
The blind spots on chest computed tomography: what do we miss
Introduction
As the most traditional imaging method for diagnosing lung diseases, the chest radiograph is a two-dimensional projection that captures information from a three-dimensional volume, thereby leading to the superimposition of anatomical structures. Therefore, chest radiograph often leads to missed diagnosis of lesions. The common sites of undetected pulmonary nodules and disease, often referred to as “blind spots”, are key teaching points of chest radiography for medical students and imaging beginners. The most common blind areas of chest radiographs include the apical area of lung, hilar area of lung, projection area after heart shadow, retrosternal space, subphrenic projection area (1,2). With the widespread increase in the use of chest computed tomography (CT), the importance of chest radiography has gradually decreased. Because chest CT avoids anatomic overlap and improves spatial resolution, the performance of detecting chest lesions, especially pulmonary nodules, is significantly improved. In comparison to chest radiography, chest CT provides enhanced visualization and characterization of thoracic anatomy and abnormalities. Even low-dose CT screening is associated with a 20% reduction in lung cancer mortality compared with chest radiography (3), Therefore, chest CT examination has become increasingly common as the initial imaging modality in assessing patients with pulmonary symptoms, helping us increase the diagnostic accuracy of related diseases, such as pulmonary embolism and coronavirus disease 2019 (COVID-19) (4-6).
Despite its widespread clinical usefulness, the interpretation of chest CT scans still involves errors. In addition, chest CT images may possess inherent blind spots, leading to potential oversight of abnormalities. Certain errors, such as the failure to detect lung nodules indicative of early-stage lung cancer or the oversight of a pneumothorax necessitating immediate intervention, can have detrimental implications for patients. These types of errors frequently serve as the basis for medical malpractice lawsuits (7,8). Understanding the commonly overlooked findings of chest CT scans, their underlying causes, and the potential repercussions is crucial for devising strategies aimed at minimizing and addressing these errors.
The special features in this article discuss commonly missed blind spots on chest CT and provide actual examples of clinical significance. In addition, we present proposed solutions to effectively address these errors, thereby providing valuable support to radiologists in their clinical practice.
The blind spots on chest CT
The common blind spots on chest CT are summarized and illustrated in Figure 1. These blind spots are detailed in the following paragraphs.
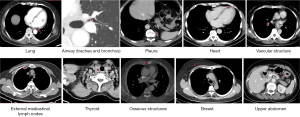
Lung
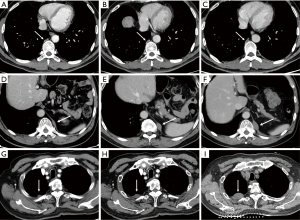
Due to the presence of air, the lungs have a naturally good contrast, and the contrast of lung lesions is good. In contrast to chest radiography, CT scans provide enhanced visualization of pulmonary structures and abnormalities by eliminating the interference caused by overlapping structures. Nonetheless, despite these advantages, lung nodules on chest CT scans can still have a chance for being overlooked. Studies examining patients with surgically removed pulmonary nodules have reported that CT scans can fail to identify up to 27–47% of these nodules (9,10), in which inferior lobe nodules (73%) and ground glass nodules (91%) are the majority (11,12). Missing diagnosis of lung cancer and lung metastasis can have serious consequences for patients. Small metastatic tumors, in particular, are highly susceptible to being overlooked, thereby influencing the formulation of effective diagnostic and treatment strategies for the patients (Figure 2).
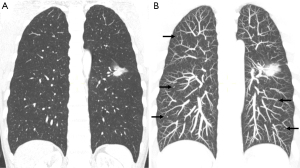
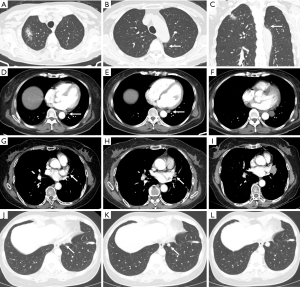
There are still areas within the pulmonary parenchyma that have been consistently identified as blind spots, where missed lesions are more frequently observed (14). The central areas within the pulmonary parenchyma, such as the perihilar and paramediastinal regions, are more prone to harbor missed lesions. It is reported that central lung cancers, including perihilar and para mediastinal lung regions are at higher risk of being overlooked (11,15). The perihilar area is affected by the pulmonary hilar and bronchial vascular bundles. The paramediastinal area, para-aortic area, pericardiac area and para-diaphragm area are all easily neglected because they lie on the edge of the lung field close to the mediastinum, aorta, and pericardium respectively (Figure 3).
