
Impact of antifibrotic therapy on lung cancer incidence and mortality in patients with idiopathic pulmonary fibrosis
Highlight box
Key findings
• In patients with both idiopathic pulmonary fibrosis (IPF) and lung cancer, pirfenidone treatment lowers all-cause mortality compared to those who did not receive it.
What is known, and what is new?
• Patients with IPF have a higher risk of developing lung cancer, even after adjusting for age and smoking history.
• The incidence of lung cancer was higher among IPF patients treated with pirfenidone, but there was no significant difference in mortality compared to those not treated with pirfenidone.
• Pirfenidone therapy showed a significantly better survival rate in IPF patients with lung cancer compared to those without pirfenidone therapy.
What is the implication, and what should change now?
• This study suggests that pirfenidone may have anti-tumor effects and improve survival in IPF patients with lung cancer, while also calling for more research on the link between IPF and lung cancer.
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common type of interstitial lung disease (ILD), accounting for about 20% of ILDs, with its prevalence and incidence are rising globally (1,2). It is characterized by a chronic, progressive fibrosis of the lungs arising from an unknown etiology with a poor prognosis and mean survival of 2 to 3 years before the advances of antifibrotic therapy, which is comparable or even worse than that of several malignant cancers (1-3). IPF typically occurs in the sixth to eighth decades of life and is associated with smoking, which also related to the development of lung cancer (1,2,4-6). IPF and lung cancer frequently co-exist, with a lung cancer prevalence of approximately 10% to 15% among IPF patients (7,8). The risk of lung cancer development in IPF is approximately 6 to 8 times higher than in the general population even after adjusting for age and smoking history (8,9). These two diseases share clinical risk factors such as aging, environmental factors (e.g., cigarette smoke and air pollution), and also share similar biological signaling pathways, making patients with IPF vulnerable to the development of lung cancer (10-12).
Concomitant lung cancer in patients with IPF is difficult to treat and patients with both diseases have poorer clinical outcomes compared to patients with either IPF or lung cancer alone. Even in early-stage lung cancer, impaired lung function and old age reduce the likelihood of surgical treatment (13), while a high incidence of postoperative acute exacerbation (AE) could affect negatively clinical outcomes (14). Moreover, chemotherapy in patients with IPF and lung cancer can induce various cytotoxic effects, and chemotherapy agents and radiotherapy have the potential to induce AE events and treatment-related pneumonitis, which worsen outcomes (15-17). Furthermore, the severity of IPF itself is closely related to the clinical course of lung cancer. The more severe IPF is, the earlier lung cancer will develop and progress to advanced stage (18-20) and weaken treatment effects for lung cancer irrespective of cancer type (21-23).
Pirfenidone is a well-known therapeutic agent that not only slows disease progression, but also reduces rates of AE in IPF, with an acceptable safety and tolerability profile (24-27). Interestingly, based on in vitro studies, the anti-tumor potential of pirfenidone in lung cancer development and progression has recently been gaining attention. Although interest in the association between IPF and lung cancer is growing, data on the incidence of lung cancer and the effect of antifibrotic therapy on the outcomes remains limited. This study aimed to address the lack of comprehensive data in the incidence of lung cancer in IPF patients. Moreover, by evaluating the impact of antifibrotic therapy, we aimed to offer insights into the interaction between IPF and lung cancer, highlighting the potential benefits of antifibrotic treatment. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1356/rc).
Methods
Study population
The National Health Insurance System (NHIS), a single mandatory health coverage system in South Korea, covers the health insurance of almost all Korean citizens. The Korean Health Insurance Review and Assessment Service (HIRA) is a government-affiliated organization that reviews and evaluates the adequacy of all claims data from hospitals. The HIRA database contains information on the details of healthcare utilization for both inpatients and outpatients allowing us to assess major and minor diagnoses using International Classification of Diseases 10th Revision (ICD-10) codes and procedures and prescription data.
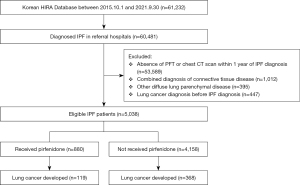
IPF was defined based on both the ICD-10 code for IPF (J841 or J8418) and the Rare Intractable Diseases (RID) code V236. IPF is a disease requiring multidisciplinary discussion on diagnosis and management. Therefore, we only included claims data from referral hospitals. As shown in Figure 1, 60,481 IPF patients were identified and 53,589 subjects whose spirometry and/or chest computed tomography (CT) scans within a year of IPF diagnosis were not available were excluded. To clarify the definition of IPF, connective tissue disease associations and other diffuse lung parenchymal diseases were also excluded. Additional details are available in Table S1. To investigate the association between the incidence of lung cancer and antifibrotic therapy, patients who were diagnosed with lung cancer before IPF diagnosis were excluded.
