (0.96, 0.99) | >0.99 | 0.56 |
| FVC% pred | 0.98 (0.97, 0.98) | 0.98 (0.97, 0.99) | >0.99 | 0.90 |
| DLCO | 0.96 (0.95, 0.98) | 0.96 (0.93, 1.00) | >0.99 | 0.62 |
| DLCO% pred | 0.98 (0.97, 0.99) | 0.98 (0.97, 0.99) | 0.73 | 0.38 |
| 6MWD | 1.00 (1.00, 1.00) | 1.00 (0.99, 1.00) | 0.22 | 0.42 |
| SaO2 | 0.98 (0.96, 1.00) | 1.04 (0.87, 1.25) | – | – |
| GAP index | 1.62 (1.37, 1.92) | 1.70 (1.20, 2.40) | – | – |
| CRP | 1.01 (0.97, 1.04) | 1.02 (0.90, 1.15) | >0.99 | 0.87 |
| Lung cancer | 2.18 (1.64, 2.90) | 2.75 (1.23, 6.15) | – | – |
| Pulmonary hypertension | 1.06 (0.75, 1.51) | 1.11 (0.68, 1.82) | – | – |
| GERD | 0.64 (0.26, 1.60) | 0.49 (0.03, 8.81) | – | – |
| Heart disease | 1.28 (0.69, 2.37) | 1.28 (0.69, 2.37) | – | – |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; FVC, forced vital capacity; FVC% pred, forced vital capacity to predicted value ratio; DLCO, diffusing capacity of the lungs for carbon monoxide; DLCO% pred, diffusing capacity of the lungs for carbon monoxideto predicted value ratio; 6MWD, 6-minute walk distance; SaO2, oxygen saturation; GAP, gender-age-physiology; CRP, C-reactive protein; GERD, gastro-oesophageal reflux disease.
Discussion
This study included only cohort studies that conducted multivariable analysis to enhance the reliability of the results. In total, 18 articles comprising 8,408 IPF patients from six different countries were included. The methodological quality of the included studies was notably high, with NOS scores ranging from 8 to 9. The findings revealed that age, FVC, FVC% pred, DLCO% pred, GAP index, and lung cancer were established as mortality risk factors among IPF patients. Nevertheless, the effects of gender, smoking, DLCO, SaO2, CRP, 6MWD, BMI, pulmonary hypertension, GERD, and heart disease on mortality still need further studies to explore.
In this study, we found an association between age and risk of mortality in patients with IPF, which was in consistent with previous studies (23,24). According to du Bois et al. (25), patients over the age of 70 years had almost twice the risk of death as those below 60 years. Leuschner et al. (26) observed an 11.2% higher mortality rate in patients aged 75 and older compared to those below. This could be attributed to age-related comorbidities such as cognitive impairment, malnutrition, and frailty, which accelerate disease progression, and elevate the risk of mortality (27).
Regarding lung function, FVC is a common endpoint of lung function, multiple studies have identified lower FVC as an independent predictor of mortality in patients with IPF (28,29), our meta-analysis showed similar results. FVC is negatively correlated with interstitial involvement in patients with IPF, and its reduction indicates more severe lung injury and poorer prognosis, studies have reported that FVC in IPF patients can decrease by more than 200 mL/year (30,31). DLCO is also a widely accepted predictor of poor prognosis in patients with IPF (32), our included studies also suggest unanimous conclusion. However, our results showed no statistical association between them. Given the high heterogeneity due to differences in population, region, and sample size, we tend to be conservative about the association between DLCO and IPF mortality, and look forward to high-quality clinical trials providing more data to help draw more firm evidence. Additionally, our findings show that reductions in FVC% pred and DLCO% pred were also associated with IPF patient mortality, as corroborated by multiple previous studies (33,34). Snyder et al. (35) found that for per 10% decrease in FVC% pred predicted at enrollment, the risk of death or lung transplant increased by 28%, similarly, for per 10% decrease in DLCO% pred at enrollment, the risk of death or lung transplant increased by 25%. The decreases in FVC% pred and DLCO% pred indicate limited lung ventilation and impaired gas diffusion, which is characteristic of advanced IPF. As the disease progresses, lung function further deteriorates, ultimately leading to respiratory failure and death, critically impacting prognosis (36,37).