Airway (trachea and bronchus)
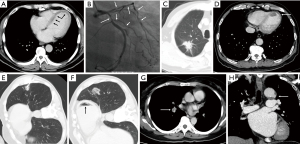
A study on missed diagnosis of lung cancers has determined that most lung cancers that have been missed on CT are endobronchial in location, with a mean diameter of 1.2 cm (11). These findings emphasize the significance of assessing the central airways to ensure the exclusion of endotracheal or endobronchial lesions. For a considerable time, the trachea has been acknowledged as a frequently overlooked blind spot or neglected area by radiologists during the interpretation of chest CT scans (16) (Figure 4). Evaluating this region is of significant importance, as tracheal abnormalities commonly manifest on chest CT scans before the onset of symptomatic presentation in patients. The primary and peripheral bronchus lesions are also easily missed due to obstructive pneumonia interference.
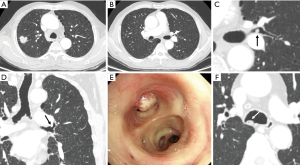
The trachea and primary bronchi are common sites of tumor development, and the most common types are squamous cell carcinoma and carcinoid, with squamous cell carcinoma and carcinoid tumors being the predominant subtypes. Specifically, they include mucoepidermoid carcinoma and adenoid cystic carcinoma. Malignant lesions within the trachea and bronchi may manifest as endoluminal nodules, with subtle wall thickening, or irregular luminal contours. A meticulous evaluation of the airways, focusing on intraluminal lesions and wall thickening, serves as a critical approach to improve the detection of bronchial and tracheal neoplasms. Benign tumors of the trachea are rare. These tumors should be distinguished from foreign bodies and intraluminal secretions (Figure 4). The aspiration of foreign bodies can lead to notable complications, including recurrent atelectasis or pneumonia. Secretions within the trachea are often located in the posterior wall of the trachea due to gravity and are often accompanied by gas. Furthermore, contrast medium could also be useful to distinguish neoplasms from secretions in airways.
Pleura
Due to their peripheral location, small pleural lesions often evade detection by radiologists. Therefore, it is imperative to thoroughly examine the pleural surface, including the diaphragmatic pleura, for any signs of nodularity during the interpretation of all chest CT scans. In addition, pleural lesions are easy to be misdiagnosed as intrapulmonary lesions due to localization errors (Figure 5), so thin-slice CT observation is very important.
The pleura is a common metastatic site for lung cancer and other malignant tumors, which indicate an advanced stage. In patients with confirmed or suspected malignancy, the presence of multiple or newly identified pleural or fissural nodules should raise consideration for pleural metastases. Vigilant scrutiny of pleural nodularity or thickening holds significant importance in patients with inexplicable pleural effusion, as it serves as a vital diagnostic criterion for malignancy. The primary lesions of the pleura include solitary fibrous tumors and mesothelioma. Mesothelioma is difficult to distinguish from metastases on CT (17).
Heart
The enhanced speed of CT scanning, which minimizes cardiac pulsation artifacts, has significantly improved the clarity of the heart in routine chest CT examinations. Given that cardiac abnormalities often lack symptoms or present with non-specific symptoms like chest pain and dyspnea, routine chest CT scans may occasionally serve as the initial diagnostic study where these incidental abnormalities are first detected. As reported in a study, a significant proportion of scans (approximately 78%) exhibited incidental cardiac findings. Surprisingly, only a minimal percentage (3%) of these findings were explicitly documented or referenced within the scope of the study (18).
Coronary calcification and cardiomegaly are very common cardiac imaging abnormalities that can be recognized on routine chest CT, both findings are associated with an increased risk of future cardiovascular events (19,20). Congenital abnormalities, such as anomalous course of coronary arteries and small septal defects can also be detected at routine enhanced chest CT although not common. Myocardial infarction can be detected occasionally according to the subtle density changes in the myocardium. Sometimes intracardiac mural thrombi can be observed on chest CT in patients with heart disease (Figure 6). For patients who underwent CT-guided biopsy, cardiac air embolism should never be neglected because this rare complication is fatal (21). Primary heart tumors are uncommon, but the cancer tends to metastasize to the pericardium. Careful inspection of the pericardium for effusion and nodular thickening is warranted, particularly in patients with known malignancy.