Exposure
In Korea, pirfenidone has been covered by health insurance since October 2015. Therefore, we collected prescription data on pirfenidone from October 2015 to September 2021. The codes for specific drugs identifiable in the claims database enable us to assess the association with pirfenidone and lung cancer in IPF patients.
Outcomes
The main outcome of this study was to assess (I) the risk of lung cancer incidence depending on pirfenidone therapy. New diagnoses of lung cancer were identified by the ICD-10 code of C34 after IPF diagnosis. The other main outcome was to analyze (II) the risk of mortality stratified by lung cancer development and pirfenidone therapy. Mortality was defined by the absence of claims data from any healthcare facilities for more than a year.
Ethics statement
The study protocol was approved by the institutional review board (IRB) of Seoul St Mary’s Hospital (No. KC22ZASE0545). The requirement for informed consent was waived due to the retrospective nature of the study and anonymity of the HIRA database. The study was performed in accordance with the principles of the Declaration of Helsinki (as revised in 2013) concerning the ethical principles for medical research.
Statistical analysis
Study participants were divided into with pirfenidone and without pirfenidone groups for comparison using Student’s t-test and χ2 test for continuous and categorical variables, respectively. Data are presented as number (%) and mean with standard deviation. To evaluate the incidence of lung cancer in both groups of IPF patients, the incidence rate [per 100 person-years (PY)] was compared, and the risk ratio was calculated to identify the effects of pirfenidone therapy. Cox proportional hazard analysis was used to analyze the risk of mortality according to pirfenidone treatment, and hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated. Age and sex were adjusted as covariates. Then, we used the cumulative incidence function to analyze lung cancer development with or without pirfenidone therapy. All-cause mortality was compared using incidence rate (per 100 PY), and survival curves were applied to assess the impact of lung cancer on mortality. Patients were stratified into four groups based on lung cancer development and pirfenidone use, with mortality risk evaluated using survival curves. Statistical significance between the groups were determined with the log-rank test.
All analyses were two-sided and conducted at a significance level of 0.05 unless otherwise noted. All analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
Among the 5,038 patients with IPF, pirfenidone was administered to 880 patients (Figure 1). Baseline clinical features were described in Table 1. Mean age was 73.3 years old and 72.4% were male. Among the various comorbid conditions, cardiovascular diseases including congestive heart failure, atrial fibrillation and hypertension, diabetes mellitus, and chronic kidney disease were more common in subjects undergoing pirfenidone therapy.
Table 1
| Characteristics | IPF patients | P value | ||
|---|---|---|---|---|
| All patients (N=5,038) | With pirfenidone (N=880) | Without pirfenidone (N=4,158) | ||
| Demographic data | ||||
| Age, years | 73.29±9.68 | 73.48±8.36 | 73.25±9.94 | <0.001 |
| Sex (male) | 3,646 (72.4) | 720 (81.8) | 2,926 (70.4) | <0.001 |
| Insurance type | ||||
| Health insurance | 4,260 (84.6) | 742 (84.3) | 3,518 (84.6) | 0.829 |
| Medical aid | 778 (15.4) | 138 (15.7) | 640 (15.4) | |
| Comorbidity | ||||
| Myocardial infarction | 1,630 (32.4) | 297 (33.8) | 1,333 (32.1) | 0.330 |
| Congestive heart failure | 1,209 (24.0) | 246 (28.0) | 963 (23.2) | 0.003 |
| Atrial fibrillation | 541 (10.7) | 117 (13.3) | 424 (10.2) | 0.007 |
| Hypertension | 3,070 (60.9) | 586 (66.6) | 2,484 (59.7) | <0.001 |
| Peripheral vascular disease | 761 (15.1) | 140 (15.9) | 621 (14.9) | 0.464 |
| CVA or TIA | 881 (17.5) | 155 (17.6) | 726 (17.5) | 0.913 |
| Diabetes mellitus | 2,933 (58.2) | 470 (53.4) | 1,923 (46.3) | <0.001 |
| Chronic kidney disease | 426 (8.5) | 105 (11.9) | 321 (7.7) | <0.001 |
| PTB | 317 (6.3) | 41 (4.7) | 276 (6.6) | 0.028 |
| Malignancy except lung cancer | 1,126 (22.4) | 193 (21.9) | 933 (22.4) | 0.743 |
| Death during follow-up | 1,232 (24.5) | 223 (25.3) | 999 (24.0) | 0.124 |
Data are presented as mean ± standard deviation for continuous variables and number (%) for categorical variables. CVA, cerebrovascular accident; IPF, idiopathic pulmonary fibrosis; TIA, transient ischemic attack; PTB, pulmonary tuberculosis.