Consider the GAP index, our result suggested that it was an independent risk factors for mortality in IPF patients, aligning with the findings reported by Lee et al. (38). Introduced by Ley et al. (39) in 2012, the GAP index encompassed four variables: gender, age, FVC% pred, and DLCO% pred. These metrics are widely employed globally to analyze the mortality prognosis of IPF patients (40-42). Oldham et al. (43) determined that each progression of one stage in the GAP index system corresponded to approximately double the risk of death in IPF patients. Furthermore, other studies have established median survival periods of 64, 45, and 17 months for stage 1, stage 2, and stage 3 of the GAP index, respectively (44). Precisely evaluating the GAP index can enhance the predictive accuracy concerning IPF mortality rates, facilitating the implementation of effective measures to improve the prognosis of IPF patients.
This study found a significant association between the risk of mortality in patients with IPF and lung cancer, and it has been consistently demonstrated in previous research (45,46). Tomassetti et al. (47) discovered that IPF patients with coexisting lung cancer had a considerably shorter median survival time compared to those without, specifically 38.7 and 63.9 months, respectively. Another study revealed that approximately 10.20% of IPF-related deaths were attributed to lung cancer (48). This can be attributed to two primary factors. Firstly, the incidence of lung cancer is notably higher among individuals with IPF, with lung cancer being the most prevalent complication accompanying IPF. Research indicates that IPF patients are five times more likely to develop lung cancer than the general population, with a 10-year incidence rate of 54.7% (49,50). Secondly, there are common biological pathways between lung cancer and IPF, resulting in their frequent co-occurrence in clinical settings, this overlap exacerbates the prognosis, leading to a worse outcome for IPF patients who have also been diagnosed with lung cancer compared to those with IPF alone (51).
6MWD was a widely utilized measure to assess the overall functioning of the pulmonary, cardiovascular, peripheral circulatory, and muscular systems in individuals with chronic respiratory ailments (52,53). In a study by du Bois et al. (25), it was observed that a baseline 6MWD of less than 250 m was linked to a twofold increase in the risk of death, while a decline of more than 50 m in 6MWD after 24 weeks correlated with an almost threefold increase in the risk of death. However, our study found no significant association between the 6MWD and the mortality risk in patients diagnosed with IPF, although opposite results were observed in almost all included studies. Further investigation is necessary to ascertain the potential relevance of 6MWD as a prognostic factor for mortality in IPF.
In addition, pulmonary hypertension and hypoxemia are common manifestations of advanced IPF and are also considered to be important factors for poor prognosis. Lai et al. (15) reported that SpO2 <90% increases the risk of death by more than 5 times in patients with IPF. However, in our meta-analysis of pulmonary hypertension and SaO2, we found no statistically association between them and IPF mortality. Definitive relationship between pulmonary hypertension, hypoxemia, and mortality needs to be confirmed by further studies. Our results showed no association between the gender of patients with IPF and the risk of death. Presently, there is insufficient evidence to support notable differences in mortality risk mechanisms between males and females. Consequently, this study suggested that gender is unlikely to be a contributing risk factor for mortality in IPF patients. Due to the limited number of studies and available data on variables such as BMI, CRP, GERD, and heart disease, the potential impact of these factors on outcomes remains uncertain, necessitating further investigation into their relationship with mortality in IPF patients.
Several limitations exist in this study. Firstly, over two-thirds of the included studies were conducted in Asia, which raises the possibility of result bias. Second, the included studies did not perform subgroup analyses for age, FVC% pred, BMI, and 6MWD, limiting the exploration of potential differences in the mortality risk between different age groups and different levels of FVC%, BMI, and 6MWD. Thirdly, the available literature on some risk factors was limited, potentially influencing the study outcomes. Fourth, in terms of treatment, as is known that the use of drugs, especially anti-fibrotic drugs, are believed to have certain impact on the prognosis of IPF. However, due to limited reporting, we were unable to perform subgroup analyses based on pre- and post-therapy, which may have contributed to results bias. In addition, the studies we included in the meta-analysis covered a period of time, during which great changes had taken place in the treatment for IPF, and the use of different drugs also may increase bias of results. Moreover, certain risk factors may be associated with mortality, due to the scarcity of studies, we were unable to conduct a meta-analysis to evaluate their impact on mortality in patients with IPF.