Vascular structure
Chest enhanced scan is a good way to show the blood vessels in the chest, most importantly the pulmonary artery and the thoracic aorta. Pulmonary embolism is the most common thoracic vascular disease and can be seen on routine chest contrast-enhanced CT incidentally appearing as the filling defect. In a retrospective study involving cancer patients who underwent chest CT, incidental pulmonary emboli were identified in 4% of cases (Figure 6). However, it was found that only 25% of these incidental pulmonary emboli were initially documented in the radiology findings (22). Common and important lesions of the aorta include penetrating aortic ulcers, dissecting, aneurysms, and vasculitides (Figure 6). Although the incidence of vascular disease is low, it has greater health risks and belongs to emergency reporting items in the radiology apartment. A thorough examination of all vascular structures is crucial to optimize detection accuracy and minimize the risk of diagnostic omissions.
External mediastinal lymph nodes
Like the lungs, the mediastinum is the focus of chest CT observations. Mediastinal lymph node partition is the key knowledge of chest CT scans, it is the basis of N stage lung cancer. Some of the lymph node areas in chest CT are not within the partition and belong to the M stage, such as the internal mammary area, pericardial diaphragm group, the supraclavicular area, paravertebral and intercostal lymph nodes (23). These areas are also often the blind spots to observe the lymph nodes (Figure 7). Precise detection of lymphadenopathy in these specific regions assumes critical importance in the staging of lung cancer, as the involvement of such nodes renders patients ineligible for surgical resection. A study utilizing PET-CT imaging has revealed a notable tendency to overlook supraclavicular lymphadenopathy during CT examinations (24). In the initial stages of a chest CT scan, the presence of numerous rounded structures, including craniocaudally oriented vessels and musculature within this region, may contribute to the inadvertent oversight of supraclavicular lymphadenopathy. Internal mammary areas are the common sites for breast cancer. In addition to lymph nodes, Pericardial cysts and thymomas can also be missed in the cardiophrenic angle region (Figure 7).
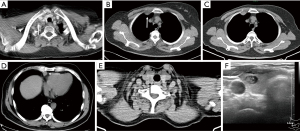
Thyroid
Chest CT includes partial thyroid, but because the thyroid belongs to the cervical organ tube, it is easy to be ignored in the chest CT scan. Incidental thyroid nodules (ITNs) on chest CT are becoming more and more common (Figure 7), with the reported prevalence over 25% of contrast-enhanced chest CT scans, but the malignancy rate of occasional thyroid nodules of chest CT ranges from only 0% to 11%. The excessive screening, diagnosis, and treatment of thyroid cancer have aroused widespread concern and debate (25,26). Despite the ongoing uncertainty surrounding the optimal management of incidentally detected thyroid nodules on CT scans, it is prudent to assign a diagnosis and provide recommendations for additional examination for all identified thyroid nodules. The CT study should first be evaluated for suspicious features associated with the ITN, including abnormal lymph nodes or signs of local invasion, neither of which is likely in a patient without thyroid-related symptoms. In general, thyroid nodules measuring 1 cm or larger, particularly those displaying invasion of neighboring structures or accompanied by adjacent lymphadenopathy (abnormal lymph nodes are defined as enlarged nodes or those with cystic change, calcification, or increased enhancement), warrant appropriate referral for ultrasound evaluation, with consideration for fine-needle aspiration (FNA) (27,28).
Osseous structures
Osseous structures of the thorax, including the vertebral bodies, ribs, sternum, clavicles, proximal portion of the humeri, and scapulae, can be involved in pathologic processes potentially visible on CT. Chest CT showed better bone than chest film. Nevertheless, subtle bony abnormalities can occasionally escape detection due to the omission of bone window images or limited time allocated by radiologists for the evaluation of multiple ribs, vertebral bodies, and other thoracic bony structures.
