Conservatism strikes back: later is better than earlier dialysis for acute kidney injury
Acute kidney injury (AKI) is not a disease but rather a clinical syndrome with multiple etiologies, and often is the result of multiple concurrent insults to the kidney. The definition of AKI is any of the following—increase in serum creatinine by 0.3 mg/dL within 48 hours; or increase in serum creatinine to 1.5 times baseline level, which is known or presumed to have occurred within the past week; or Urine volume of less than 0.5 mL/kg/h for 6 hours. Quite frequently an AKI event occurs in the setting of an underlying CKD, so that it is said that AKI is a marker of preexisting CKD (1,2). AKI per se is implicated for causing or accelerating CKD, although debate about the role of AKI events in long-standing CKD progression rate is ongoing (3) According to the Kidney Disease Improving Global Outcome (KDIGO) guidelines, patients with AKI should be monitored for serum creatinine and urine output to stage the severity, and when reaching stage 2 (i.e., a rise in creatinine of more than 1.5–1.9 times compared to base line, or decrease in urine output to less than 0.5 mg/kg/h in 6–12 hours) the clinician may consider renal replacement therapy (RRT) which often requires admission or transfer to the intensive care unit setting to initiate some type of dialysis treatment including intermittent hemodialysis or continuous hemodiafiltration therapy.
Acute tubular necrosis (ATN) is the most common clinical diagnosis of AKI in hospitalized patients. ATN is often the result of persistence or worsening pre-renal azotemia in that the renal perfusion is compromised leading to ischemic injury to the tubules followed by their annihilation. Whereas the tubular cells almost always eventually recover and regenerate as shown by mitotic figures in kidney biopsy, there may be need to support the patient during the AKI event with no urine output. Hence, RRT is embarked on in form of intermittent hemodialysis or continuous hemodiafiltration. The indications for RRT in the setting of an oligoanuric AKI, which is often due to ATN, include life threatening situations such as hyperkalemia, acidemia, lung edema and uremia complications such as pericarditis, change in mental state (uremic encephalopathy) or bleeding (see Table 1). Some of the none-life-threatening situations in an AKI that may benefit from dialysis therapy include solute control as in tumor lysis syndrome, acid base disorders such as, e.g., enabling permissive hypercapnia in a ventilated patient, and most importantly—volume overload. Accumulation of fluid is a critical consequence of oligoanuric AKI and could lead to complications. Controlling fluid overload may permit feeding without limitation and adequate drug therapy. This is often attempted by administration of loop diuretics such as intermittent intravenous or continuous infusion of frusemide, bumetanide or torsemide, in an attempt to convert oligoanuric AKI to non-oliguric AKI for better fluid management. However, some observational studies have shown worse AKI outcome after loop diuretics use (4) which may be related to confounding by indication, in that patients with poor prognosis are more likely to receive this treatment (5).
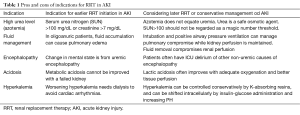
Full table
In many critical circumstance some type of RRT is initiated during the course oligoanuric AKI especially with multiple organ system failure. The timing of RRT should bring into consideration the broad clinical context, when RRT could make a difference in patient outcomes including both patient and kidney survival, rather than relating solely to laboratory data or removing “retained fluid”. An earlier start of dialysis in AKI has the advantage of avoiding volume overload, improving acid-base and electrolyte balance, preventing more severe AKI complications and enhancing toxin removal. However, an early dialysis initiation exposes the patient to unnecessary risks related to the dialysis catheter placement and complications of dialysis therapy including infection, volume depletion, low blood pressure episodes, compromised renal perfusion and other ischemic events. These events may prolong or worsen ATN, which may hinder the chance of faster recovery of renal structure and function.
In a recent single-center randomized clinical trial (RCT) of 231 critically ill patients with AKI stage 2 (≥2 times baseline or urinary output <0.5 mL/kg/h for ≥12 hours) in Germany, the early (within 8 hours of AKI diagnosis; n=112) compared with delayed (within 12 hours of stage 3 AKI or no initiation; n=119) initiation of RRT, showed reduced mortality over the first 90 days (4). An invited commentary on this study by Winkelmayer and Chertow failed to discuss scientifically as to how a modest 24-hour difference in initiation of dialysis would explains such a dramatic difference in outcome (6). In an earlier RCT by Bourman et al. in 2002 in 106 critically ill patients with AKI, who were randomized to early vs. late initiation of RRT (7), the early initiation group started RRT within 12 hours of oliguria (30 mL/h for 6 hours, not responding to diuretics or hemodynamic optimization) or creatinine clearance below 20 mL/min, while the late-initiation group started RRT when classic indications were met. The study did not find differences in ICU or hospital mortality, or in renal recovery among survivors. Of note, 16% of the patients in the delayed group were never treated with RRT.
