
Impact of preoperative brain natriuretic peptide level for predicting postoperative respiratory complications
Highlight box
Key findings
• Preoperative brain natriuretic peptide (BNP) ≥35 pg/mL was associated with an increased risk for postoperative respiratory complications (PRCs).
What is known and what is new?
• The incidence of PRCs among patients who had BNP levels ≥35 pg/mL was significantly higher than that among patients with <35 pg/mL.
• In patients with preoperative BNP levels ≥35 pg/mL, preservation of lung parenchyma may be an important factor influencing the short-term prognosis following pulmonary resection.
What is the implication, and what should change now?
• We emphasize the importance of evaluating preoperative BNP level before pulmonary surgery.
Introduction
Prevention and prediction of postoperative respiratory complications (PRCs) in pulmonary resection are important to thoracic surgeons. The occurrence rate of PRCs is approximately 12.9% to 17% (1-3). In Japan, the 30-day mortality rate among patients who underwent surgical resection for malignant pulmonary tumors was 0.4% in 2021 (4). Furthermore, risk factors for PRCs can be divided into patient- and surgical-related factors. Preoperative patient-related risk factors for predicting PRCs include older age, heavy smoking, interstitial pneumonia (IP), and obesity (5-8). Emphysema is also a risk factor for PRCs, and a better short-term prognosis may be achieved in patients with emphysematous lungs through the preservation of more lung parenchyma (1). Surgical-related risk factors include operative time, bleeding, and intraoperative blood transfusion (9-11), which are known to strongly influence short-term prognosis.
Brain natriuretic peptide (BNP) is globally used for diagnosing heart failure (12). It is mainly produced in cardiac ventricles upon rapid induction of gene expression, protein synthesis, and secretion in response to myocardial stretching (13-16). In addition, BNP acutely acts on vascular smooth muscle cells and chronically affects the permeability of vascular endothelial cells to regulate blood pressure (16,17). An elevated BNP level suggests a structural or functional cardiac abnormality, or both, and a BNP level ≥35 pg/mL is increasingly recognized as a reliable international cut-off value for diagnosing heart failure. Referral to a cardiovascular specialist is recommended for patients with overt heart failure symptoms, such as exertional dyspnea, general fatigue, or edema (12,18). The incidence of pneumonia is high in patients with heart failure (19), and a correlation may exist between preoperative BNP levels and PRCs, including pneumonia.
High preoperative BNP levels affect the incidence of postoperative atrial fibrillation (POAF) after pulmonary resection (20,21); however, the relationship between preoperative BNP levels and PRCs remains unclear. Hence, we hypothesized that high BNP levels affect the incidence of PRCs and that the development of PRCs may be lower if lung preservation surgery is performed in patients with high BNP levels.
This study aimed to clarify the impact of evaluating preoperative BNP levels in predicting PRCs following pulmonary resection and whether preserving lung parenchyma in patients with high BNP levels decreases the risk of PRCs. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1248/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research ethics committee of Shinshu University approved this study (No. 4938). Owing to the retrospective nature of the study, the requirement for written informed consent was waived.
Patients and study design
This retrospective study included almost the same study cohort for whom PRCs and low-attenuation areas on preoperative computed tomography had previously been reported (1).
We evaluated patients with primary or metastatic lung cancer who underwent surgical resection between 2017 and 2021. The exclusion criteria were preoperative radiotherapy, history of pulmonary resection, and incomplete data. Patient data regarding age, sex, body mass index, smoking history, respiratory function, surgical approach, and postoperative complications were retrieved from the medical records of the hospital. Preoperative BNP levels were evaluated using the most recent blood data, which were obtained within at least 2 months before surgery.
PRCs were defined as Clavien-Dindo Grade II or higher prolonged air leakage, pneumonia, bronchial fistula, acute IP exacerbation, empyema, acute respiratory distress syndrome, respiratory failure, pleural effusion requiring fine-needle aspiration, asthma, and intrapulmonary hemorrhage observed within 30 days after surgery (22). POAF was defined as the occurrence of POAF within 30 days after surgery.