Median follow-up duration was 4,872.8 person-years for the group receiving pirfenidone and 23,612.1 person-years for the group not receiving pirfenidone.
Incidence and risk of lung cancer in patients with IPF
Overall, lung cancer occurred in 487 patients: 119/880 (13.5%) in the pirfenidone group and 368/4,158 (8.9%) in the non-pirfenidone group. The incidence of lung cancer was 2.44 per 100 PY (95% CI, 2.02–2.92) in the pirfenidone group and 1.56 per 100 PY (95% CI, 1.40–1.73) in the non-pirfenidone group. Compared to the non-pirfenidone group, the risk ratio for the lung cancer incidence was higher in the pirfenidone group (risk ratio, 1.56; 95% CI, 1.27–1.92) (Table 2).
Table 2
| Events | All IPF subjects | With pirfenidone | Without pirfenidone | Risk ratio [pirfenidone (+) vs. pirfenidone (−) group] |
|---|---|---|---|---|
| Lung cancer cases | ||||
| Number (%) | 487 (9.7) | 119 (13.5) | 368 (8.9) | |
| Person-years | 28,484.93 | 4,872.84 | 23,612.08 | |
| Incidence, per 100 person-years (95% CI) | 1.71 (1.56–1.87) | 2.44 (2.02–2.92) | 1.56 (1.40–1.73) | 1.56 (1.27–1.92) |
| All-cause mortality | ||||
| Number (%) | 1,232 (24.5) | 223 (25.3) | 999 (24.0) | |
| Person-years | 26,955.69 | 4,777.33 | 22,178.36 | |
| Incidence, per 100 person-years (95% CI) | 4.57 (4.32–4.83) | 4.67 (4.08–5.32) | 4.50 (4.23–4.79) | 1.03 (0.89–1.19) |
The risk ratio, with a 95% CI, is calculated in relation to pirfenidone treatment status, with the untreated group as the reference. CI, confidence interval; IPF, idiopathic pulmonary fibrosis.
Effect of pirfenidone therapy on mortality in patients with IPF
Death events occurred in 1,232 patients with IPF: 223 (26.5%) in the pirfenidone group and 999 (24.0%) in the non-pirfenidone group. The incidence of mortality was 4.67 per 100 PY (95% CI, 4.08–5.32) and 4.50 per 100 PY (95% CI, 4.23–4.79) in subjects with and without pirfenidone therapy, respectively. The risk of mortality did not differ between the two groups (risk ratio, 1.03; 95% CI, 0.89–1.19) (Table 2, Figure S1).
The probability of survival of patients with IPF depending on lung cancer development is shown in Figure S2. Compared to those without lung cancer, patients with IPF who developed lung cancer were significantly more likely to die from any cause (log-rank test ≤0.001). Mortality risk was further assessed by lung cancer development and pirfenidone therapy. The comparison of cumulative probability for survival is presented in Figure 2. Patients with IPF were stratified into four groups by lung cancer development and pirfenidone treatment. In IPF patients without lung cancer, pirfenidone therapy was not associated with a difference in mortality risk. However, in patients with lung cancer, the pirfenidone therapy group exhibited significantly better survival compared to those without pirfenidone therapy (log-rank test ≤0.001).

In multivariate Cox proportional hazard analysis, pirfenidone treatment was associated with the survival benefit for mortality in IPF patients with lung cancer (HR, 0.61; 95% CI, 0.43–0.85) (Table 3). Otherwise, the risk for mortality in IPF patients without lung cancer was not affected by pirfenidone use or not.