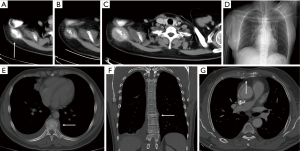
The most common and important of the missed bone diseases are fractures and metastases (29). Bone metastasis detected on chest CT is important for cancer staging (Figure 8). Among malpractice claims against radiologists, missed fractures rank as the second most frequent cause. In addition, incidental osteoporotic vertebral compression fractures are common on body CT in adults, with a prevalence of 10–35%, but it is often underreported (30-32). The utilization of sagittal multiplanar reformation images is especially valuable for expediting the evaluation of spinal alignment and vertebral body height. Conversely, the comprehensive assessment of images across all three planes (axial, sagittal, and coronal) enhances the detection of inconspicuous lesions in the clavicles, scapulae, sternum, or ribs. Although infrequent, intramedullary metastases and primary spinal neoplasms, including ependymoma and meningioma, may occur (33).
Breast
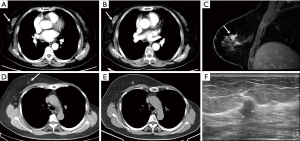
CT is not the preferred or ideal imaging technique for assessing breast tissue, but typically, a portion, if not all, of the breast tissue can be visualized on chest CT. A retrospective study found that incidental breast lesions were detected in 7.63% of female patients who underwent chest CT scans for various clinical indications. Among these incidental findings, 1.85% were identified as breast cancer (34). About 30% of incidental breast lesions detected on CT prove to be unsuspected breast cancers (35). These results underscore the critical need for careful evaluation of the breast tissue on chest CT images, as incidental findings, though infrequent, may have significant clinical implications, including the early detection of malignancies that might otherwise go unnoticed. Routine contrast-enhanced chest CT can reveal sufficient details to allow for the detection of unsuspected breast cancer (Figure 9). An irregular margin of incidental enhancing breast lesion and non-mass-like enhancement can be considered a suggestive sign of malignancy (36). Breast calcifications seen on CT are nearly all benign.
Upper abdomen
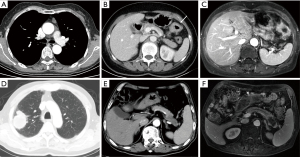
Chest CT typically captures a portion of several upper abdominal organs, including but not limited to the liver, adrenal gland, kidney, gastrointestinal tract, and pancreas (Figure 10). These are often the chest CT scans of the blind area due to the location of the last few images. The adrenal gland demonstrates heightened vulnerability to metastatic involvement originating from lung cancer (37). Therefore, even if a patient has a malignant tumor in another part of the body, when a CT scan detects an adrenal nodule incidentally, the possibility of an adrenal adenoma should be considered first, rather than metastasis. In addition asymptomatic occasional pheoc hromocytoma should be identified, because 30–50% pheochromocytomas are found incidentally (38,39). Furthermore, there is a possibility of incidental asymptomatic enteric abnormalities, such as colon or gastric cancer, being present in certain cases (Figure 10) (40).
Techniques to avoid errors
Error reasons and types
Awareness of commonly missed chest CT findings, their causes, and their consequences is important in developing approaches to reduce and mitigate these errors if managed within a just, supportive culture. Image diagnosis errors can be divided into perceptual errors and cognitive errors. Missed diagnosis is a perceptual error, accounting for the majority of imaging diagnostic errors about 60–80% (41,42), and is more likely to cause serious consequences. Limited time to read the image, lack of attention due to fatigue, lesions located in the blind area and so on are the reasons for missed diagnosis (43).
Certain perceptual errors can be attributed to cognitive biases inherent in the human brain, with the most prevalent being the satisfaction of search phenomenon. This phenomenon entails a diminished level of vigilance towards additional abnormalities after the detection of the initial lesion, leading to premature termination of examination and unintentional omission of other critical lesions. The satisfaction of search bias is a widespread perceptual error, accounting for approximately 22% of diagnostic inaccuracies (44). Of chest CT exams, 43% of patients with missed lung cancer were found to have significant distracting findings in other regions of the thorax (11). A majority of missed lung cancers were accompanied by underlying lung diseases such as tuberculosis, emphysema, or interstitial fibrosis (12). The other recognition of thinking pitfalls is inattentional blindness, which describes findings that can be missed because of their location (e.g., the last sections of the acquisition or the periphery of the field of view) or because of the unexpected nature of the findings when one is engaged in a demanding task (45,46), such as missing clavicle missed on chest radiographs in 60% of readers (47).