We set the cut-off value of an elevated BNP level as 35 pg/mL, which is a clinically meaningful cut-off value for suggesting cardiac abnormality and heart failure (12,18). We evaluated the frequency of PRCs and POAF in each group of patients with a BNP level ≥35 and <35 pg/mL. Subsequently, we sub-analyzed the BNP ≥35 pg/mL group and evaluated whether the surgical procedure, number of resected subsegments, and surgical approach influenced the development of PRCs. The number of resected subsegments in patients who underwent wedge resection was counted as 0.5, and we set the cut-off value of the resected subsegments to 5, as applied in previous studies (1,23). All patients, as well as patients who underwent anatomical pulmonary resection (except wedge resection), were analyzed.
Management of pulmonary resection
As previously described (1), lobectomy, segmentectomy, or wedge resection was decided based on the size and location of tumors and the performance status, preoperative pulmonary function, and nutritional status of patients. Curability and surgical margins were considered in each case. While patients with primary lung cancer usually underwent anatomical resection (segmentectomy or lobectomy) and lymph node dissection, some underwent wedge resection depending on the size or location of the tumor. We selected a surgical procedure to achieve an adequate surgical margin for metastatic tumors. Lymph node dissection was performed if the size of the metastatic tumor exceeded 3 cm. Although minimally invasive surgery (MIS) was planned initially, the final decision regarding the surgical approach (thoracotomy or MIS, three or four-port video-assisted thoracoscopic surgery, or five-port robot-assisted thoracoscopic surgery) was at the surgeon’s discretion, depending on factors such as tumor size and lymph node involvement.
To avoid postoperative air leaks, fibrin glue and polyglycolic acid sheets were used at the staple line or intersegmental plane as needed. Continuous suction was performed at −10 cmH2O through a 20- or 24-Fr chest drain tube placed in the thorax.
In segmentectomy, the intersegmental plane was identified by either intravenous indocyanine green injection, inflation-deflation, or both. A stapler and electrocautery were used for generally and partially dissecting the intersegmental plane, respectively.
Statistical analysis
Categorical variables were analyzed using Chi-squared or Fisher’s exact test, and continuous variables using the Student’s t-test. Univariate and multivariate logistic regression was used to determine the risk factors for PRCs in analyses.
All statistical analyses were performed using SPSS (version 29; Chicago, IL, USA) and STATA (version 18.0; Stata Corp., College Station, TX, USA). Statistical significance was set at P<0.05 for all analyses.
Results
Patient characteristics
Figure S1 shows the patient selection flowchart. This study included 383 patients. Overall, 120 (31%) patients had BNP ≥35 pg/mL and 263 (69%) had BNP <35 pg/mL, respectively. Figure S2 shows a histogram of BNP levels in all patients; the median BNP level was 23.7 pg/mL.
Table 1 shows the patient characteristics. The BNP ≥35 pg/mL group had a significantly higher median age than the BNP <35 pg/mL group (P<0.001). No significant differences were observed between the groups in terms of sex, body mass index, smoking history, chronic obstructive pulmonary disease history, or IP history; however, there was a significant difference in cardiac surgery history (P<0.001). Regarding the preoperative pulmonary function test, patients with BNP ≥35 pg/mL had a poorer median diffusing capacity of the lung for carbon monoxide (%DLco) than those with BNP <35 pg/mL (P=0.03). However, regarding the surgical approach, the thoracotomy rate was higher among patients who had BNP levels ≥35 pg/mL compared with those who had BNP <35 pg/mL (P=0.003).
