
Thoracoscopic anatomical sublobar resection for deep interlobar lung cancer with fused fissure
Highlight box
Key findings
• Anatomical resection of part of the adjacent lobe provides a wider surgical margin and superior local tumor control compared to non-anatomical extended resection for the removal of deep interlobar lung cancer with fused fissures.
What is known and what is new?
• The deep location and fused fissures pose two significant challenges: precise localization of non-palpable nodules and complicating decisions regarding the extent of resection.
• Anatomical resection of part of the adjacent lobe has been successfully applied to this population, offering comparable perioperative outcomes, providing a more adequate margin, and reducing the need for preoperative localization.
What is the implication, and what should change now?
• Anatomical resection of partial of the adjacent lobe is a feasible surgical strategy for deep interlobar lung cancer with fused fissures.
Introduction
The management of non-small cell lung cancer (NSCLC) located deeply within fused fissures remains a topic of controversy. The deep location and fused fissure may pose two significant challenges in this scenario, hindering precise localization of the non-palpable nodules during surgery and determination of its lobar location from the preoperative computed tomography (CT) scans, thereby complicating decisions regarding the extent of resection. Mistaking the deep interlobar lung cancer with fused fissure (DILCFF) could compromise the surgical margin or result in unnecessary resection of pulmonary parenchyma.
Traditionally, the management for such lung cancers typically involved pneumonectomy of the left side or bilobectomy of the right side to ensure complete resection (1). However, the survival of patients after pneumonectomy was worse than those with bilobectomy or lobectomy plus partial adjacent lobe resection (LPR) (2). Furthermore, the lobectomy plus LPR had a lower occurrence of immediate surgical morbidity than pneumonectomy or bilobectomy (odds ratio, 0.13; 95% confidence interval: 0.02–0.74; P=0.02), and it did not demonstrate any significant association with an increased risk of recurrence or a reduction in survival time (3). The LPR could be the non-anatomical extended resection (NER) or the anatomical (sub)segmentectomy of the adjacent lobe. However, no comparative studies to date have been conducted to evaluate the outcomes of the anatomical versus non-anatomical resections (ARs) of the adjacent lobe.
The consideration of oncological outcomes arises when contemplating a lesser resection of the adjacent lobes. Patients with small, peripherally located early-stage NSCLC, who underwent sublobar resections, exhibited non-inferior overall survival (OS) and disease-free survival (DFS) compared to those after lobar resection (4,5). Notably, as emphasized by Rusch, the margin of resection is a crucial aspect that surgeons must meticulously consider in sublobar resection (6). Regarding the non-anatomical wedge resection, the challenge persists with attempt to obtain a safe margin for nodules located in the base of the lung, deep in the fissure, or on the large ovoid surface (7). In the DILCFF setting, the nodule may be deeply located in the fuse fissures and the removal of it with a safe margin is challenging.
The aims of this study were to evaluate the technical feasibility of performing an AR of the adjacent lobe with a safe margin for cT1N0M0 DILCFFs, and to compare the recurrence patterns between anatomical and non-ARs. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-84/rc).
Methods
Patients
This study was approved by The First Affiliated Hospital of Nanjing Medical University Institutional Review Board (No. 2022-SR-760) and waiver of consent was granted due to its retrospective nature. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Retrospective analysis was conducted on cT1N0M0 NSCLC patients who underwent complete resection at The First Affiliated Hospital of Nanjing Medical University from January 2013 to December 2022. The eligible patients should have a solitary nodule that was deeply located within fused fissures between adjacent lobes. Exclusion criteria were: (I) patients with tumors invading adjacent lobe through complete fissure; (II) patients with peripherally located tumors within incomplete fissures; (III) patients who received any neoadjuvant therapy or had a history of other malignancies in the past 5 years.