Techniques to avoid errors
In addition to careful review of images, there are several techniques that radiologists can use to avoid or reduce errors due to blind spots during film reading and reporting. These techniques include multiplanar reconstruction (MPR) (Figure 8), maximum intensity projection (MIP), and artificial intelligence (AI). MPR images are an important supplement to transverse CT scanning. It can help to fully observe the images from multiple angles and reduce the missed diagnosis of blind area lesions, such as sagittal reconstruction for vertebrae morphology assessment. MIP images can increase the reading speed and sensitivity of lung nodule detection, by providing fewer images and less time to review images. MIP images can improve the sensitivity of detection for small solid pulmonary nodules and increase reader confidence level (Figure 2) (48). In addition, there was a significant reduction in the number of images to review and, consecutively, in reporting time with MIP presentation (49). AI in diagnostic radiology is undergoing rapid development. Even though limitations such as the significant number of false-positive findings and the bias in different AI algorithms remain (50), AI algorithms have shown the potential to support radiologists in their day-to-day tasks of evaluating chest CT scans by automating lung nodule detection and alleviating the workload of doctors (51,52). In clinical practice, using AI software to initially detect lung nodules, followed by a manual review, helps to reduce the risk of missed diagnoses. This approach leverages the efficiency of AI for preliminary screening and the expertise of human interpretation for final assessment, significantly improving diagnostic accuracy. Many radiologists are trying to use structured reporting. Structured reports have many benefits, such as standardized format, improved report completeness, easier to compare to previous reports, facilitates decision-making for treatment, etc. The structured report format resembles a diagnostic checklist during the interpretation of CT images, aiming to mitigate the risk of inadvertent omission of clinically significant findings that are frequently missed. It has been reported that inexperienced readers using structured reporting (SR) templates from the Royal College of Radiologists (RCR-SR) and the Italian Society of Medical and Interventional Radiology (SIRM-SR) demonstrated significantly higher completeness (90% for RCR-SR and 100% for SIRM-SR) and accuracy (70% for SIRM-SR) compared to traditional non-systematic reports (NSR) (70% completeness and 55% accuracy), indicating their potential to enhance reporting skills for non-small-cell lung cancer (NSCLC) staging (53). Moreover, if a structured report is not used, radiologist can adopt a mental scheme to remember all the districts to look at.
Conclusions
This article summarizes and analyzes the blind spots and related diagnostic errors that are easily missed on chest CT through the presentation of specific cases, and then proposes feasible methods to avoid these errors in the process of image reading. This has important implications for the daily imaging diagnosis and continuing education of physicians.
Acknowledgments
Funding: This work was supported by
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1125/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1125/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gefter WB, Post BA, Hatabu H. Commonly Missed Findings on Chest Radiographs: Causes and Consequences. Chest 2023;163:650-61. [Crossref] [PubMed]
- Gefter WB, Hatabu H. Reducing Errors Resulting From Commonly Missed Chest Radiography Findings. Chest 2023;163:634-49. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Wang RC, Miglioretti DL, Marlow EC, et al. Trends in Imaging for Suspected Pulmonary Embolism Across US Health Care Systems, 2004 to 2016. JAMA Netw Open 2020;3:e2026930. [Crossref] [PubMed]
- Inui S, Gonoi W, Kurokawa R, et al. The role of chest imaging in the diagnosis, management, and monitoring of coronavirus disease 2019 (COVID-19). Insights Imaging 2021;12:155. [Crossref] [PubMed]