Table 1
| Characteristics | All cases (n=383) | BNP ≥35 pg/mL (n=120) | BNP <35 pg/mL (n=263) | P value |
|---|---|---|---|---|
| Age (years) | 70±10.2 | 73.6±6.5 | 68±11.1 | <0.001 |
| Sex | 0.74 | |||
| Male | 231 [60] | 74 [62] | 157 [60] | |
| Female | 152 [40] | 46 [38] | 106 [40] | |
| BMI (kg/m2) | 22.9±3.4 | 22.6±3.6 | 23±3.3 | 0.20 |
| Smoking history | 0.91 | |||
| No | 227 [59] | 72 [60] | 155 [59] | |
| Yes | 156 [41] | 48 [40] | 108 [41] | |
| History of COPD | 0.25 | |||
| No | 287 [75] | 85 [71] | 202 [77] | |
| Yes | 96 [25] | 35 [29] | 61 [23] | |
| History of IP | 0.56 | |||
| No | 317 [83] | 97 [81] | 220 [84] | |
| Yes | 66 [17] | 23 [19] | 43 [16] | |
| History of cardiac surgery | <0.001 | |||
| No | 348 [91] | 91 [76] | 257 [98] | |
| Yes | 35 [9] | 29 [24] | 6 [2] | |
| Respiratory function test | ||||
| %FEV1.0 (%) | 98.5±20 | 96.9±20.8 | 99.3±20.8 | 0.27 |
| %DLCO (%) | 84.1±23.5 | 80.2±22.6 | 86±23.7 | 0.03 |
| Diagnosis | 0.25 | |||
| Primary lung cancer | 318 [83] | 102 [85] | 216 [82] | |
| Metastatic tumor | 65 [17] | 18 [15] | 47 [18] | |
| Surgical approach | 0.003 | |||
| MIS | 334 [87] | 95 [79] | 239 [91] | |
| Thoracotomy | 49 [13] | 25 [21] | 24 [9] | |
| Surgical procedure | 0.46 | |||
| Wedge | 68 [18] | 20 [17] | 48 [18] | |
| Segmentectomy | 135 [35] | 38 [31] | 97 [37] | |
| Lobectomy | 180 [47] | 62 [52] | 118 [45] | |
| Resected side | 0.91 | |||
| Right | 220 [57] | 68 [57] | 152 [58] | |
| Left | 163 [43] | 52 [43] | 111 [42] |
Data are expressed as number [%] and mean ± SD. BNP, brain natriuretic peptide; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IP, interstitial pneumonia; FEV1.0, forced expiratory volume in one second; DLCO, diffusing capacity of the lung for carbon monoxide; MIS, minimally invasive surgery; SD, standard deviation.
The risk of PRCs and POAF based on preoperative BNP levels
First, we compared the frequency of PRCs in the BNP ≥35 pg/mL and BNP <35 pg/mL groups. In all 383 patients, the incidence of PRCs was significantly higher in patients with BNP ≥35 pg/mL than in those with BNP <35 pg/mL (14% vs. 24%, P=0.02) (Figure 1A). Figure 1B shows the incidence of PRCs based on BNP levels in patients who underwent anatomical pulmonary resection; 17% (n=36/215) of patients with BNP <35 pg/mL and 28% (n=28/100) of those with BNP ≥35 pg/mL showed PRCs (P=0.02).

Subsequently, we compared POAF frequency in the BNP ≥35 pg/mL and BNP <35 pg/mL groups. Figure S3A shows POAF incidence based on BNP levels in all patients. POAF was observed in 6% (n=16/263) and 12% (n=14/120) of patients in the BNP <35 pg/mL and BNP ≥35 pg/mL groups, respectively (P=0.07). Figure S3B shows POAF incidence based on BNP levels in patients who underwent anatomical pulmonary resection; 7% (16/215) of patients in the BNP <35 pg/mL group and 14% (14/100) in the BNP ≥35 pg/mL group showed POAF (P=0.10).
The types of PRCs between patients in both groups are presented in Table 2 (all patients) and Table S1 (patients who underwent anatomical resection). No significant differences were observed in the development of prolonged air leakage, pneumonia, atelectasis, acute exacerbation of IP, empyema, acute respiratory distress syndrome, bronchopleural fistula, or intrapulmonary hemorrhage. However, rates of pleural effusion, which required invasive procedures (including aspiration to the thoracic cavity), and respiratory failure tended to be higher (approximately two-fold) in patients in the BNP ≥35 pg/mL group than in those in the BNP <35 pg/mL group; however, the difference was insignificant.