We restaged all patients using the tumor node metastasis (TNM) Classification of Malignant Tumors 8th edition (8). The clinical T stage was classified according to the solid component size of the nodule, which was not upstaged when the tumor invaded the adjacent lobe through fused fissures (9). Endobronchial ultrasonography, mediastinoscopy, and 18F-positron emission tomography/computed tomography (PET/CT) were not routinely employed in all patients but PET/CT was recommended for patients with solid component of nodules larger than 10 mm. The suspicion of positive node metastasis arose when a swollen node measured short axis of more than 1 cm on high-resolution computed tomography (HRCT), and 18F-fluorodeoxyglucose (FDG) accumulated an SUVmax of more than 1.5 in the lymph node on PET/CT.
Definition of DILCFFs
The fissure integrity classification proposed by Craig and Walker was adopted (10) (Figure 1). Only patients with grade 3 and 4 fissure integrity were enrolled. Two authors (Z.H. and W.X.) independently reviewed all radiological images and determined the fissure integrity of each patient. Any discrepancy was referred to the senior authors for consultation (L.C. and W.W.).

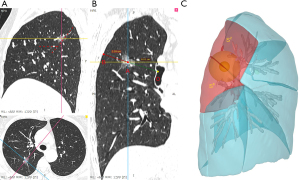
The definition of a deep nodule was consistent with our previous research (11). In this study, the depth ratio of the target nodule was determined by dividing the distance from the core of the nodule to the parietal pleura by the distance from the bronchial bifurcation at the second carina to the parietal pleura. Nodules with depth ratios larger than 33.3% were considered as deep nodules (Figure 2).
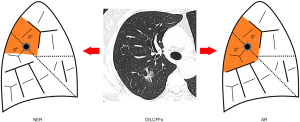
Group
Eligible patients were assigned to two arms at the surgeon’s discretion. They were the NER and AR arms (Figure 3). The NER suggested either a lobectomy or a segmentectomy for the predominant location of the DILCFF, with an extended wedge resection of portion of an adjacent lobe. While AR referred to combined a lobectomy with a (sub)segmentectomy of an adjacent lobe, or combined (sub)segmentectomies of two affected lobes where the DILCFF was located.
Surgery
The surgical procedure was tailored based on the fissure integrity and the target nodule depth (Figure S1). A three-dimensional computed tomographic bronchography and angiography (3D-CTBA) reconstruction was routinely performed for all patients using our self-developed software ‘DeepInsight’ (Neusoft, Liaoning, China) or commercially available software ‘InferOperate Thoracic Surgery’ (Infervision Medical Technology Co., Ltd., Beijing, China). The landmark hilar structure in the 3D-CTBA is utilized for intraoperative localization of the non-palpable nodules. Otherwise, preoperative CT-guided localization was deemed necessary. The procedure was demonstrated in Video 1.
For deep nodules, the surgical margin was defined as the minimum distance from the resected or the stapled bronchi stump or the parenchymal margin to the nodule detected in the fresh deflated specimen. The final margin was determined based on either of the narrower margin widths.
The evaluation involved a systematic dissection or sampling of lymph nodes. If present, station 11 located between two affected lobes, as well as stations 12 and 13, was taken for pathological evaluation in the AR arm. However, retrieval of these lymph nodes in the adjacent lobe sometimes proved challenging in the NER arm due to the non-anatomical dissection technique.
Follow-up
All patients were followed up with chest CT scans every 3–6 months for the first two years after surgery and annually thereafter. The needle biopsy or PET/CT was performed for any suspicious tumor recurrence. Local relapse referred to the recurrence at surgical margin and the same residual lobe. OS was defined as the time from surgery to any cause of death. DFS was determined by the time from surgery to recurrence.
Statistical analysis
Student’s t-test and Chi-squared test (or Fisher’s exact test) were used for the comparison of continuous variables and categorical variables, respectively. All the analyses were performed using R (version 4.1.2), and the significance level was set at P<0.05.
Results
Patients’ characteristics and margin
A total of 113 patients underwent resection for tumors located between adjacent lobes. Among these, 39 patients with tumors invading adjacent lobes through complete fissure and 31 patients with peripheral tumors removed by wedge resection were excluded. Thus, of the 43 eligible patients, 17 underwent NER and 26 underwent AR. More patients in the NER group underwent preoperative CT-guided nodule localization than those in the AR group (P=0.01). There were significantly more tumors located within the left posterior oblique fissures and less tumors located within the right horizontal fissures in the AR group than that in the NER group (P=0.02). Of note, the median surgical margin in the AR and NER arms were 2.52 and 1.27 cm, respectively (P<0.001). There were more cases with the ratio of margin to tumor size ≥1 in the AR arm (P=0.01), and shorter postoperative hospital stay (P=0.01) than those in the NER arm (Table 1).