Table 3
| Treatment status | HR | 95% CI | P value | Age, sex-adjusted | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | ||||
| Pirfenidone use | 1.07 | 0.93–1.24 | 0.330 | 1.06 | 0.92–1.23 | 0.399 |
| Lung cancer patients | 2.35 | 2.03–2.72 | <0.001 | 2.43 | 2.09–2.82 | <0.001 |
| Pirfenidone (+) | 0.58 | 0.41–0.82 | 0.002 | 0.61 | 0.43–0.85 | 0.005 |
| Pirfenidone (−) | Reference | Reference | ||||
CI, confidence interval; IPF, idiopathic pulmonary fibrosis; HR, hazard ratio.
Discussion
In this large national cohort study, we investigated the risk of lung cancer development in patients with IPF and the effect of antifibrotic agents on mortality. Antifibrotic treatment was associated with improved survival in patients with IPF who developed lung cancer, despite a higher incidence of lung cancer in those receiving antifibrotic therapy compared to those who did not. These findings suggest that pirfenidone may not only slow the progression of fibrosis in IPF patients but also exert potential antitumor effects in patients with both IPF and lung cancer.
Although the pathogenetic link between IPF and lung cancer remains unclear, pulmonary fibrosis itself is regarded as a risk factor for lung cancer development beyond the shared risk factors of both diseases such as advanced age and smoking exposure (28-30). These two diseases also share biological signaling pathways such as transforming growth factor (TGF)-β, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF). These signaling pathways enhance epithelial-mesenchymal transition (EMT), cause tissue damage and abnormal repair leading to a disruption in tissue architecture and contribute to fibrosis and carcinogenesis (10,11,31). Multiple genetic and environmental factors are related to repetitive epithelial injury and the remodeling process leading to progressive fibrosis in IPF (32), and these aberrant chronic wound healing processes are associated with not only pulmonary fibrosis, but also tumor occurrence (31). In this process, a key player cells with a similar role to myofibroblasts in IPF has been introduced. The role of cancer-associated fibroblast (CAF) in lung cancer has recently gained attention. It plays various roles in cancer progression including DNA damage, inflammatory signals, oxidative stress, metabolic effects, and matrix remodeling (28,33).
The mechanisms by which antifibrotic therapy reduces the risk of lung cancer development in IPF are not clearly investigated. However, several in vitro and in vivo studies of pirfenidone exhibited potential anti-tumor effects by reverting EMT with the suppression of the TGF- β and FGF pathway (29,34,35) and by inducing apoptosis in CAF (29,36). Furthermore, nintedanib was originally developed as an anticancer drug and approved for non-small cell carcinoma in few countries (29,37). Recently reported in vitro tumor model, pirfenidone demonstrated antineoplatic effects not only by inhibiting TGF-β but also by altering metabolic properties, such as reducing lactate and adenosine 5’-triphosphate (ATP) production, thereby inhibiting glycolysis (38). In addition, when combined with cisplatin, pirfenidone enhanced chemosensitization in cancer cell lines. Although this study did not provide a direct anticancer role for antifibrotics in patients with both IPF and lung cancer, the observed effects of antifibrotic agents suggest that it may have both anti-fibrotic and anti-tumor properties, which could account for the reduced mortality in IPF patients with lung cancer undergoing pirfenidone treatment.
Despite of potential anti-tumor effects of pirfenidone, the incidence of lung cancer was higher in those undergoing pirfenidone therapy in this study. The development of lung cancer in IPF patients may be influenced by shared risk factors such as smoking, but IPF itself is regarded as an independent risk factor for lung cancer (8). Additionally, the severity of IPF plays a significant role in the risk of lung cancer development. Studies have reported that a lower forced vital capacity (FVC) and a more rapid decline in FVC are associated with an increased risk of lung cancer (39,40). Furthermore, lung cancer frequently located in areas abutting or within fibrosis (40). Like other claim data-based studies, we could not accurately assess the severity of IPF using physiological indices such as FVC, diffusing capacity for carbon monoxide (DLCO), or the 6-minute walk test. In South Korea, the reimbursement criteria for the prescription of pirfenidone in IPF are based on a lung function impairment. Pirfenidone was approved for IPF treatment by the Korean Food and Drug Administration in 2012 and it has been covered by the health insurance system since October 2015. Unfortunately, the reimbursement criteria for pirfenidone were strict and it was available to a limited number of patients whose high-resolution CT or surgical lung biopsy findings were compatible with definite interstitial pneumonia patterns and with FVC ≤90% or DLCO ≤80%. Patients with preserved pulmonary function could not be prescribed pirfenidone under health insurance coverage until January 2019. Afterward, patients with FVC >90% and DLCO >80% are eligible to use pirfenidone through health insurance coverage when they met two or more following additional criteria: (I) a decline in the FVC of at least 10% of the predicted value per year or reduction of the FVC more than 200 mL per year; (II) worsening of disease-related symptoms; and (III) deterioration of chest radiographic findings. As a result, patients with mild disease whose lung function was preserved often face difficulty in disease targeted therapy. Therefore, the use of pirfenidone itself can be regarded as a marker reflecting the severity of the disease. As results of this study, the risk of developing lung cancer may have been higher in the pirfenidone treated group with reduced lung function, indicating greater disease severity.