Table 2
| Complications | BNP ≥35 pg/mL (n=120) | BNP <35 pg/mL (n=263) |
|---|---|---|
| Prolonged air leakage | 8 [7] | 13 [5] |
| Pneumonia | 5 [4] | 11 [4] |
| Pleural effusion | 8 [7] | 9 [3] |
| Respiratory failure | 5 [4] | 4 [2] |
| Atelectasis | 3 [3] | 3 [1] |
| Acute exacerbation of IP | 2 [2] | 4 [2] |
| Empyema | 2 [2] | 1 [0.4] |
| ARDS | 2 [2] | 1 [0.4] |
| Asthma | 1 [1] | 0 |
| Intrapulmonary hemorrhage | 1 [1] | 1 [0.4] |
| Bronchopleural fistula | 2 [2] | 0 |
Data are expressed as number of patients [%]. ARDS, acute respiratory distress syndrome; BNP, brain natriuretic peptide; IP, interstitial pneumonia.
Figure S4A shows PRC incidence among all patients who underwent MIS (except thoracotomy) (n=334). PRCs were observed in 14% (n=30/239) and 20% (n=19/95) of patients with BNP <35 and ≥35 pg/mL, respectively (P=0.09). Figure S4B shows PRC incidence among patients who underwent anatomical resection (except wedge resection) and MIS (n=268). PRCs were observed in 15% (n=29/192) and 25% (n=19/76) of patients with BNP <35 and ≥35 pg/mL, respectively (P=0.06).
The risk of PRCs in patients with a preoperative BNP level ≥35 pg/mL
Figure 2A shows PRC incidence based on the surgical procedure in all patients in the BNP ≥35 pg/mL group (n=120). PRCs were observed in 14% (n=8/58) and 34% (n=21/62) of patients who underwent sublobar resection and lobectomy, respectively (P=0.01). Figure 2B shows PRC incidence based on the surgical procedure in the BNP ≥35 pg/mL group for patients who underwent anatomical resection (except wedge resection) (n=100). PRCs were observed in 18% (n=7/38) and 34% (n=21/62) of patients who underwent segmentectomy and lobectomy, respectively (P=0.11).
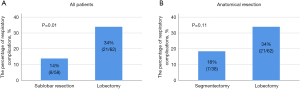
Figure 3A shows the incidence of PRCs based on the number of resected subsegments in all patients in the BNP ≥35 pg/mL group (n=120). PRCs were observed in 15% (n=9/59) and 33% (n=20/61) of patients with <5 and ≥5 resected subsegments, respectively (P=0.03). Figure 3B shows PRC incidence based on the surgical procedure in the BNP ≥35 pg/mL group for patients who underwent anatomical resection (n=100). PRCs were observed in 21% (n=8/39) and 33% (n=20/61) of patients with <5 and ≥5 resected subsegments (P=0.25).

Figure S5A shows PCR incidence based on the surgical approach among all patients in the BNP ≥35 pg/mL group (n=120). PRCs were observed in 20% (n=19/95) and 40% (n=10/25) of patients who underwent MIS and thoracotomy, respectively (P=0.06). Figure S5B shows PRC incidence based on the surgical approach in patients in the BNP ≥35 pg/mL group who underwent anatomical resection (n=100). PRCs were observed in 25% (n=19/76) and 38% (n=9/24) of patients who underwent MIS and thoracotomy, respectively (P=0.30).
Risk factors of PRCs
Table 3 shows the univariate and multivariate analysis results of the preoperative clinical parameters of PRCs for the entire cohort. Age [odds ratio (OR): 1.939, 95% confidence interval (CI): 1.097–3.427], male sex (OR: 4.006, 95% CI: 2.021–7.94), FEV1.0% <70% (OR: 2.698, 95% CI: 1.546–4.709), IP history (OR: 2.821, 95% CI: 1.538–5.173), and a preoperative BNP level ≥35 pg/mL (OR: 1.947, 95% CI: 1.13–3.352) were significantly associated with PRCs in the univariate analysis. Moreover, multivariate analysis identified male sex (OR: 2.858, 95% CI: 1.363–5.994), IP history (OR: 2.002, 95% CI: 1.052–3.807), and a preoperative BNP level ≥35 pg/mL (OR: 1.838, 95% CI: 1.032–3.273) as factors significantly associated with PRCs.