In a 2015 study, Wald and colleagues examined patients with significant AKI but without immediate biochemical or clinical indication for RRT and randomized 101 patients to “accelerated” RRT, commencing within 12 hours, or, “standard” RRT on indication, including clinician judgement that mandated RRT (8). Thirteen of 52 patients in the standard RRT avoided RRT and survived, while six others died without receiving RRT. There was no significant difference in 90-day survival between the groups. It is overwhelming that 25% of patients receiving RRT could have survived without it, and that this therapeutic modality that was according to the clinician discretion could not alter disease course (8,9). In 2011, a meta-analysis of 15 papers, reviewed the data on this subject (10). Only two of the studies listed were RCT, and the rest were observational studies. The analysis concluded that early RRT improved mortality after 28 days; however, the selected studies were heterogeneous and the may have been associated with publication bias. Indeed, the pooled analysis of secondary outcomes was not statistically significant but described a trend in favor of early RRT in terms of renal function recovery.
In the largest ever RCT published most recently in NEJM entitled, “Initiation Strategies for Renal Replacement therapy in the Intensive Care Unit” with over 600 French patients, the investigators have sought to address the debate of timing of RRT in critically ill patients who develop AKI but who have no potentially life-threatening complication directly related to renal failure (11). The so called “Artificial Kidney Initiation in Kidney Injury” (AKIKI) study included patients from 31 ICUs in France, who were intubated and catecholamine infusion dependent. Inclusion criteria were stage 3 AKI, i.e., a serum creatinine concentration >4 mg/dL or 3 times over the baseline, oliguria for a day, or anuria for 12 hours. Patients were included if they suffered ischemic or toxic ATN, and excluded if other etiology was identified as the cause of AKI, or had urgent indication to start RRT. A total of 620 patients were randomized within 5 hours to either start RRT, choice of method was left to the discretion of clinician, or to delay treatment until an urgent indication arises (11). In the early RRT group (n=311 patients) all except six received RRT within two hours of randomization, i.e., 4.3 hours after stage 3 AKI was documented. In the late RRT group (n=308 patients), only 51% were dialyzed, after a median of 57 hours and within 4.7 hours from urgent indication. The majority of dialyzed patients, some 70%, were either oliguric or developed biochemical indication meaning a rise in BUN. The two groups did not differ in mortality rates after 60 days of follow-up. Regarding secondary outcome—the groups did not differ in length of hospital or ICU stay, intubation free days or vasopressor free days. In a post-hoc analysis comparing patients who never received RRT with those who received it, either early or late, the lowest mortality rate was found among patients who never received RRT, while the mortality was higher among patients who received late therapy. The authors explain this difference in term of illness severity—the patients that were never dialysed were the healthiest, and the patients in the late group were the most severely ill (11). As a group, the delayed RRT patients needed less dialysis treatment, but each patient needed more treatments. Patients in the delayed RRT group resumed urine output earlier than patients in the early RRT group. The delayed strategy prevented the need for dialysis in nearly half of patients, and did not affect overall mortality. Delaying dialysis however enabled faster and more uneventful kidney recovery. This, the authors conclude, could, not necessarily be interpreted as if the ‘wait and see’ approach is always better, but rather that a close surveillance is needed to identify early as possible the complications of AKI (11). In a commentary by Mehta, he highlighted that there was no difference between the groups in term of kidney recovery or the risk for CKD, and that it was surprising that intermittent dialysis was used in most patients, while continuous therapy only in 30% of patients (12).
Is adopting a conservative approach to AKI by postponing dialysis initiation a wiser decision after all? We believe that a conservative approach to AKI is a better choice. Dialysis therapy is inevitably associated with certain adverse consequences on the hemodynamics of the injured kidney (Table 1). Renal perfusion should not be compromised during AKI recovery or it may cause additional ischemic injury and prolonged ATN (Figure 1). Even in the best run ICU, dialysis and hemofiltration therapies are coined with drops in blood pressure as a result of fluid removal. Once dialysis has started the urge to remove more fluid is often uncontrollable, given the pressure by different teams including surgery, pulmonary, cardiology, etc. to “take off more fluid”, and the nephrologist or intensivist in charge of running the RRT often tends to give up to the demand. Whereas delaying RRT may lead to worsening azotemia and other metabolic deteriorations, higher serum urea level per se should never be an indication. Indeed, urea can be safely injected as an osmotic agent, e.g., in association with mannitol for management of cerebral edema (13). Fluid overload can be managed conservatively or in extreme circumstances that a patient needed to be intubated by positive airway pressure ventilation. Keeping patient on the “wet side” is not an unwise approach, and it is routinely practiced by transplant nephrologists upon placement an ATN kidney in the body of the recipient. Change in mental state in ICU is rarely if ever, clearly related to uremic encephalopathy. Perhaps the only reasonable indication for dialysis in AKI is worsening or persistent acidosis, given multi-organ consequences of metabolic acidosis, but this is mostly related to lactic acidosis (14), which should be managed by improving oxygenation and organ perfusion without dialysis, although a non-ischemic (Type B) lactic acidosis may require RRT. Current literature shows wide variations in practice in the use of RRT for AKI. Attention should focus on whether early vs. late RRT can improve patient outcome and not fluid management to make ICU colleagues happier.
