
A new selective mediastinal lymph node dissection for clinical peripheral stage IA invasive non-small-cell lung cancer: a propensity-score matching study
Highlight box
Key findings
• This new strategy is of equal effectiveness to complete mediastinal lymph node dissection (C-MLND) in terms of prognosis, perioperative recovery, and recurrence control and has advantages in reducing postoperative complications.
What is known and what is new?
• A recent prospective, multicenter, clinical trial validated the staging accuracy of a new strategy of selective mediastinal lymph node dissection (S-MLND) in clinical stage IA non-small cell lung cancer (NSCLC), but there is a lack of survival comparison analysis.
• We used propensity score matching analysis to minimize the influence of confounding factors and compared this new lymph node dissection strategy with C-MLND in terms of prognosis, perioperative recovery, and recurrence.
What is the implication, and what should change now?
• The new S-MLND has the potential to replace C-MLND as the standard surgical procedure in surgery for certain clinical stage IA NSCLC.
Introduction
Lung cancer represents the most prevalent and lethal form of cancer worldwide (1). The increased use of computed tomography (CT) screening has led to a notable rise in the detection rate of early-stage lung cancer patients (2,3). In East Asians, the proportion of patients diagnosed with stage 0 or IA lung cancer has increased from 32.2% in 2008 to an impressive 73.5% in 2017 (4). Lobectomy with complete mediastinal lymph node dissection (C-MLND), previously referred to as systemic lymph node resection (SND), remains the standard treatment for early-stage non-small cell lung cancer (NSCLC) (5). Although current guidelines recommend C-MLND for all resectable NSCLC, regardless of tumor location and stage (6), it is important to consider individual factors when determining the appropriate lymph node assessment approach.
The study conducted by Lee and colleagues indicates that a uniform extent of lymph node (LN) assessment may not be an appropriate approach for all lung cancers. The study revealed that a less rigorous LN evaluation did not result in a poorer prognosis for clinical stage I NSCLC, as well as adenocarcinoma (ADC) with a lepidic component subtype (7). These findings indicate that a tailored approach to LN assessment may prove more effective in certain cases. A number of previous studies have investigated detailed patterns of LN metastasis based on the location of tumor (8-10). These studies have demonstrated that lobe-specific lymph node dissection (L-SND) can be a viable alternative to C-MLND for the treatment of early-stage NSCLC (11-14). Zhang et al. (15) put forth a novel selective mediastinal lymph node dissection (S-MLND) strategy, one that is contingent upon the segment location, the specific subtype of lung ADC and consolidation to tumor ratio (CTR). In his multi-centre, prospective clinical study, he successfully verified the staging accuracy of the S-MLND strategy in cT1N0 invasive NSCLC (16). Although the results suggest that this new S-MLND may be feasible in selected cT1N0 NSCLC, it is unclear whether S-MLND is equal to C-MLND in terms of perioperative parameters, postoperative complications and prognosis due to the lack of relevant research to date.
The objective of this study was to assess surgical outcomes and postoperative complications for clinical stage IA NSCLC based on the extent of mediastinal LN dissection (MLND) using the propensity score matching (PSM) method. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1346/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committees of The First Hospital of Lanzhou University (No. LDYYLL2024-35), Henan Cancer Hospital (No. 2024-182-043), Affiliated Hospital of North Sichuan Medical College (No. 2024ER350-13), The First Affiliated Hospital of Chengdu Medical College (No. 2024CYFYIRBSQ-15), and Henan Provincial People’s Hospital (No. 2024-23). Patient consent has been waived due to the retrospective nature of the analysis. All participating hospitals were informed and agreed with the study.