Table 3
| Characteristics | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age ≥72 (vs. <72 years old) | 1.939 | 1.097–3.427 | 0.02 | 1.312 | 0.711–2.421 | 0.39 | |
| Male sex (vs. female) | 4.006 | 2.021–7.94 | <0.001 | 2.858 | 1.363–5.994 | 0.005 | |
| FEV1.0% <70% (vs. ≥70%) | 2.698 | 1.546–4.709 | <0.001 | 1.665 | 0.909–3.05 | 0.10 | |
| IP history (vs. none) | 2.821 | 1.538–5.173 | <0.001 | 2.002 | 1.052–3.807 | 0.03 | |
| BMI ≥22 (vs. <22 kg/m2) | 0.665 | 0.39–1.133 | 0.13 | – | – | – | |
| BNP ≥35 (vs. <35 pg/mL) | 1.947 | 1.13–3.352 | 0.02 | 1.838 | 1.032–3.273 | 0.04 | |
PRCs, postoperative respiratory complications; FEV1.0, forced expiratory volume in one second; IP, interstitial pneumonia; BMI, body mass index; BNP, brain natriuretic peptide; OR, odds ratio; CI, confidence interval.
Discussion
This study revealed that patients with a preoperative BNP level ≥35 pg/mL, a cut-off value for suggesting cardiac abnormality, diagnosing heart failure, or both, may be at risk for PRCs. In addition, preserving lung parenchyma may reduce the incidence of PRCs in patients with a BNP level ≥35 pg/mL. We emphasize the importance of evaluating preoperative BNP levels before pulmonary surgery to predict PRCs.
We assessed whether the preoperative BNP level was a predictive factor for PRCs. An elevated BNP level (BNP ≥35 pg/mL) is a proposed contemporary universal criterion for diagnosing heart failure (18). This cut-off value could be useful for predicting PRCs in our study, suggesting that patients with possible heart failure preoperatively were more likely to develop PRCs. Some reports have referred to the relationship between POAF and preoperative BNP levels, including our previous report (20,21). However, few studies have evaluated the relationship between postoperative complications and preoperative BNP levels (24-26). Nojiri et al. investigated 80 patients aged ≥75 years who underwent pulmonary resection for lung cancer. They concluded that preoperative BNP level could be useful in predicting postoperative cardiopulmonary complications in elderly patients (24). Although the number of patients is relatively small and limited to elderly patients, their finding supports that of our study. Leuchte et al. suggested BNP as a prognostic marker and screening parameter for significant pulmonary hypertension in chronic lung disease (27). Thus, BNP evaluation is likely useful in assessing lung disease progression.
Although statistical differences were not identified owing to the small number of patients, the incidence rates of pleural effusion and respiratory failure tended to be higher in patients with BNP levels ≥35 pg/mL than in those with levels <35 pg/mL. All patients who developed pleural effusion required invasive treatment, such as reinsertion of a chest drainage tube or thoracentesis. Conversely, no differences were observed in other major respiratory complications, such as pneumonia or prolonged air leakage, between the two groups.
In patients with preoperative BNP levels ≥35 pg/mL, the occurrence rate of PRCs tended to be higher in those who underwent lobectomy (with ≥5 resected subsegments) and thoracotomy. We suggest that lung parenchyma preservation and MIS may be important factors in the short-term prognosis of pulmonary resection. Although curability should be emphasized, less invasive surgeries should be considered for patients with elevated preoperative BNP levels. On the other hand, the incidence rates of PRCs were higher in patients with BNP ≥35 pg/mL even after limiting analysis to patients who underwent MIS (excluding thoracotomy). Therefore, we suggest that patients with elevated BNP levels should be closely monitored regardless of the surgical approach.
We also evaluated the relationship between BNP levels and POAF. Some studies reported that preoperative BNP levels are associated with a significantly higher risk of POAF after pulmonary resection (20,21); this was not the case in this study. However, the incidence of POAF tended to be higher in patients with BNP levels ≥35 pg/mL than in those with levels <35 pg/mL. We previously reported that POAF is a risk factor for predicting 1-year non-cancer-related adverse events (20). Preventing and predicting POAF is highly likely to lead to further prognostic evaluations; therefore, evaluating preoperative BNP levels at the point of POAF prediction, similar to PRC prediction, is crucial.