- Raju S, Ghosh S, Mehta AC, Chest CT. Signs in Pulmonary Disease: A Pictorial Review. Chest 2017;151:1356-74. [Crossref] [PubMed]
- Waite S, Scott J, Gale B, et al. Interpretive Error in Radiology. AJR Am J Roentgenol 2017;208:739-49. [Crossref] [PubMed]
- Brady A, Laoide RÓ, McCarthy P, et al. Discrepancy and error in radiology: concepts, causes and consequences. Ulster Med J 2012;81:3-9. [PubMed]
- Parsons AM, Ennis EK, Yankaskas BC, et al. Helical computed tomography inaccuracy in the detection of pulmonary metastases: can it be improved? Ann Thorac Surg 2007;84:1830-6. [Crossref] [PubMed]
- Peuchot M, Libshitz HI. Pulmonary metastatic disease: radiologic-surgical correlation. Radiology 1987;164:719-22. [Crossref] [PubMed]
- White CS, Romney BM, Mason AC, et al. Primary carcinoma of the lung overlooked at CT: analysis of findings in 14 patients. Radiology 1996;199:109-15. [Crossref] [PubMed]
- Li F, Sone S, Abe H, et al. Lung cancers missed at low-dose helical CT screening in a general population: comparison of clinical, histopathologic, and imaging findings. Radiology 2002;225:673-83. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Wu CC, Khorashadi L, Abbott GF, et al. Common blind spots on chest CT: where are they all hiding? Part 1-airways, lungs, and pleura. AJR Am J Roentgenol 2013;201:W533-8. [Crossref] [PubMed]
- Gurney JW. Missed lung cancer at CT: imaging findings in nine patients. Radiology 1996;199:117-22. [Crossref] [PubMed]
- Al-Qadi MO, Artenstein AW, Braman SS. The "forgotten zone": acquired disorders of the trachea in adults. Respir Med 2013;107:1301-13. [Crossref] [PubMed]
- Seely JM, Nguyen ET, Churg AM, et al. Malignant pleural mesothelioma: computed tomography and correlation with histology. Eur J Radiol 2009;70:485-91. [Crossref] [PubMed]
- Foley PW, Hamaad A, El-Gendi H, et al. Incidental cardiac findings on computed tomography imaging of the thorax. BMC Res Notes 2010;3:326. [Crossref] [PubMed]
- Jacobs PC, Gondrie MJ, Mali WP, et al. Unrequested information from routine diagnostic chest CT predicts future cardiovascular events. Eur Radiol 2011;21:1577-85. [Crossref] [PubMed]
- Wong ND, Gransar H, Shaw L, et al. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc Imaging 2009;2:319-26. [Crossref] [PubMed]
- Pigaiani N, Barbiero G, Balestro E, et al. Fatal cardiac air embolism after CT-guided percutaneous needle lung biopsy: medical complication or medical malpractice?. Forensic Sci Med Pathol 2024;20:199-204. [Crossref] [PubMed]
- Gladish GW, Choe DH, Marom EM, et al. Incidental pulmonary emboli in oncology patients: prevalence, CT evaluation, and natural history. Radiology 2006;240:246-55. [Crossref] [PubMed]
- El-Sherief AH, Lau CT, Wu CC, et al. International association for the study of lung cancer (IASLC) lymph node map: radiologic review with CT illustration. Radiographics 2014;34:1680-91. [Crossref] [PubMed]
- Vinjamuri M, Craig M, Campbell-Fontaine A, et al. Can positron emission tomography be used as a staging tool for small-cell lung cancer? Clin Lung Cancer 2008;9:30-4. [Crossref] [PubMed]
- Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer "epidemic"--screening and overdiagnosis. N Engl J Med 2014;371:1765-7. [Crossref] [PubMed]
- Ahn HS, Welch HG. South Korea's Thyroid-Cancer "Epidemic"--Turning the Tide. N Engl J Med 2015;373:2389-90. [Crossref] [PubMed]
- Ahmed S, Horton KM, Jeffrey RB Jr, et al. Incidental thyroid nodules on chest CT: Review of the literature and management suggestions. AJR Am J Roentgenol 2010;195:1066-71. [Crossref] [PubMed]
- Hoang JK, Langer JE, Middleton WD, et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR Incidental Thyroid Findings Committee. J Am Coll Radiol 2015;12:143-50. [Crossref] [PubMed]
- Donald JJ, Barnard SA. Common patterns in 558 diagnostic radiology errors. J Med Imaging Radiat Oncol 2012;56:173-8. [Crossref] [PubMed]
- Woo EK, Mansoubi H, Alyas F. Incidental vertebral fractures on multidetector CT images of the chest: prevalence and recognition. Clin Radiol 2008;63:160-4. [Crossref] [PubMed]