Table 1
| Variable | All (n=43) | AR (n=26) | NER (n=17) | P |
|---|---|---|---|---|
| Age (years) | 62.79±11.02 | 59.62±8.98 | 62.59±13.68 | 0.39 |
| Sex (female) | 24 (55.8) | 14 (53.8) | 10 (58.8) | 0.75 |
| Smoke | 9 (20.9) | 7 (26.9) | 2 (11.8) | 0.42 |
| Pulmonary function | ||||
| FVC, L | 3.09±0.77 | 3.11±0.83 | 3.08±0.71 | 0.92 |
| FEV1, L | 2.56±0.63 | 2.61±0.72 | 2.49±0.50 | 0.63 |
| Total tumor size (cm) | 2.05±0.76 | 1.91±0.64 | 2.26±0.90 | 0.14 |
| Solid component size (cm) | 1.14±0.85 | 1.06±0.75 | 1.27±0.98 | 0.43 |
| Nodule type | >0.99 | |||
| Sub-solid | 32 (74.4) | 19 (73.1) | 13 (76.5) | |
| Pure solid | 11 (25.6) | 7 (26.9) | 4 (23.5) | |
| Location | 0.02 | |||
| Right anterior oblique fissure | 2 (4.7) | 1 (3.8) | 1 (5.9) | |
| Right posterior oblique fissure | 15 (34.9) | 8 (30.8) | 7 (41.2) | |
| Right horizontal fissure | 11 (25.6) | 3 (11.5) | 8 (47.1) | |
| Left anterior oblique fissure | 5 (11.6) | 5 (19.2) | 0 (0.0) | |
| Left posterior oblique fissure | 10 (23.3) | 9 (34.6) | 1 (5.9) | |
| Degree of fused fissures | 0.86 | |||
| Grade 3 | 26 (60.5) | 16 (61.5) | 10 (58.8) | |
| Grade 4 | 17 (39.5) | 10 (38.5) | 7 (41.2) | |
| Preoperative nodule localization | 7 (16.3) | 1 (3.8) | 6 (35.3) | 0.01 |
| Operative time (min) | 138.74±39.53 | 137.92±39.92 | 140.06±40.17 | 0.87 |
| Intraoperative hemorrhage (mL) | 32.96±4.89 | 33.32±5.17 | 32.54±4.39 | 0.62 |
| Air leakage (≥5 days) | 2 (4.7) | 1 (3.8) | 1 (5.9) | >0.99 |
| Postoperative hospital days | 4.64±2.12 | 4.00±1.36 | 5.69±2.70 | 0.01 |
| Number of lymph nodes removed | 7.37±3.98 | 7.42±3.73 | 7.29±4.44 | 0.92 |
| Number of lymph node resection stations | 4.05±1.76 | 3.92±1.52 | 4.24±2.11 | 0.58 |
| Lymph node metastasis | 2 (4.7) | 2 (7.7) | 0 (0.0) | 0.51 |
| Margin/tumor size ratio | 0.01 | |||
| <1 | 18 (41.9) | 7 (26.9) | 11 (64.7) | |
| ≥1 | 25 (58.1) | 19 (73.1) | 6 (35.3) | |
| Histological types | 0.44 | |||
| AIS | 1 (2.3) | 1 (3.8) | 0 (0.0) | |
| MIA | 7 (16.3) | 3 (11.5) | 4 (23.5) | |
| IAC | 35 (81.4) | 22 (84.6) | 13 (76.5) | |
| Pathological stage | 0.44 | |||
| 0 | 1 (2.3) | 1 (3.8) | 0 (0.0) | |
| IA | 36 (83.7) | 20 (76.9) | 16 (94.1) | |
| IB | 4 (9.3) | 3 (11.5) | 1 (5.9) | |
| IIIA | 2 (4.7) | 2 (7.7) | 0 (0.0) | |
| Local recurrence at surgical margin | 5 (11.6) | 0 (0.0) | 5 (29.4) | 0.006 |
Data are presented as mean ± SD for continuous data and n (%) for categorical data. AR, anatomical resection; NER, non-anatomical extended resection; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; AIS, adenocarcinoma in situ; MIA, microinvasive adenocarcinoma; IAC, invasive adenocarcinoma; SD, standard deviation.