Patients
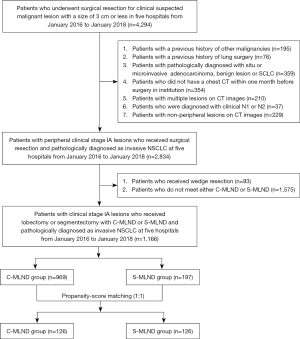
The data were retrospectively collected on patients who underwent surgical resection for clinically suspected malignant lesions with a size of 3 cm or less in five Chinese hospitals between January 2016 and January 2018. A total of 2,834 Asian patients with peripheral clinical stage IA lesions who underwent surgical resection and were pathologically diagnosed with invasive NSCLC were included in the study. Patients who underwent wedge resection and those who did not meet the criteria for either C-MLND or S-MLND were further excluded from the subsequent analysis. Finally, the study enrolled 1,166 patients with peripheral clinical stage IA lesions who underwent lobectomy or segmentectomy with S-MLND or C-MLND and were pathologically diagnosed with invasive NSCLC (Figure 1). No patients received induction chemotherapy or radiotherapy. The clinical and pathological tumor-node-metastasis (TNM) stages were diagnosed in accordance with the eighth edition of the TNM classification for lung cancer (17). Clinical N0 classification was defined as the absence of LNs with a short axis measuring more than 1cm on CT or proven to be metastatic by positron emission tomography/computed tomography (PET/CT). It is noteworthy that mediastinoscopy and endobronchial ultrasonography are not performed as a matter of routine.
Evaluation of CT imaging features
Maximum tumor size and maximum consolidation size were measured on thin-section CT images in the lung window setting (window level, –600 HU, window width: 1,600 HU) by two researchers blinded to clinical and pathological information. Tumor location was first classified as central or peripheral (tumors located within the outer one third of the lung on CT scans) and then segment location was classified. The CTR was defined as the ratio of the maximum size of the consolidation to the maximum size on the CT scan. Any discrepancy was resolved by discussion. The detailed CT scan parameters are shown in the Supplementary file (Appendix 1).
Surgical procedures and definition of lymph node dissection
All patients included in the study underwent lobectomy or segmentectomy with C-MLND or S-MLND by video-assisted thoracic surgery, with LNs categorized according to the International Association for the Study of Lung Cancer (IASLC) map (18). In the C-MLND group, LN stations 5, 6, 7 and 8 were dissected for all left-sided tumors and stations 2R, 4R, 7 and 8 for right-sided tumors. LN stations 3 for right-sided tumors, station 4 for left-sided tumors and station 9 for upper and middle lobe tumors were not routinely removed. However, these LN stations would be dissected if the surgeons suspected LN metastasis. In addition, hilar LN stations (10-12) were also dissected. According to a new selective LN dissection strategy proposed by Zhang et al. (15), patients who met one of six criteria were assigned to the S-MLND group based on pathological results including lung ADC subtypes, hilar LN status and visceral pleural invasion (VPI) status, as well as CTR and extent of LN resection (16). The six criteria are: (I) patients with CTR ≤0.5 do not require MLND; (II) patients with pathological diagnosis of lepidic-predominant adenocarcinoma (LPA) do not require MLND; (III) patients with tumor located in the apical segment do not require resection of inferior mediastinal lymph nodes (MLNs); (IV) if both VPI and hilar LNs were negative, it is not necessary to resect inferior MLNs in patients with non-apical upper lobe tumors; (V) if hilar LNs were negative, it is not necessary to resect 4L in patients with left superior segment tumors; (VI) if hilar LNs were negative, it is not necessary to resect superior MLNs in patients with left basal segment tumors. The description of lymph node resection is shown in Table 1.
Table 1
| C-MLND | S-MLND |
|---|---|
| Left upper lobe: (4L), 5, 6, 7, 8, (9) | CTR ≤0.5/lepidic-predominant: no need for MLND |
| Left lower lobe: (4L), 5, 6, 7, 8, 9 | Left apical segments: (4L), 5, 6; right apical segments: 2R, 4R |
| Right upper/middle lobe: 2R, 4R, 7, 8, (9) | Negative VPI and hilar LN: right non-apical segment upper lobe: 2R, 4R; left non-apical segment upper lobe: (4L), 5, 6 |
| Right lower lobe: 2R, 4R, 7, 8, 9 | Negative hilar LN: left superior segment tumors: 5, 6, 7, 8, 9; left basal segment tumors: 7, 8, 9 |
Lymph node outside the bracket was constantly dissected while those enclosed in the bracket was removed only if suspicious of metastases. C-MLND, complete mediastinal lymph node dissection; S-MLND, selective mediastinal lymph node dissection; CTR, consolidation to tumor ratio; MLND, mediastinal lymph node dissection; VPI, visceral pleural invasion; LN, lymph node.