Conclusions
In conclusion, this study applies the meta-analysis method to analyze and summarize the risk factors associated with mortality in patients with IPF. The identified potential risk factors include age, FVC, FVC% pred, DLCO% pred, GAP index, and lung cancer. However, it is important to note that the conclusions drawn from this analysis are subject to certain limitations stemming from restrictions in both the number and quality of the included studies. Therefore, further research is necessary to validate these findings. Moving forward, it is recommended to place greater emphasis on these identified risk factors during the diagnostic and treatment process for IPF. This enhanced focus will enable clinicians to make more informed clinical decisions and effectively reduce the mortality risk for patients with IPF.
Acknowledgments
Funding: This article was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1908/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1908/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1908/coif). All authors report that this study was supported by the National Natural Science Foundation of China’s Young Scientists Fund (No. 82105048), the National Chinese Medicine Inheritance and Innovation Team Project for the Prevention and Treatment of Respiratory Diseases (No. ZYYCXTD-C-202206), the Henan Province Special Scientific Research Project on Chinese Medicine (Nos. 2021JDZY029 and 2022ZY1040), and the Doctoral Scientific Research Foundation of the First Affiliated Hospital of Henan University of Traditional Chinese Medicine (No. 2021BSJJ001). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maher TM, Bendstrup E, Dron L, et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res 2021;22:197. [Crossref] [PubMed]
- Fernández Fabrellas E, Peris Sánchez R, Sabater Abad C, et al. Prognosis and Follow-Up of Idiopathic Pulmonary Fibrosis. Med Sci (Basel) 2018;6:51. [Crossref] [PubMed]
- Glass DS, Grossfeld D, Renna HA, et al. Idiopathic pulmonary fibrosis: Current and future treatment. Clin Respir J 2022;16:84-96. [Crossref] [PubMed]
- Suzuki Y, Aono Y, Kono M, et al. Cause of mortality and sarcopenia in patients with idiopathic pulmonary fibrosis receiving antifibrotic therapy. Respirology 2021;26:171-9. [Crossref] [PubMed]
- Mochizuka Y, Suzuki Y, Kono M, et al. Geriatric Nutritional Risk Index is a predictor of tolerability of antifibrotic therapy and mortality risk in patients with idiopathic pulmonary fibrosis. Respirology 2023;28:775-83. [Crossref] [PubMed]
- Ghang B, Lee J, Chan Kwon O, et al. Clinical significance of autoantibody positivity in idiopathic pulmonary fibrosis. Respir Med 2019;155:43-8. [Crossref] [PubMed]
- Lee JK, Chung C, Kim J, et al. Clinical impact of weight loss on mortality in patients with idiopathic pulmonary fibrosis: a retrospective cohort study. Sci Rep 2023;13:5774. [Crossref] [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Biondini D, Cocconcelli E, Bernardinello N, et al. Prognostic role of MUC5B rs35705950 genotype in patients with idiopathic pulmonary fibrosis (IPF) on antifibrotic treatment. Respir Res 2021;22:98. [Crossref] [PubMed]
- Caminati A, Bianchi A, Cassandro R, et al. Walking distance on 6-MWT is a prognostic factor in idiopathic pulmonary fibrosis. Respir Med 2009;103:117-23. [Crossref] [PubMed]
- Furukawa T, Taniguchi H, Ando M, et al. The St. George's Respiratory Questionnaire as a prognostic factor in IPF. Respir Res 2017;18:18. [Crossref] [PubMed]
- Hachisu Y, Murata K, Takei K, et al. Possible Serological Markers to Predict Mortality in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Medicina (Kaunas) 2019;55:132. [Crossref] [PubMed]