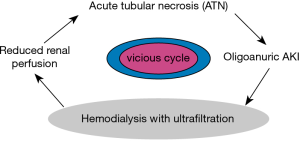
Preservation of urine output is an important prognostic indicator in the setting of AKI. In a post hoc analysis of an ATN trial, the difference in urine output 7 days after randomization corresponded to mortality difference after 28 days of follow-up (15). Patients in this study were randomized to either more or less intense dialysis treatment, and patients in the less intense dialysis group had higher urine output and greater survival. An important factor that may contribute to differences in urine output according to RRT intensity could be hemodynamic instability as a result of more frequent dialysis treatment. Frequent and intense dialysis treatment could create a vicious cycle of hemodynamic instability, diminish peripheral perfusion, and aggravate renal ischemia and injury (15-17). There is biological plausibility to suggest that preservation of urine output be an important clinical management goal. Current literature shows wide variations in practice in the use of RRT in AKI. The study by Gaudry and colleagues (11) aids clinicians in decision making regarding whether to dialysis patients in the ICU and when. Since RRT does not appear to affect mortality in short-term, the major questions should be as what the hospitalization course and the overall prognosis are and how well can conservative treatments balance the patient’s clinical need and outcome. The clinician has to bear in mind that early dialysis may prevent kidney spontaneous recovery (Figure 1), and that any CRRT poses the unnecessary risk of bloodstream infections and hypotensive episodes (Table 1). As to whether early vs late RRT can improve or confound fluid management or prevent fluid overload is an individualized question and differs case-by-case. Sometimes less (dialysis) is more. As the basic and primary directive of medicine guides us—PRIMUM NON NOCERE.
Acknowledgements
Funding: The authors are supported by research grants from the NIH/NIDDK including K24-DK091419 (KKZ), R01-DK078106 (KKZ), and philanthropist grants from Mr. Harold Simmons, Dr Joseph Lee and Mr. Louis Chang.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Zhiheng Xu (State Key Laboratory of Respiratory Disease, Guangzhou Institute of Respiratory Disease, Department of Intensive Care, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rifkin DE, Coca SG, Kalantar-Zadeh K. Does AKI truly lead to CKD? J Am Soc Nephrol 2012;23:979-84. [Crossref] [PubMed]
- Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014;371:58-66. [Crossref] [PubMed]
- Hsu CY. Yes, AKI truly leads to CKD. J Am Soc Nephrol 2012;23:967-9. [Crossref] [PubMed]
- Mehta RL, Pascual MT, Soroko S, et al. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA 2002;288:2547-53. [Crossref] [PubMed]
- Kalantar-Zadeh K. Diuretics in critically ill patients with acute renal failure. JAMA 2003;289:1380; author reply 1380-1. [Crossref] [PubMed]
- Chertow GM, Winkelmayer WC. Early to Dialyze: Healthy and Wise? JAMA 2016;315:2171-2. [Crossref] [PubMed]
- Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, et al. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med 2002;30:2205-11. [Crossref] [PubMed]
- Wald R, Adhikari NK, Smith OM, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int 2015;88:897-904. [Crossref] [PubMed]
- Prowle JR, Davenport A. Does early-start renal replacement therapy improve outcomes for patients with acute kidney injury? Kidney Int 2015;88:670-3. [Crossref] [PubMed]
- Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care 2011;15:R72. [Crossref] [PubMed]
- Gaudry S, Hajage D, Schortgen F, et al. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med 2016;375:122-33. [Crossref] [PubMed]
- Mehta RL. Renal-Replacement Therapy in the Critically Ill--Does Timing Matter? N Engl J Med 2016;375:175-6. [Crossref] [PubMed]
- Otvos B, Kshettry VR, Benzel EC. The history of urea as a hyperosmolar agent to decrease brain swelling. Neurosurg Focus 2014;36:E3. [Crossref] [PubMed]
- Kalantar-Zadeh K. Case 23-2013: a 54-year-old woman with metformin toxicity. N Engl J Med 2013;369:1769. [PubMed]
- Mc Causland FR, Asafu-Adjei J, Betensky RA, et al. Comparison of Urine Output among Patients Treated with More Intensive Versus Less Intensive RRT: Results from the Acute Renal Failure Trial Network Study. Clin J Am Soc Nephrol 2016;11:1335-42. [Crossref] [PubMed]
- VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008;359:7-20. [Crossref] [PubMed]
- Mathew A, Obi Y, Rhee CM, et al. Treatment frequency and mortality among incident hemodialysis patients in the United States comparing incremental with standard and more frequent dialysis. Kidney Int 2016. [Epub ahead of print]. [Crossref] [PubMed]