Follow-up and evaluation
Postoperative follow-up examination included chest, abdominal and brain CT or PET-CT scans, blood tests and physical examinations were performed every 3 months for the first 2 years and every 6 months thereafter at each hospital. Tissue biopsy was recommended if recurrence was uncertain based on imaging examinations alone. Patients who were lost to follow-up at the operating hospital were contacted by telephone. According to the recommendations of the Thoracic Surgeons Workforce, recurrences were classified as locoregional recurrence (tumor recurrence within the hilar or mediastinal LN stations or in the same ipsilateral hemithorax) and distant recurrence (tumor recurrence in another lobe or organs outside the hemithorax). Information on postoperative complications was recorded and adjudicated according to the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) (19). Recurrence-free survival (RFS) was defined as the time from surgery to the date of recurrence, death, or last follow-up. Overall survival (OS) was defined as the time from surgery to the date of death from any cause or last follow-up.
Statistical analysis
Clinicopathological variables were collected from the medical records of each participating hospital. These variables included age, sex, smoking history, Charlson/Deyo Comorbidity Index (CCI), operative approach, intraoperative blood loss, operative time, total pleural drainage, duration of chest tube placement, postoperative length of stay, postoperative complications such as pneumonitis, chylothorax, prolonged air leak (≥7 days), arrhythmia, pulmonary embolism and histological type, histological subtype, VPI, lymphovascular invasion (LVI), spread through air space (STAS), clinical T stage (cT), pathological T stage (pT), pathological N stage (pN), number of resected MLNs, number of resected MLN stations and postoperative adjuvant therapy. The ADC was further classified into several subtypes according to the 2015 World Health Organization lung cancer classification (20). All pathological assessments were performed by pathologists at each hospital. The PSM method was used to reduce bias due to confounding factors. Patients in the C-MLND group were matched with those in the S-MLND group in a 1:1 ratio based on their PS, which was calculated by logistic regression using factors such as age, sex, histological type, CCI, smoking history, CTR, cT, surgical approach and tumor location. These factors were carefully selected because they are known to be associated with the choice of extent of lymphadenectomy.
Chi-squared test or Fisher’s exact test was used to compare the characteristics in the two groups for categorical variables and Student t-test for continuous variables with normal distribution, Mann-Whitney U test for continuous variables with abnormal distribution. Survival curves were estimated using the Kaplan-Meier method and prognosis was compared between groups using the log-rank test. All statistical analyses were performed using IBM SPSS software version 24.0 (IBM Corp., Armonk, NY, USA) and R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). Results were considered statistically significant when the P<0.05.
Results
A total of 1,166 patients were included in the study, with 969 patients classified as C-MLND and 197 patients classified as S-MLND according to our criteria. The comparison of clinicopathological characteristics based on the extent of MLND is shown in Table S1. The C-MLND group had fewer patients with left lower lobe tumors and LPA, but more patients with left upper lobe, lobectomy, solid predominant (CTR >0.5), VPI positive tumors, advanced cT, pT, and pN2 than the S-MLND group. On the other hand, the C-MLND group included more patients who dissected a greater number of MLNs and MLN stations and received postoperative adjuvant therapy more frequently than the S-MLND group. There were no significant differences in age, gender, smoking history, histological type, LVI, STAS, EGFR mutation and CCI.
Therefore, we performed a PSM analysis to control for confounders and prognostic imbalance between the C-MLND and S-MLND groups. After matching, there were 126 patients in each group and 60, 43, 14, 8, 31 and 9 patients in the S-MLND group fulfilled criteria 1, 2, 3, 4, 5 and 6 respectively. Clinicopathological variables were well balanced between the groups except for the number of MLNDs and MLND stations, which may have resulted from the different extent of MLN dissection. There was also no significant difference in the positive rate of pN2 between the groups (Table 2). The comparison of perioperative parameters as well as postoperative complications between C-MLND and S-MLND groups after matching is shown in Table 3. There were no significant differences in intraoperative blood loss (P=0.34), total pleural drainage (P=0.74), operative time (P=0.18), duration of chest tube placement (P=0.42), postoperative length of stay (P=0.34) between the groups. However, the incidence of postoperative complications was significantly higher in the C-MLND group than in the S-MLND group (23.0% vs. 12.7%, P=0.03). Similarly, the incidence of chylothorax was also significantly higher in the C-MLND group than in the S-MLND group (3.2% vs. 0.0%, P=0.04). There were no perioperative deaths in either group. Meanwhile, there was no significant difference in the incidence of pneumonitis or other complications such prolonged air leak (≥7 days), arrhythmia and pulmonary embolism, when each postoperative complication was analyzed separately. Similar results were observed in the lobectomy subgroup (Table S2).