- Hyldgaard C, Møller J, Bendstrup E. Changes in management of idiopathic pulmonary fibrosis: impact on disease severity and mortality. Eur Clin Respir J 2020;7:1807682. [Crossref] [PubMed]
- Kim TH, Shin YY, Kim HJ, et al. Impact of body weight change on clinical outcomes in patients with idiopathic pulmonary fibrosis receiving pirfenidone. Sci Rep 2022;12:17397. [Crossref] [PubMed]
- Lai RS, Chen CF, Chu KA, et al. The effect of emphysema on survival in patients with idiopathic pulmonary fibrosis: A retrospective study in Taiwan. J Chin Med Assoc 2019;82:922-8. [Crossref] [PubMed]
- Loeh B, Brylski LT, von der Beck D, et al. Lung CT Densitometry in Idiopathic Pulmonary Fibrosis for the Prediction of Natural Course, Severity, and Mortality. Chest 2019;155:972-81. [Crossref] [PubMed]
- Moon SW, Choi JS, Lee SH, et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir Res 2019;20:35. [Crossref] [PubMed]
- Nakano A, Ohkubo H, Taniguchi H, et al. Early decrease in erector spinae muscle area and future risk of mortality in idiopathic pulmonary fibrosis. Sci Rep 2020;10:2312. [Crossref] [PubMed]
- Oda K, Yatera K, Fujino Y, et al. Respiratory comorbidities and risk of mortality in hospitalized patients with idiopathic pulmonary fibrosis. Respir Investig 2018;56:64-71. [Crossref] [PubMed]
- Sonaglioni A, Caminati A, Re M, et al. Prognostic role of CHA(2)DS(2)-VASc score for mortality risk assessment in non-advanced idiopathic pulmonary fibrosis: a preliminary observation. Intern Emerg Med 2023;18:755-67. [Crossref] [PubMed]
- Tseng CM, Chen MY, Kao CY, et al. Investigation of clinical predictors of survival in idiopathic pulmonary fibrosis patients: A cohort study in Taiwan. J Chin Med Assoc 2022;85:578-83. [Crossref] [PubMed]
- Yoon HY, Lee SH, Ha S, et al. The Value of (18)F-FDG PET/CT in Evaluating Disease Severity and Prognosis in Idiopathic Pulmonary Fibrosis Patients. J Korean Med Sci 2021;36:e257. [Crossref] [PubMed]
- Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356-63. [Crossref] [PubMed]
- Zubairi ABS, Ahmad H, Hassan M, et al. Clinical characteristics and factors associated with mortality in idiopathic pulmonary fibrosis: An experience from a tertiary care center in Pakistan. Clin Respir J 2018;12:1191-6. [Crossref] [PubMed]
- du Bois RM, Albera C, Bradford WZ, et al. 6-Minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J 2014;43:1421-9. [Crossref] [PubMed]
- Leuschner G, Klotsche J, Kreuter M, et al. Idiopathic Pulmonary Fibrosis in Elderly Patients: Analysis of the INSIGHTS-IPF Observational Study. Front Med (Lausanne) 2020;7:601279. [Crossref] [PubMed]
- Collard HR. The age of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2010;181:771-2. [Crossref] [PubMed]
- du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011;184:1382-9. [Crossref] [PubMed]
- Reichmann WM, Yu YF, Macaulay D, et al. Change in forced vital capacity and associated subsequent outcomes in patients with newly diagnosed idiopathic pulmonary fibrosis. BMC Pulm Med 2015;15:167. [Crossref] [PubMed]
- Nathan SD, Yang M, Morgenthien EA, et al. FVC variability in patients with idiopathic pulmonary fibrosis and role of 6-min walk test to predict further change. Eur Respir J 2020;55:1902151. [Crossref] [PubMed]
- Kolb M, Richeldi L, Behr J, et al. Nintedanib in patients with idiopathic pulmonary fibrosis and preserved lung volume. Thorax 2017;72:340-6. [Crossref] [PubMed]
- Mononen ME, Kettunen HP, Suoranta SK, et al. Several specific high-resolution computed tomography patterns correlate with survival in patients with idiopathic pulmonary fibrosis. J Thorac Dis 2021;13:2319-30. [Crossref] [PubMed]