Surgical procedure
In the AR arm, a lobectomy plus an adjacent (sub)segmentectomy occurred in 6 cases, the combined (sub)segmentectomies in 20 cases (76.9%). Twelve patients underwent an AR in the right side, 14 in the left side. Unexpected N2 positive nodes were found in two patients. One patient underwent left lower lobe and lingular resections, while the other received right middle lobectomy with S3 segmentectomy. In the NER arm, only 1 patient underwent a left upper division resection (LS1+2 + S3) plus a NER of adjacent left lower lobe. Of 16 patients with DILCFFs in the right side, 6 patients received a segmentectomy plus a NER of the adjacent lobe, 10 with the lobectomy plus NER (Table 2). No patients in both arms experienced severe morbidity such as persistent air leak, hemoptysis, or pneumonia.
Table 2
| AR | n | NER | n |
|---|---|---|---|
| RS1a + S2 + S6a | 1 | RS2 + NER (RLL) | 2 |
| RS2a + S6 | 1 | RS3 + NER (RML) | 2 |
| RS2 + S6a + c | 1 | RS6 + NER (RUL) | 2 |
| RS2 + S6 | 4 | RUL + NER (RML) | 2 |
| RS3 + S4 | 1 | RUL + NER (RLL) | 3 |
| RUL + S6 | 1 | RML + NER (RUL) | 3 |
| RML + S3b | 1 | RML + NER (RLL) | 1 |
| RML + S3 | 1 | RLL + NER (RUL) | 1 |
| RML + S7+8 | 1 | LS1+2 + S3 + NER (LLL) | 1 |
| LS1+2a + b + S6 | 1 | ||
| LS1+2b + c + S6 | 1 | ||
| LS1+2c + S6a | 1 | ||
| LS1+2c + S6 | 3 | ||
| LS1+2 + S6 | 2 | ||
| LS3a + S6 | 1 | ||
| LS3a + S4+5 + S6b + S8 | 1 | ||
| LS4+5 + S8 | 2 | ||
| LLL + S4+5 | 2 |
AR, anatomical resection; NER, non-anatomical extended resection; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; LLL, left lower lobe.
Aberrant interlobar vessel
A total of 10 types of interlobar vessels were identified with an overall incidence of 88.4% (38/43). The predominant type of interlobar veins observed were the V3b within the right fused horizontal fissure (81.8%), followed by the V6, converged into the central vein (73.3%), and the V2 draining into V6 (20%) within the fused right oblique fissure. The V6 flowing into V1+2 within the left fused oblique fissure was observed in 70% patients (7/10). The prevalence of aberrant arteries in the fused fissure was relatively lower than that of veins (Table 3).
Table 3
| Location | Interlobar vessels | Anatomical position | Number | Frequency |
|---|---|---|---|---|
| Right anterior oblique fissure | V5 | Interlobar vein | 1/2 | 50.0% |
| Right posterior oblique fissure | V2 | Flow into V6 | 3/15 | 20.0% |
| V6 | Flow into central vein | 11/15 | 73.3% | |
| A2 + A6 | Originate from a common trunk | 1/15 | 6.7% | |
| Right horizontal fissure | V3b | Interlobar vein | 9/11 | 81.8% |
| V4 | Flow into central vein | 2/11 | 18.2% | |
| Left anterior oblique fissure | A5 | Originate from A8 | 2/5 | 40.0% |
| Inter.V | Flow into SPV | 1/5 | 20.0% | |
| Left posterior oblique fissure | V1+2 | Flow into V6 | 1/10 | 10.0% |
| V6 | Flow into V1+2 | 7/10 | 70.0% |
SPV, superior pulmonary vein.