Table 2
| Variables | C-MLND (n=126) | S-MLND (n=126) | P |
|---|---|---|---|
| Age (years) | 62.5 [55.0–66.0] | 60.0 [54.8–66.0] | 0.34 |
| Sex (male/female) | 55 (43.7)/71 (56.3) | 57 (45.2)/69 (54.8) | 0.80 |
| Smoking history (no/yes) | 103 (81.7)/23 (18.3) | 101 (80.2)/25 (19.8) | 0.75 |
| Tumor location | 0.36 | ||
| RUL/RML/RLL | 43 (34.1)/7 (5.6)/13 (10.3) | 40 (31.7)/4 (3.2)/9 (7.1) | |
| LUL/LLL | 29 (23.0)/34 (27.0) | 25 (19.8)/48 (38.1) | |
| CTR (≤0.5 vs. >0.5) | 68 (54.0)/58 (46.0) | 60 (47.6)/66 (52.4) | 0.31 |
| cT (T1a/T1b/T1c) | 6 (4.8)/79 (62.7)/41 (32.5) | 4 (3.2)/80 (63.5)/42 (33.3) | 0.81 |
| Histology (ADC/SCC/others) | 117 (92.9)/5 (4.0)/4 (3.2) | 113 (89.7)/8 (6.3)/5 (4.0) | 0.65 |
| ADC histologic subtype | 0.57 | ||
| LPA/APA/PPA | 36 (28.6)/59 (46.8)/14 (11.1) | 43 (34.1)/54 (42.9)/11 (8.7) | |
| MPA/SPA | 1 (0.8)/7 (5.6) | 2 (1.6)/3 (2.4) | |
| EGFR mutation† (yes/no) | 69 (75.0)/23 (25.0) | 59 (72.0)/23 (28.0) | 0.36 |
| Others‡ (yes/no) | 4 (4.3)/88 (95.7) | 9 (11.0)/73 (89.0) | 0.10 |
| Operative approach (lobectomy/segmentectomy) | 88 (69.8)/38 (30.2) | 86 (68.3)/40 (31.7) | 0.79 |
| LVI positive | 0 (0.0) | 0 (0.0) | |
| VPI positive | 11 (8.7) | 4 (3.2) | 0.06 |
| STAS positive | 4 (3.2) | 3 (2.4) | >0.99 |
| No. of resected MLN stations | 5 [5–6] | 0 [0–3] | <0.001 |
| No. of resected MLNs | 11 [7–14] | 0 [0–6] | |
| pT | 0.12 | ||
| T1a/T1b/T1c | 9 (7.1)/74 (58.7)/30 (23.8) | 21 (16.7)/71 (56.3)/27 (21.4) | |
| T2a/T2b | 12 (9.5)/1 (0.8) | 7 (5.6)/0 (0.0) | |
| pN (N0/N1/N2) | 114 (90.5)/4 (3.2)/8 (6.3) | 120 (95.2)/0 (0.0)/6 (4.8) | 0.11 |
| Adjuvant therapy (yes/no) | 33 (26.2)/93 (73.8) | 25 (19.8)/101 (80.2) | 0.23 |
| CCI (0/1–2/3) | 99 (78.6)/26 (20.6)/1 (0.8) | 101 (80.2)/25 (19.8)/0 (0.0) | 0.60 |
Data are expressed as median [first quartile and third quartile] or n (%). †, 174 patients had genetic sequencing test after surgery, with 92 in the C-MLND group and 82 in the S-MLND group. ‡, others included KRAS mutation, BRAF mutation, ROS1 fusion, EML4-ALK fusion. C-MLND, complete mediastinal lymph node dissection; S-MLND, selective mediastinal lymph node dissection; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; CTR, consolidation tumor ratio; ADC, adenocarcinoma; SCC, squamous cell carcinoma; LPA, lepidic-predominant adenocarcinoma; APA, acinar-predominant adenocarcinoma; PPA, papillary-predominant adenocarcinoma; MPA, micropapillary-predominant adenocarcinoma; SPA, solid-predominant adenocarcinoma; LVI, lymphovascular invasion; VPI, visceral pleural invasion; STAS, spread through air space; MLN, mediastinal lymph node; CCI, Charlson/Deyo comorbidity index.