- Paterniti MO, Bi Y, Rekić D, et al. Acute Exacerbation and Decline in Forced Vital Capacity Are Associated with Increased Mortality in Idiopathic Pulmonary Fibrosis. Ann Am Thorac Soc 2017;14:1395-402. [Crossref] [PubMed]
- Brown KK, Inoue Y, Flaherty KR, et al. Predictors of mortality in subjects with progressive fibrosing interstitial lung diseases. Respirology 2022;27:294-300. [Crossref] [PubMed]
- Snyder L, Neely ML, Hellkamp AS, et al. Predictors of death or lung transplant after a diagnosis of idiopathic pulmonary fibrosis: insights from the IPF-PRO Registry. Respir Res 2019;20:105. [Crossref] [PubMed]
- Behr J, Nathan SD, Costabel U, et al. Efficacy and Safety of Pirfenidone in Advanced Versus Non-Advanced Idiopathic Pulmonary Fibrosis: Post-Hoc Analysis of Six Clinical Studies. Adv Ther 2023;40:3937-55. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2022;205:e18-47. [Crossref] [PubMed]
- Lee SH, Kim SY, Kim DS, et al. Predicting survival of patients with idiopathic pulmonary fibrosis using GAP score: a nationwide cohort study. Respir Res 2016;17:131. [Crossref] [PubMed]
- Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684-91. [Crossref] [PubMed]
- Abe M, Tsushima K, Yoshioka K, et al. The Gender-Age-Physiology system as a prognostic model in patients with idiopathic pulmonary fibrosis treated with nintedanib: a longitudinal cohort study. Adv Respir Med 2020;88:369-76. [Crossref] [PubMed]
- Suzuki Y, Mori K, Aono Y, et al. Combined assessment of the GAP index and body mass index at antifibrotic therapy initiation for prognosis of idiopathic pulmonary fibrosis. Sci Rep 2021;11:18579. [Crossref] [PubMed]
- Du K, Zhu Y, Mao R, et al. Medium-long term prognosis prediction for idiopathic pulmonary fibrosis patients based on quantitative analysis of fibrotic lung volume. Respir Res 2022;23:372. [Crossref] [PubMed]
- Oldham JM, Kumar D, Lee C, et al. Thyroid Disease Is Prevalent and Predicts Survival in Patients With Idiopathic Pulmonary Fibrosis. Chest 2015;148:692-700. [Crossref] [PubMed]
- Fukuda CY, Soares MR, de Castro Pereira CA. A score without diffusion capacity of the lung for carbon monoxide for estimating survival in idiopathic pulmonary fibrosis. Medicine (Baltimore) 2020;99:e20739. [Crossref] [PubMed]
- Lee KJ, Chung MP, Kim YW, et al. Prevalence, risk factors and survival of lung cancer in the idiopathic pulmonary fibrosis. Thorac Cancer 2012;3:150-5. [Crossref] [PubMed]
- Liu Y, Zhu M, Geng J, et al. Incidence and radiologic-pathological features of lung cancer in idiopathic pulmonary fibrosis. Clin Respir J 2018;12:1700-5. [Crossref] [PubMed]
- Tomassetti S, Gurioli C, Ryu JH, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015;147:157-64. [Crossref] [PubMed]
- Brown SW, Dobelle M, Padilla M, et al. Idiopathic Pulmonary Fibrosis and Lung Cancer. A Systematic Review and Meta-analysis. Ann Am Thorac Soc 2019;16:1041-51. [Crossref] [PubMed]
- Fulton BG, Ryerson CJ. Managing comorbidities in idiopathic pulmonary fibrosis. Int J Gen Med 2015;8:309-18. [PubMed]
- Karampitsakos T, Tzilas V, Tringidou R, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther 2017;45:1-10. [Crossref] [PubMed]
- Kinoshita T, Goto T. Molecular Mechanisms of Pulmonary Fibrogenesis and Its Progression to Lung Cancer: A Review. Int J Mol Sci 2019;20:1461. [Crossref] [PubMed]
- Lancaster LH. Utility of the six-minute walk test in patients with idiopathic pulmonary fibrosis. Multidiscip Respir Med 2018;13:45. [Crossref] [PubMed]
- Serajeddini H, Rogliani P, Mura M. Multi-dimensional Assessment of IPF Across a Wide Range of Disease Severity. Lung 2018;196:707-13. [Crossref] [PubMed]