Prognostic outcome
The median follow-up time was 21.4 months. No instance of postoperative 90-day mortality was observed in both arms. Five patients in the NER group and one in the AR group experienced tumor recurrence (29.4% vs. 3.8%, P=0.04). In the NER arm, local relapse at the margin occurred in 5 patients (29.4%) (Table 4). In contrast, no margin relapse occurred in the AR arm except one patient with distant metastasis (local recurrence rate: 29.4% vs. 0%, P=0.006). This female patient underwent a left lower lobectomy plus lingular segmentectomy (surgical margin: 2.0 cm). However, an unexpected positive node was detected in station 7 and the tumor itself was grade III differentiated invasive adenocarcinoma at the final pathology note. She then received adjuvant chemotherapy and finally died of contralateral lung metastasis and severe pneumonia (DFS: 25 months, OS: 38 months). The Kaplan-Meier curves revealed no significant difference in OS and DFS between the two groups (Figure S2).
Table 4
| No. | Age | Sex | Total tumor size, cm | Surgical type | Histological types | Pathological stage | Surgical margin | Interval to recurrence | Recurrence site | Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | F | 2.6 | LLL + S4+5 | IAC | IIIA | 2.0 | 25 mo | Contralateral lung | Chemotherapy | 38 mo, dead |
| 2 | 43 | M | 1.1 | RS2 + NER (RLL) | MIA | IA | 0.3 | 28 mo | Surgical margin | RUL + S6 | 69 mo, alive |
| 3 | 84 | F | 2.7 | RS3 + NER (RML) | IAC | IA | 1.0 | 11 mo | Surgical margin | Chemotherapy | 25 mo, dead |
| 4 | 53 | M | 3.0 | RUL + NER (RML) | IAC | IA | 0.5 | 25 mo | Surgical margin | Chemotherapy | 59 mo, alive |
| 5 | 58 | F | 2.0 | RML + NER (RLL) | IAC | IA | 0.3 | 8 mo | Surgical margin | RLL | 29 mo, alive |
| 6 | 53 | M | 3.7 | RML + NER (RUL) | IAC | IA | 0.4 | 46 mo | Surgical margin | RUL | 110 mo, alive |
F, female; M, male; LLL, left lower lobe; IAC, invasive adenocarcinoma; mo, months; NER, non-anatomical extended resection; RLL, right lower lobe; MIA, microinvasive adenocarcinoma; RUL, right upper lobe; RML, right middle lobe.
Discussion
The optimal surgical management of the DILCFF remains a subject of controversy due to its challenging localization. How to technically remove the target nodule with a safe margin is yet to be comprehensively elucidated. Patients who underwent lobectomy plus LPR of the adjacent lobe demonstrated comparable survival outcomes and lower incidence of surgery-related morbidity than those undergoing more extended resections such as pneumonectomy and bilobectomy (2,3). The LPR, instead of the complete lobectomy, resulted in high quality of postoperative life and increased opportunities for subsequent resection because these early-stage NSCLC patients survived long enough to be at risk for developing second or even third NSCLC (12,13). Our preliminary data showed that AR of the adjacent lobe, as opposed to NER, provided a wider surgical margin, and resulted in a superior local tumor control.
The post-hoc analysis of CALGB140503 (Alliance) data indicated that anatomical segmental resection yielded comparable survival outcomes to non-anatomical wedge resection. However, the study specifically focused on early-stage peripheral lung cancers, and the selection of surgical intervention was based on surgeons’ preferences (14). Surgeons tended to exhibit bias in favor of performing wedge resections for nodules located in easily manageable areas, while opting for segmentectomies for challenging nodules (15). Thus, we specifically included subjects of DILCFFs (cT1N0M0) within such challenging locations.