Table 3
| Variables | C-MLND (n=126) | S-MLND (n=126) | P |
|---|---|---|---|
| Intraoperative blood loss (mL) | 50.0 [50.0–100.0] | 50 [20.0–100.0] | 0.34 |
| Total pleural drainage (mL) | 430.0 [320.0–600.0] | 390 [257.5–362.5] | 0.74 |
| Operative time (min) | 122.0 [94.8–161.3] | 120.0 [85.0–155.0] | 0.18 |
| Duration of chest tube placement (d) | 3 [3–4] | 3 [3–4] | 0.42 |
| Postoperative length of stay (d) | 4 [3–5] | 4 [3–5] | 0.34 |
| Postoperative complications | 29 (23.0) | 16 (12.7) | 0.03 |
| Pneumonitis | 12 (9.5) | 13 (10.3) | 0.83 |
| Prolonged air leakage (≥7 d) | 6 (4.8) | 1 (0.8) | 0.06 |
| Chylothorax | 4 (3.2) | 0 (0.0) | 0.04 |
| Arrhythmia | 6 (4.8) | 2 (1.6) | 0.26 |
| Pulmonary embolism | 1 (0.8) | 0 (0.0) | 0.32 |
Data are expressed as median [first quartile and third quartile] or n (%). C-MLND, complete mediastinal lymph node dissection; S-MLND, selective mediastinal lymph node dissection.
The comparison of postoperative recurrence patterns between C-MLND and S-MLND groups after matching is shown in Table 4. There was no significant difference in the overall recurrence rate (11.9% vs. 16.7%, P=0.28), the incidence of locoregional recurrence (1.6% vs. 4.8%, P=0.28), and distant metastasis (10.3% vs. 11.9%, P=0.69) between the C-MLND and S-MLND groups. These results were consistent with those observed in the lobectomy subgroup (Table S3).
Table 4
| Variables | C-MLND (n=126) | S-MLND (N=126) | P |
|---|---|---|---|
| Recurrence | 15 (11.9) | 21 (16.7) | 0.28 |
| Locoregional recurrence | 2 (1.6) | 6 (4.8) | 0.28 |
| Distant metastasis | 13 (10.3) | 15 (11.9) | 0.69 |
Data are expressed as n (%). C-MLND, complete mediastinal lymph node dissection; S-MLND, selective mediastinal lymph node dissection.
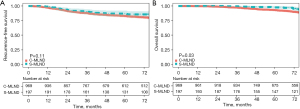
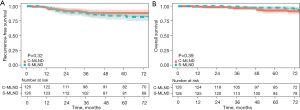
The median follow-up time for the C-MLND and S-MLND groups was 75.6 (range, 3.1–96.5) and 74.4 (range, 1.2–96.6) months respectively. The study showed that there was no significant difference in 5-year RFS (C-MLND vs. S-MLND, 82.9% vs. 86.2%; P=0.11) between the two groups. However, the 5-year OS of the S-MLND group was better than that of the C-MLND group (C-MLND vs. S-MLND, 92.9% vs. 97.5%; P=0.03) before matching (Figure 2). After matching, 5-year RFS (C-MLND vs. S-MLND, 87.5% vs. 82.9%; P=0.32) and 5-year OS (C-MLND vs. S-MLND, 92.0% vs. 95.9%; P=0.39) showed no significant differences between the groups (Figure 3). Similar results were seen in the lobectomy subgroup (Figure S1).