- Urrutia J, Besa P, Piza C. Incidental identification of vertebral compression fractures in patients over 60 years old using computed tomography scans showing the entire thoraco-lumbar spine. Arch Orthop Trauma Surg 2019;139:1497-503. [Crossref] [PubMed]
- Bartalena T, Giannelli G, Rinaldi MF, et al. Prevalence of thoracolumbar vertebral fractures on multidetector CT: underreporting by radiologists. Eur J Radiol 2009;69:555-9. [Crossref] [PubMed]
- Meyer CA, Vagal AS, Seaman D. Put your back into it: pathologic conditions of the spine at chest CT. Radiographics 2011;31:1425-41. [Crossref] [PubMed]
- Hussain A, Gordon-Dixon A, Almusawy H, et al. The incidence and outcome of incidental breast lesions detected by computed tomography. Ann R Coll Surg Engl 2010;92:124-6. [Crossref] [PubMed]
- Moyle P, Sonoda L, Britton P, et al. Incidental breast lesions detected on CT: what is their significance? Br J Radiol 2010;83:233-40. [Crossref] [PubMed]
- Lin WC, Hsu HH, Li CS, et al. Incidentally detected enhancing breast lesions on chest computed tomography. Korean J Radiol 2011;12:44-51. [Crossref] [PubMed]
- Ebbehoj A, Li D, Kaur RJ, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol 2020;8:894-902. [Crossref] [PubMed]
- Baguet JP, Hammer L, Mazzuco TL, et al. Circumstances of discovery of phaeochromocytoma: a retrospective study of 41 consecutive patients. Eur J Endocrinol 2004;150:681-6. [Crossref] [PubMed]
- Kopetschke R, Slisko M, Kilisli A, et al. Frequent incidental discovery of phaeochromocytoma: data from a German cohort of 201 phaeochromocytoma. Eur J Endocrinol 2009;161:355-61. [Crossref] [PubMed]
- Wu CC, Khorashadi L, Abbott GF, et al. Common blind spots on chest CT: where are they all hiding? Part 2, Extrapulmonary structures. AJR Am J Roentgenol 2013;201:W671-7. [Crossref] [PubMed]
- Bruno MA, Walker EA, Abujudeh HH. Understanding and Confronting Our Mistakes: The Epidemiology of Error in Radiology and Strategies for Error Reduction. Radiographics 2015;35:1668-76. [Crossref] [PubMed]
- Zhang L, Wen X, Li JW, et al. Diagnostic error and bias in the department of radiology: a pictorial essay. Insights Imaging 2023;14:163. [Crossref] [PubMed]
- Degnan AJ, Ghobadi EH, Hardy P, et al. Perceptual and Interpretive Error in Diagnostic Radiology-Causes and Potential Solutions. Acad Radiol 2019;26:833-45. [Crossref] [PubMed]
- Kim YW, Mansfield LT. Fool me twice: delayed diagnoses in radiology with emphasis on perpetuated errors. AJR Am J Roentgenol 2014;202:465-70. [Crossref] [PubMed]
- Drew T, Võ ML, Wolfe JM. The invisible gorilla strikes again: sustained inattentional blindness in expert observers. Psychol Sci 2013;24:1848-53. [Crossref] [PubMed]
- Busby LP, Courtier JL, Glastonbury CM. Bias in Radiology: The How and Why of Misses and Misinterpretations. Radiographics 2018;38:236-47. [Crossref] [PubMed]
- Potchen EJ. Measuring observer performance in chest radiology: some experiences. J Am Coll Radiol 2006;3:423-32. [Crossref] [PubMed]
- Coakley FV, Cohen MD, Johnson MS, et al. Maximum intensity projection images in the detection of simulated pulmonary nodules by spiral CT. Br J Radiol 1998;71:135-40. [Crossref] [PubMed]
- Diederich S, Lentschig MG, Overbeck TR, et al. Detection of pulmonary nodules at spiral CT: comparison of maximum intensity projection sliding slabs and single-image reporting. Eur Radiol 2001;11:1345-50. [Crossref] [PubMed]
- Sourlos N, Wang J, Nagaraj Y, et al. Possible Bias in Supervised Deep Learning Algorithms for CT Lung Nodule Detection and Classification. Cancers (Basel) 2022;14:3867. [Crossref] [PubMed]
- Chamberlin J, Kocher MR, Waltz J, et al. Automated detection of lung nodules and coronary artery calcium using artificial intelligence on low-dose CT scans for lung cancer screening: accuracy and prognostic value. BMC Med 2021;19:55. [Crossref] [PubMed]
- Gu Y, Chi J, Liu J, et al. A survey of computer-aided diagnosis of lung nodules from CT scans using deep learning. Comput Biol Med 2021;137:104806. [Crossref] [PubMed]
- Cereser L, Cortiula F, Simiele C, et al. Assessing the impact of structured reporting on learning how to report lung cancer staging CT: A triple cohort study on inexperienced readers. Eur J Radiol 2024;171:111291. [Crossref] [PubMed]