This study had some limitations. First, this was a retrospective, single-institute study with a small sample size. In addition, the exclusion of 428 patients due to incomplete data might have introduced selection bias. Second, while a BNP cut-off value of 35 pg/mL indicates cardiac abnormality and heart failure, it remains unclear whether this value is the most appropriate for predicting PRCs. However, similar results were observed when PRCs were evaluated using a median BNP level of 23.7 pg/mL, suggesting that highly elevated preoperative BNP levels could predict PRCs. Third, preoperative echocardiography to assess cardiac structure and function was not included in this study. Evaluating the correlation between the actual ejection fraction and BNP levels, as well as ejection fraction changes before and after pulmonary resection, could enable further advanced studies. Fourth, preoperative interventions for heart failure were not considered in this study, which may have affected the results. Finally, we did not assess postoperative BNP levels after pulmonary resection. We hypothesized that postoperative elevation of BNP levels is related to long-term survival and short-term complications and that the elevation would be strongly related to the resected lung volume. Therefore, we conducted a prospective study to evaluate changes in pre- to postoperative BNP levels and their relationship with long-term survival. However, this is the first study to report the relationship between preoperative BNP levels and PRCs. Further prospective studies to evaluate changes in pre- to postoperative BNP levels and their relationship with short- and long-term survival are needed.
Conclusions
We emphasized that evaluating preoperative BNP levels before pulmonary resection is important for predicting PRCs. A BNP level ≥35 pg/mL, a novel cut-off value for suggesting a structural or functional cardiac abnormality, or both, and used in heart failure diagnosis, may be a valuable tool for identifying patients with elevated risk for PRCs before pulmonary surgery. Based on the results of this study, further prospective studies should be conducted to clarify the relationship between BNP levels before and after pulmonary resection and short- and long-term prognoses.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1248/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1248/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1248/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1248/coif). K.K. and his department have received research grants or contracts from Kowa Co., Ltd., AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Novo Nordisk Pharma Ltd., Amgen, Janssen Pharmaceutical K.K., Parexel International Inc., Astellas Pharma Inc., Otsuka Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., Nippon Boehringer Ingelheim Co., Ltd., Kyowa Kirin Co., Ltd., Medtronic Japan Co. Ltd., Boston Scientific Japan K.K., Abbott Japan LLC, Japan Lifeline Co., Ltd., Biotronik Japan, Terumo Corporation, Nipro Corporation, Cordis Japan G.K., and have consulted for Astellas Pharma Inc., AstraZeneca, MSD, Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Kowa Co., Ltd., Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd. (Sumitomo Pharma Co., Ltd.), Mitsubishi Tanabe Pharma Corp., Eli Lilly Japan, Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma, Novo Nordisk Pharma Ltd., Bayer Yakuhin, Ltd., Pfizer Japan Inc., Janssen Pharmaceutical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the research ethics of Shinshu University (No. 4938). Owing to the retrospective nature of the study, written informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miura K, Ide S, Minamisawa M, et al. Sublobar resection or lobectomy and postoperative respiratory complications in emphysematous lungs. Eur J Cardiothorac Surg 2024;65:ezae061. [Crossref] [PubMed]
- Makino Y, Shimada Y, Hagiwara M, et al. Assessment of emphysema severity as measured on three-dimensional computed tomography images for predicting respiratory complications after lung surgery. Eur J Cardiothorac Surg 2018;54:671-6. [Crossref] [PubMed]
- Tane S, Nishikubo M, Kitazume M, et al. Cluster analysis of emphysema for predicting pulmonary complications after thoracoscopic lobectomy. Eur J Cardiothorac Surg 2021;60:607-13. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgeries in Japan during 2021 : Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2024;72:254-91. [Crossref] [PubMed]