Discussion
The management of lymph nodes in the early-stage NSCLC remains a topic of debate in the medical community. Excessive MLND may be futile or even harmful. Previous studies have demonstrated that the resection of non-metastatic LNs not only fails to provide survival benefits (21), but may also impair anti-tumor immunity, which can affect the effectiveness of subsequent immunotherapy (22). Accordingly, the optimal extent of LN resection for early-stage NSCLC is to accurately and thoroughly remove positive LNs while preserving healthy ones to the greatest extent possible. In recent years, numerous researchers have dedicated significant effort to investigating the optimal extent of LN dissection for early-stage NSCLC. A number of previous studies have compared the prognosis, perioperative parameters and postoperative complications of C-MLND with lobe-specific LN dissection (L-SND) or LN sampling. However, no consistent conclusions have been reached. Previous studies reported that C-MLND enabled more accurate staging, guided optimal treatment and achieved better local control, which subsequently improved postoperative survival (23-25). In a randomized trial, Wu et al. demonstrated that C-MLND had a superior 5-year survival rate compared to MLN sampling (82.2% vs. 57.5%) in stage I NSCLC (26). However, another randomized trial (ACOSOGZ0030) indicated that C-MLND was unable to improve survival compared with MLN sampling in early-stage NSCLC (27). Bille et al. (25) retrospectively analyzed the distribution and incidence of pN1 and pN2 metastases in 1,667 patients and concluded that C-MLND should be performed in cI NSCLC, as 16% of patients had upstaging beyond the lobe-specific lymphatic drainage. However, the studies conducted by Adachi et al. (13) and Hishida et al. (14) suggested that L-SND could be a viable alternative to C-MLND for patients with c-stage I or II NSCLC.
In 2020, Zhang et al. (15) conducted an analysis of segment-specific lymph node metastasis patterns based on a comprehensive retrospective database. The findings of this study indicated that a segment-specific LN metastasis pattern was a more reliable indicator than a lobe-specific metastasis pattern. Furthermore, the study proposed a new S-MLND. Subsequently, a multicentre, prospective clinical trial was conducted to further validate the staging accuracy of this novel strategy (16). However, there was a dearth of comparative analysis of surgical and pathological results. The objective of this study was to compare surgical and oncological outcomes between C-MLND and S-MLND in a multi-institutional cohort. The PSM method was employed to achieve as much balance as possible between the confounding factors.
No significant differences were observed in perioperative parameters between the groups. A multitude of factors, including the surgeon’s surgical technique, the surgical approach, the resection scope and the patient’s personal condition, can exert an influence on intraoperative blood loss and operation time. It is possible that the perioperative results may vary among different institutions. However, the surgeons who participated in this study have considerable experience in pulmonary surgeries, which serves to minimize differences between institutions. The results demonstrated that the perioperative recovery was comparable between the C-MLND and S-MLND groups.
The incidence of postoperative complications in the C-MLND group was significantly higher than in the S-MLND group (23.0% vs. 12.7%, P=0.03). As demonstrated in previous studies, C-MLND may increase the risk of damage to normal vascular, neurogenic, and lymphatic structures in the mediastinum, which may in turn lead to complications such as chylothorax (2–2.3%) (28,29) and recurrent laryngeal nerve injury (1%) (30). The incidence of chylothorax in the C-MLND group was 3.2%, whereas no cases were observed in the S-MLND group. The underlying causes of postoperative chylothorax may include poor thoracic duct exposure, extensive lymph node removal and inadequate intraoperative identification. A strategy to prevent chylothorax that has been demonstrated to be effective is to be familiar with the anatomical configuration of the thoracic duct, to ensure full exposure of the thoracic duct during MLN resection and to use high-energy devices. The incidence of prolonged air leakage in the C-MLND group was higher than that in the S-MLND group, although this difference was not statistically significant (P=0.06). This may be attributable to a combination of factors, including chronic obstructive pulmonary disease, diffuse emphysema with large bullae, history of smoking and female gender, which have been demonstrated to increase the risk of air leaks following lung resection (31).