Several studies have reported the beneficial effects of pirfenidone on reducing the risk of lung cancer and improving survival in patients with IPF. Miura et al. (41) investigated lung cancer incidence in patients with IPF from two hospitals in Japan and demonstrated that patients who were treated with pirfenidone were associated with a lower incidence of lung cancer (HR, 0.11; 95% CI, 0.03–0.46). There were only two lung cancer cases in 83 pirfenidone treated patients compared to 39 cases in 178 patients not treated with pirfenidone. More recently, Naoi et al. (42) also showed lower lung cancer incidence (risk ratio, 0.235; 95% CI, 0.092–0.602) and lung cancer-related mortality (risk ratio, 0.106; 95% CI, 0.025–0.450) in patients with IPF treated with antifibrotic therapy of either pirfenidone or nintedanib through 345 patients with IPF. However, there were 5 and 30 lung cancer cases in patients treated and not treated with antifibrotic therapy, respectively. Both studies included a small number of lung cancer cases, which may have contributed to the positive outcomes. Further research is needed to clarify the preventive effects of pirfenidone in patients with IPF. Although the risk for lung cancer incidence was not reduced by pirfenidone therapy in this study, potential survival benefits of pirfenidone in patients with IPF and lung cancer were suggested. We used a nationwide database of 5,038 IPF patients and lung cancer developed in 487 of those patients. Enough lung cancer cases made the results of the study more reliable. However, we could not assume possible mechanisms or causal relationships between pirfenidone treatment and reduced all-cause mortality in patients with IPF and lung cancer due to the retrospective nature of the study. Additional studies are warranted to allow for a better understanding of the relationship between lung cancer and IPF, and antifibrotic agents should be initiated or continued when lung cancer is present unless drug related adverse effects outweigh the risk of unfavorable outcomes from IPF and/or lung cancer.
The present study has several limitations. First, the disease severity of IPF based on pulmonary function test (PFT) and the extent of fibrosis on imaging was not available. Therefore, we could not assess the association between IPF severity and lung cancer incidence and mortality. Second, mortality cases were identified by the absence of claims for more than a year, and the cause of death could not be determined from the claims dataset, which is a potential limitation inherent to studies using claim databases. Third, data on lung cancer stage and anticancer treatments were not available. Fourth, another antifibrotic agent, nintedanib, was not included in the study because it is not covered by the NHIS for IPF treatment yet and its high cost has resulted in limited access (43). Fifth, we were unable to obtain smoking data from the claims database, even though smoking is a well-known risk factor for both IPF and lung cancer. Lastly, there is a lack of external validation.
Conclusions
Lung cancer frequently occurred in IPF patients and was associated with higher mortality. Although antifibrotic therapy with pirfenidone did not lead to survival benefit in IPF patients without lung cancer, it did reduce the risk of mortality in patients with lung cancer. While our findings are promising, further prospective studies are required to establish the pathogenetic link between IPF and lung cancer, as well as to assess the potential synergistic anti-tumor effects of antifibrotic therapy.
Acknowledgments
The content of this paper was presented as a poster at the 2023 European Respiratory Society Conference (European Respiratory Journal 2023;62:PA2880; DOI: 10.1183/13993003.congress-2023.PA2880).