- Shiono S, Yoshida J, Nishimura M, et al. Risk factors of postoperative respiratory infections in lung cancer surgery. J Thorac Oncol 2007;2:34-8. [Crossref] [PubMed]
- Simonsen DF, Søgaard M, Bozi I, et al. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med 2015;109:1340-6. [Crossref] [PubMed]
- Guerrera F, Brunelli A, Falcoz PE, et al. Video-assisted thoracic surgery or thoracotomy for lung cancer surgery in obese patients? An analysis of the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2023;64:ezad368. [Crossref] [PubMed]
- Ohsawa M, Tsutani Y, Fujiwara M, et al. Predicting Severe Postoperative Complication in Patients With Lung Cancer and Interstitial Pneumonia. Ann Thorac Surg 2020;109:1054-60. [Crossref] [PubMed]
- Gómez-Hernández MT, Forcada C, Varela G, et al. Operating time: an independent and modifiable risk factor for short-term complications after video-thoracoscopic pulmonary lobectomy. Eur J Cardiothorac Surg 2022;62:ezac503. [Crossref] [PubMed]
- Shiono S, Abiko M, Sato T. Postoperative complications in elderly patients after lung cancer surgery. Interact Cardiovasc Thorac Surg 2013;16:819-23. [Crossref] [PubMed]
- Kim HJ, Cha SI, Kim CH, et al. Risk factors of postoperative acute lung injury following lobectomy for nonsmall cell lung cancer. Medicine (Baltimore) 2019;98:e15078. [Crossref] [PubMed]
- Tsutsui H, Albert NM, Coats AJS, et al. Natriuretic Peptides: Role in the Diagnosis and Management of Heart Failure: A Scientific Statement From the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. J Card Fail 2023;29:787-804. [Crossref] [PubMed]
- Mäntymaa P, Vuolteenaho O, Marttila M, et al. Atrial stretch induces rapid increase in brain natriuretic peptide but not in atrial natriuretic peptide gene expression in vitro. Endocrinology 1993;133:1470-3. [Crossref] [PubMed]
- Nakagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an "emergency" cardiac hormone against ventricular overload. J Clin Invest 1995;96:1280-7. [Crossref] [PubMed]
- Potter LR, Yoder AR, Flora DR, et al. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol 2009;341-66. [Crossref] [PubMed]
- Kuwahara K. The natriuretic peptide system in heart failure: Diagnostic and therapeutic implications. Pharmacol Ther 2021;227:107863. [Crossref] [PubMed]
- Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res 2006;69:318-28. [Crossref] [PubMed]
- Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352-80. [Crossref] [PubMed]
- Shen L, Jhund PS, Anand IS, et al. Incidence and Outcomes of Pneumonia in Patients With Heart Failure. J Am Coll Cardiol 2021;77:1961-73. [Crossref] [PubMed]
- Eguchi T, Ide S, Matsuoka S, et al. Predicting 1-year non-cancer-related adverse events after lung resection. Interdiscip Cardiovasc Thorac Surg 2023;37:ivad199. [Crossref] [PubMed]
- Zhang L, Li X, Wu H, et al. Risk factors associated with atrial fibrillation following lung cancer surgery: A multi-center case-control study. Asian J Surg 2024;47:176-83. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Nomori H, Shiraishi A, Yamazaki I, et al. Extent of Segmentectomy That Achieves Greater Lung Preservation Than Lobectomy. Ann Thorac Surg 2021;112:1127-33. [Crossref] [PubMed]
- Nojiri T, Inoue M, Yamamoto K, et al. B-type natriuretic Peptide as a predictor of postoperative cardiopulmonary complications in elderly patients undergoing pulmonary resection for lung cancer. Ann Thorac Surg 2011;92:1051-5. [Crossref] [PubMed]
- Singh A, Kumar A, Hai AA, et al. Serum B-type natriuretic peptide levels (BNP) can be used as a predictor of complications in patients undergoing non-cardiac surgery: a prospective observational study. Open Heart 2023;10:e002256. [Crossref] [PubMed]
- Fox AA, Shernan SK, Collard CD, et al. Preoperative B-type natriuretic peptide is as independent predictor of ventricular dysfunction and mortality after primary coronary artery bypass grafting. J Thorac Cardiovasc Surg 2008;136:452-61. [Crossref] [PubMed]
- Leuchte HH, Baumgartner RA, Nounou ME, et al. Brain natriuretic peptide is a prognostic parameter in chronic lung disease. Am J Respir Crit Care Med 2006;173:744-50. [Crossref] [PubMed]