No significant difference was observed in the 5-year RFS between the two groups. However, the S-MLND group exhibited a higher 5-year OS than the C-MLND group (92.9% vs. 97.5%; P=0.03) prior to matching. This discrepancy may be attributed to the higher proportion of patients with advanced disease in the C-MLND group prior to matching. Nevertheless, no notable discrepancy was identified in the 5-year RFS and OS after matching. Furthermore, no significant differences were observed in terms of disease-specific control, including locoregional recurrence and distant metastasis. In general, the study found that S-MLND is comparable to C-MLND in terms of prognosis and recurrence control. The rate of detection of pN2 cases did not differ significantly between the groups after matching (C-MLND vs. S-MLND, 6.3% vs. 4.8%; P=0.11), despite the smaller total number of resected MLNs and MLN stations in the S-MLND group. This may be attributed to the rarity of LN metastasis in patients with ground-glass opacity (GGO)-dominant (CTR ≤0.5) lung cancers and LPA. Similarly, Flores et al. (32) also reported that the rate of LN metastasis in GGO-dominant clinical stage IA NSCLC was closed to zero and Yu et al. (33) found no LN involvement in patients with LPA.
It is important to note that this was a multicentre retrospective study and that several limitations should be acknowledged. First, while PET/CT has the advantage over CT in the preoperative nodal staging of NSCLC, it still has a false negative rate of approximately 30% (34). However, due to the high cost of PET/CT, only a small proportion of patients underwent PET/CT examination in our study. It was unavoidable that false negatives would occur in the preoperative diagnosis of cN0. Second, although PSM was employed to minimize confounding factors, it is not possible to eliminate all potential selection bias in retrospective studies. Despite the inclusion of a relatively large number of patients meeting the criteria for C-MLND, the number of patients who met criteria IV and VI after PSM was relatively small. Further studies with a larger sample size and a more balanced number of patients meeting each criterion for S-MLND are required to validate the conclusions of this study in greater depth. Third, intraoperative evaluation of the hilar lymph nodes or VPI was not conducted as a standard procedure in this retrospective study. The scope of lymph node evaluation was determined by a combination of factors, including intraoperative freezing pathology, clinician experience and patient clinical and imaging features. The S-MLND strategy requires validation through a prospective, randomized controlled trial, with a comparison of survival and perioperative recovery outcome.
Conclusions
The findings of this retrospective study indicate that S-MLND is associated with a significantly lower incidence of postoperative complications, including a reduced prevalence of chylothorax. These results suggest that S-MLND may be a more secure method than C-MLND. Nodal upstaging and long-term outcomes did not significantly differ between the two methods. However, given that the study was not designed with the specific aim of assessing lymph node staging or long-term outcomes, and that the statistical power was not calculated, these results should be interpreted with caution. Notwithstanding these limitations, S-MLND demonstrated comparable outcomes and a reduced incidence of complications in comparison with C-MLND, thereby indicating its potential as an alternative approach for selected patients with stage IA NSCLC. It is recommended that prospective, randomized trials are conducted to confirm these findings and establish clear clinical guidelines.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1346/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1346/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1346/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1346/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committees of The First Hospital of Lanzhou University (No. LDYYLL2024-35), Henan Cancer Hospital (No. 2024-182-043), Affiliated Hospital of North Sichuan Medical College (No. 2024ER350-13), The First Affiliated Hospital of Chengdu Medical College (No. 2024CYFYIRBSQ-15), and Henan Provincial People’s Hospital (No. 2024-23). All participating hospitals were informed and agreed with the study. Written informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Deng HY, Wang YC, Ni PZ, et al. Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2017;51:203-10. [PubMed]