Funding: This research was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1356/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1356/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1356/coif). C.K.R. serves as an unpaid editorial board member of Journal of Thoracic Disease. Y.S.J. reports funding from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. NRF-2022R1I1A1A01063654 to Y.S.J.). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the institutional review board (IRB) of Seoul St Mary’s Hospital (No. KC22ZASE0545). The requirement for informed consent was waived due to the retrospective nature of the study and anonymity of the HIRA database. The study was performed in accordance with the principles of the Declaration of Helsinki (as revised in 2013) concerning the ethical principles for medical research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Chianese M, Screm G, Salton F, et al. Pirfenidone and Nintedanib in Pulmonary Fibrosis: Lights and Shadows. Pharmaceuticals (Basel) 2024;17:709. [Crossref] [PubMed]
- SEER explorer network 2000-2018. Available online: http://seer.cancer.gov/statistics-network [accessed 23 February 2023].
- Raghu G, Amatto VC, Behr J, et al. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J 2015;46:1113-30. [Crossref] [PubMed]
- Baumgartner KB, Samet JM, Stidley CA, et al. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1997;155:242-8. [Crossref] [PubMed]
- Kärkkäinen M, Kettunen HP, Nurmi H, et al. Effect of smoking and comorbidities on survival in idiopathic pulmonary fibrosis. Respir Res 2017;18:160. [Crossref] [PubMed]
- JafariNezhad A. YektaKooshali MH. Lung cancer in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. PLoS One 2018;13:e0202360. [Crossref] [PubMed]
- Brown SW, Dobelle M, Padilla M, et al. Idiopathic Pulmonary Fibrosis and Lung Cancer. A Systematic Review and Meta-analysis. Ann Am Thorac Soc 2019;16:1041-51. [Crossref] [PubMed]
- Choi WI, Park SH, Park BJ, et al. Interstitial Lung Disease and Lung Cancer Development: A 5-Year Nationwide Population-Based Study. Cancer Res Treat 2018;50:374-81. [Crossref] [PubMed]
- Vancheri C. Common pathways in idiopathic pulmonary fibrosis and cancer. Eur Respir Rev 2013;22:265-72. [Crossref] [PubMed]
- Kinoshita T, Goto T. Molecular Mechanisms of Pulmonary Fibrogenesis and Its Progression to Lung Cancer: A Review. Int J Mol Sci 2019;20:1461. [Crossref] [PubMed]
- Ruaro B, Pozzan R, Confalonieri P, et al. Gastroesophageal Reflux Disease in Idiopathic Pulmonary Fibrosis: Viewer or Actor? To Treat or Not to Treat? Pharmaceuticals (Basel) 2022;15:1033. [Crossref] [PubMed]
- Kim HC, Lee S, Song JW. Impact of idiopathic pulmonary fibrosis on clinical outcomes of lung cancer patients. Sci Rep 2021;11:8312. [Crossref] [PubMed]
- Watanabe A, Miyajima M, Mishina T, et al. Surgical treatment for primary lung cancer combined with idiopathic pulmonary fibrosis. Gen Thorac Cardiovasc Surg 2013;61:254-61. [Crossref] [PubMed]
- Kenmotsu H, Naito T, Kimura M, et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol 2011;6:1242-6. [Crossref] [PubMed]
- Goodman CD, Nijman SFM, Senan S, et al. A Primer on Interstitial Lung Disease and Thoracic Radiation. J Thorac Oncol 2020;15:902-13. [Crossref] [PubMed]
- Johkoh T, Lee KS, Nishino M, et al. Chest CT Diagnosis and Clinical Management of Drug-related Pneumonitis in Patients Receiving Molecular Targeting Agents and Immune Checkpoint Inhibitors: A Position Paper from the Fleischner Society. Radiology 2021;298:550-66. [Crossref] [PubMed]
- Tomassetti S, Gurioli C, Ryu JH, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015;147:157-64. [Crossref] [PubMed]
- Antoniou KM, Tomassetti S, Tsitoura E, et al. Idiopathic pulmonary fibrosis and lung cancer: a clinical and pathogenesis update. Curr Opin Pulm Med 2015;21:626-33. [Crossref] [PubMed]
- Lee T, Park JY, Lee HY, et al. Lung cancer in patients with idiopathic pulmonary fibrosis: clinical characteristics and impact on survival. Respir Med 2014;108:1549-55. [Crossref] [PubMed]