Achieving a safe margin becomes more “challenging” when the location deepens (11). The 5-year DFS reached 98% in the Japanese JCOG1211 trial, wherein 11% patients with deeply located nodules, 99% patients underwent R0 resection with a median pathological margin of 25 mm (range, 17–32 mm) (16). Hopefully, our retrospective analyses also showed that the safe margin (mean: 2.39 cm) for deep nodules after segmentectomy was clinically attainable and survival outcomes were comparable with those of lobectomy [DFS: hazard ratio (HR) =1.20, P=0.688; OS: HR =1.09, P=0.892] (11). Nevertheless, achieving the protocol-required margin in the removal of DILCFFs is sometimes challenging even considering bilobectomy, due to their deep locations and fused fissures.
Regarding the AR and NER techniques, there has been evidence favoring AR. The reasons may be attributed to the following aspects. Firstly, the anatomical (sub)segmentectomy provides a wider margin and reduces the likelihood of local recurrence, while wedge resection is associated with a narrower margin width and higher risk of local recurrence (17,18).
Secondly, the AR of the adjacent lobe theoretically offers the advantage of achieving a comprehensive resection of the draining lymphatics, which are commonly acknowledged as a potential source of residual cancer cells (19). Up to 85.7% nodal metastasis proven in the AR are hilar or intrapulmonary lymph nodes which are hard to be obtained by NER resection (20). Interestingly, pneumonectomy is unnecessary in patients with intraoperatively detected interlobar nodal involvement (21). Survival outcomes are comparable between anatomical segmental and lobar resections in cT1N0 patients with unsuspected N1/N2 nodal involvement. Irrespective of the extent of anatomical lung resections, the adjuvant therapy offers survival benefit for these patients (22). In this study, the number of nodal evaluations, however, did not exhibit significant difference, potentially attributed to the limited sample size. The only unexpected nodal positive patient dying of contralateral lung metastasis could be ascribed to the invasiveness of the poorly differentiated tumor itself rather than being primarily associated with the extent of surgery.
Finally, the AR technique anatomically isolates the interlobar vessels, reducing the likelihood of squeezing, or directly damaging the underlying interlobar vein (23). Our previous analyses have shown that translobar bronchovascular structures exhibited a high incidence in the incomplete interlobar fissures and 75.0% of translobar veins were mistransected during anatomical pulmonary resection, resulting in gas-exchanging dysfunction in the preserved territory (24). Although postoperative complications such as hemoptysis were not significantly observed in both arms, we technically emphasized the importance of meticulous isolation of the interlobar vessels.
Preoperative tumor marking is commonly required for deep non-palpable tumors. However, our 3D-CTBA guided AR concept resulted in a decreased use of preoperative CT-guided hookwire nodule localization compared to NER methods, reducing the incidence of localization failure and complications such as pain, pneumothorax, hemothorax and air embolism caused by the puncture of pulmonary vein (25,26). The “tunneling stapler technique” is an additional surgical approach that merits attention. The “tunnel” is created along the intersegmental lung parenchyma from the bronchus stump, facilitating placement of the anvil of a stapler into the “tunnel”, subsequently the dissection line is easily stapled (27).
However, there are some limitations in this study. Firstly, the bias inevitably existed and was likely very significant in this retrospective, single institutional, small-sized analysis. Secondly, the duration of the follow-up period was insufficient to draw a definitive conclusion on the benefits of prolonged OS or DFS. Finally, the postoperative pulmonary functional metrics were not included, thus leaving the extent of pulmonary functional loss in the two arms unknown.