- Soo RA, Stone ECA, Cummings KM, et al. Scientific Advances in Thoracic Oncology 2016. J Thorac Oncol 2017;12:1183-209. [Crossref] [PubMed]
- Zhang Y, Zheng D, Li H, et al. Changes in lung cancer incidence by sex and smoking status in China: a multicentre observational study. Lancet 2018;392:S4. [Crossref]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Lee J, Hong YS, Cho J, et al. Reclassifying the International Association for the Study of Lung Cancer Residual Tumor Classification According to the Extent of Nodal Dissection for NSCLC: One Size Does Not Fit All. J Thorac Oncol 2022;17:890-9. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Proposal for reasonable mediastinal lymphadenectomy in bronchogenic carcinomas: role of subcarinal nodes in selective dissection. J Thorac Cardiovasc Surg 1998;116:949-53. [Crossref] [PubMed]
- Shien K, Toyooka S, Soh J, et al. Clinicopathological characteristics and lymph node metastasis pathway of non-small-cell lung cancer located in the left lingular division. Interact Cardiovasc Thorac Surg 2015;20:791-6. [Crossref] [PubMed]
- Deng HY, Zhou J, Wang RL, et al. Lobe-Specific Lymph Node Dissection for Clinical Early-Stage (cIA) Peripheral Non-small Cell Lung Cancer Patients: What and How? Ann Surg Oncol 2020;27:472-80. [Crossref] [PubMed]
- Zhao Y, Mao Y, He J, et al. Lobe-specific Lymph Node Dissection in Clinical Stage IA Solid-dominant Non-small-cell Lung Cancer: A Propensity Score Matching Study. Clin Lung Cancer 2021;22:e201-10. [Crossref] [PubMed]
- Luo J, Yang S, Dong S. Selective Mediastinal Lymphadenectomy or Complete Mediastinal Lymphadenectomy for Clinical Stage I Non-Small Cell Lung Cancer: A Meta-Analysis. Adv Ther 2021;38:5671-83. [Crossref] [PubMed]
- Adachi H, Sakamaki K, Nishii T, et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol 2017;12:85-93. [Crossref] [PubMed]
- Hishida T, Miyaoka E, Yokoi K, et al. Lobe-Specific Nodal Dissection for Clinical Stage I and II NSCLC: Japanese Multi-Institutional Retrospective Study Using a Propensity Score Analysis. J Thorac Oncol 2016;11:1529-37. [Crossref] [PubMed]
- Zhang Y, Fu F, Wen Z, et al. Segment Location and Ground Glass Opacity Ratio Reliably Predict Node-Negative Status in Lung Cancer. Ann Thorac Surg 2020;109:1061-8. [Crossref] [PubMed]
- Zhang Y, Deng C, Zheng Q, et al. Selective Mediastinal Lymph Node Dissection Strategy for Clinical T1N0 Invasive Lung Cancer: A Prospective, Multicenter, Clinical Trial. J Thorac Oncol 2023;18:931-9. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Watanabe S, Asamura H. Lymph node dissection for lung cancer: significance, strategy, and technique. J Thorac Oncol 2009;4:652-7. [Crossref] [PubMed]
- Deng H, Zhou J, Chen H, et al. Impact of lymphadenectomy extent on immunotherapy efficacy in postresectional recurred non-small cell lung cancer: a multi-institutional retrospective cohort study. Int J Surg 2024;110:238-52. [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Smeltzer MP, Faris NR, Ray MA, et al. Association of Pathologic Nodal Staging Quality With Survival Among Patients With Non-Small Cell Lung Cancer After Resection With Curative Intent. JAMA Oncol 2018;4:80-7. [Crossref] [PubMed]
- Bille A, Woo KM, Ahmad U, et al. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg 2017;51:674-9. [Crossref] [PubMed]
- Wu YL, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Cho HJ, Kim DK, Lee GD, et al. Chylothorax complicating pulmonary resection for lung cancer: effective management and pleurodesis. Ann Thorac Surg 2014;97:408-13. [Crossref] [PubMed]
- Takuwa T, Yoshida J, Ono S, et al. Low-fat diet management strategy for chylothorax after pulmonary resection and lymph node dissection for primary lung cancer. J Thorac Cardiovasc Surg 2013;146:571-4. [Crossref] [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [Crossref] [PubMed]
- Kreso A, Mathisen DJ. Management of Air Leaks and Residual Spaces Following Lung Resection. Thorac Surg Clin 2021;31:265-71. [Crossref] [PubMed]
- Flores RM, Nicastri D, Bauer T, et al. Computed Tomography Screening for Lung Cancer: Mediastinal Lymph Node Resection in Stage IA Nonsmall Cell Lung Cancer Manifesting as Subsolid and Solid Nodules. Ann Surg 2017;265:1025-33. [Crossref] [PubMed]
- Yu Y, Jian H, Shen L, et al. Lymph node involvement influenced by lung adenocarcinoma subtypes in tumor size ≤3 cm disease: A study of 2268 cases. Eur J Surg Oncol 2016;42:1714-9. [Crossref] [PubMed]
- Defranchi SA, Cassivi SD, Nichols FC, et al. N2 disease in T1 non-small cell lung cancer. Ann Thorac Surg 2009;88:924-8. [Crossref] [PubMed]