- Jang HJ, Park MS, Kim YS, et al. The relationship between the severity of pulmonary fibrosis and the lung cancer stage. J Cancer 2021;12:2807-14. [Crossref] [PubMed]
- Han S, Lee YJ, Park JS, et al. Prognosis of non-small-cell lung cancer in patients with idiopathic pulmonary fibrosis. Sci Rep 2019;9:12561. [Crossref] [PubMed]
- Song MJ, Lim SY, Park JS, et al. Prognosis of Small Cell Lung Cancer with Idiopathic Pulmonary Fibrosis: Assessment according to GAP Stage. J Oncol 2019;2019:5437390. [Crossref] [PubMed]
- Petnak T, Lertjitbanjong P, Thongprayoon C, et al. Impact of Antifibrotic Therapy on Mortality and Acute Exacerbation in Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Chest 2021;160:1751-63. [Crossref] [PubMed]
- Iwata T, Yoshida S, Fujiwara T, et al. Effect of Perioperative Pirfenidone Treatment in Lung Cancer Patients With Idiopathic Pulmonary Fibrosis. Ann Thorac Surg 2016;102:1905-10. [Crossref] [PubMed]
- Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2020;8:147-57. [Crossref] [PubMed]
- Reccardini N, Chernovsky M, Salton F, et al. Pirfenidone in Idiopathic Pulmonary Fibrosis: Real-World Observation on Efficacy and Safety, Focus on Patients Undergoing Antithrombotic and Anticoagulant. Pharmaceuticals (Basel) 2024;17:930. [Crossref] [PubMed]
- Ballester B, Milara J, Cortijo J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Mechanisms and Molecular Targets. Int J Mol Sci 2019;20:593. [Crossref] [PubMed]
- Karampitsakos T, Tzilas V, Tringidou R, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther 2017;45:1-10. [Crossref] [PubMed]
- Bouros D, Hatzakis K, Labrakis H, et al. Association of malignancy with diseases causing interstitial pulmonary changes. Chest 2002;121:1278-89. [Crossref] [PubMed]
- Hua Y, Bergers G. Tumors vs. Chronic Wounds: An Immune Cell's Perspective. Front Immunol 2019;10:2178. [Crossref] [PubMed]
- Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet 2017;389:1941-52. [Crossref] [PubMed]
- Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 2020;20:174-86. [Crossref] [PubMed]
- Di Gregorio J, Robuffo I, Spalletta S, et al. The Epithelial-to-Mesenchymal Transition as a Possible Therapeutic Target in Fibrotic Disorders. Front Cell Dev Biol 2020;8:607483. [Crossref] [PubMed]
- Kurimoto R, Ebata T, Iwasawa S, et al. Pirfenidone may revert the epithelial-to-mesenchymal transition in human lung adenocarcinoma. Oncol Lett 2017;14:944-50. [Crossref] [PubMed]
- Mediavilla-Varela M, Boateng K, Noyes D, et al. The anti-fibrotic agent pirfenidone synergizes with cisplatin in killing tumor cells and cancer-associated fibroblasts. BMC Cancer 2016;16:176. [Crossref] [PubMed]
- Tzouvelekis A, Gomatou G, Bouros E, et al. Common Pathogenic Mechanisms Between Idiopathic Pulmonary Fibrosis and Lung Cancer. Chest 2019;156:383-91. [Crossref] [PubMed]
- Zhang S, Wang Y, Luo D, et al. Pirfenidone inhibits TGF-β1-induced metabolic reprogramming during epithelial-mesenchymal transition in non-small cell lung cancer. J Cell Mol Med 2024;28:e18059. [Crossref] [PubMed]
- Wasswa-Kintu S, Gan WQ, Man SF, et al. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax 2005;60:570-5. [Crossref] [PubMed]
- Yoo H, Jeong BH, Chung MJ, et al. Risk factors and clinical characteristics of lung cancer in idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med 2019;19:149. [Crossref] [PubMed]
- Miura Y, Saito T, Tanaka T, et al. Reduced incidence of lung cancer in patients with idiopathic pulmonary fibrosis treated with pirfenidone. Respir Investig 2018;56:72-9. [Crossref] [PubMed]
- Naoi H, Suzuki Y, Mori K, et al. Impact of antifibrotic therapy on lung cancer development in idiopathic pulmonary fibrosis. Thorax 2022;77:727-30. [Crossref] [PubMed]
- Jegal Y, Park JS, Kim SY, et al. Clinical Features, Diagnosis, Management, and Outcomes of Idiopathic Pulmonary Fibrosis in Korea: Analysis of the Korea IPF Cohort (KICO) Registry. Tuberc Respir Dis (Seoul) 2022;85:185-94. [Crossref] [PubMed]