Conclusions
In conclusion, AR of partial of the adjacent lobe provides a wider surgical margin than that of NER in the removal of DILCFFs, potentially accounting for the lower incidence of margin failure.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-84/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-84/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-84/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-84/coif). Z.H. gets financial supports from the Jiangsu Province Hospital (the first Affiliated Hospital with Nanjing Medical University) Clinical Capacity Enhancement Project (No. JSPH-MB-2021-11), the Bethune Charitable Foundation (No. HZB-20190528-16), and the Foundation of Jiangsu Commission of Health (No. M2021049). W.W. gets financial support from the Key Project of Jiangsu Commission of Health (No. ZD2022055). The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Riquet M, Berna P, Arame A, et al. Lung cancer invading the fissure to the adjacent lobe: more a question of spreading mode than a staging problem. Eur J Cardiothorac Surg 2012;41:1047-51. [Crossref] [PubMed]
- Demir A, Gunluoglu MZ, Sansar D, et al. Staging and resection of lung cancer with minimal invasion of the adjacent lobe. Eur J Cardiothorac Surg 2007;32:855-8. [Crossref] [PubMed]
- Smith SP, Bograd AJ, Levy G, et al. Surgical Management of Non-Small Cell Lung Cancer Invading the Fissure: Less Is More? Ann Thorac Surg 2021;111:231-6. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Rusch VW. Initiating the Era of "Precision" Lung Cancer Surgery. N Engl J Med 2023;388:557-8. [Crossref] [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE, et al. Video-assisted thoracic surgical resection of malignant lung tumors. J Thorac Cardiovasc Surg 1992;104:1679-85; discussion 1685-7.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Ohtaki Y, Hishida T, Yoshida J, et al. The clinical outcome of non-small cell lung cancer patients with adjacent lobe invasion: the optimal classification according to the status of the interlobar pleura at the invasion point. Eur J Cardiothorac Surg 2013;43:302-9. [Crossref] [PubMed]
- Craig SR, Walker WS. A proposed anatomical classification of the pulmonary fissures. J R Coll Surg Edinb 1997;42:233-4.
- Li Z, He Z, Xu W, et al. Segmentectomy versus lobectomy for deep clinical T1a-bN0M0 non-small cell lung cancer. Eur J Surg Oncol 2023;49:106946. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- Altorki N, Wang X, Damman B, et al. Lobectomy, segmentectomy, or wedge resection for peripheral clinical T1aN0 non-small cell lung cancer: A post hoc analysis of CALGB 140503 (Alliance). J Thorac Cardiovasc Surg 2024;167:338-347.e1. [Crossref] [PubMed]
- Altorki NK, Chow OS. Cancer and Leukemia Group B 140503: Is it time to turn the page on Lung Cancer Study Group 821? J Thorac Cardiovasc Surg 2024;167:367-70. [Crossref] [PubMed]
- Aokage K, Suzuki K, Saji H, et al. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): a multicentre, single-arm, confirmatory, phase 3 trial. Lancet Respir Med 2023;11:540-9. [Crossref] [PubMed]
- El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400-5. [Crossref] [PubMed]
- Sienel W, Dango S, Kirschbaum A, et al. Sublobar resections in stage IA non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg 2008;33:728-34. [Crossref] [PubMed]
- Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol 2010;5:1583-93. [Crossref] [PubMed]
- Tsutani Y, Handa Y, Shimada Y, et al. Comparison of cancer control between segmentectomy and wedge resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2021;162:1244-1252.e1. [Crossref] [PubMed]
- Gunluoglu MZ, Demir A, Turna A, et al. Extent of lung resection in non-small lung cancer with interlobar lymph node involvement. Ann Thorac Cardiovasc Surg 2011;17:229-35. [Crossref] [PubMed]
- Razi SS, Nguyen D, Villamizar N. Lobectomy does not confer survival advantage over segmentectomy for non-small cell lung cancer with unsuspected nodal disease. J Thorac Cardiovasc Surg 2020;159:2469-83.e4.
- Otsuji H, Uchida H, Maeda M, et al. Incomplete interlobar fissures: bronchovascular analysis with CT. Radiology 1993;187:541-6. [Crossref] [PubMed]
- Xu W, Li Z, He Z, et al. Anatomical distribution and clinical significance of translobar bronchi, arteries, and veins hidden in the interlobar fissure. J Thorac Dis 2024;16:901-10. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Horan TA, Pinheiro PM, Araújo LM, et al. Massive gas embolism during pulmonary nodule hook wire localization. Ann Thorac Surg 2002;73:1647-9. [Crossref] [PubMed]
- Kobayashi N, Endo K. Pulmonary segmentectomy with tunneling stapler technique. Thorac Cardiovasc Surg 2014;62:722-4. [Crossref] [PubMed]


