
Prognostic analysis of acute type A aortic dissection after different surgical interventions: a cohort study
Highlight box
Key findings
• After propensity score matching (PSM), the 3-year survival rates in the Sun procedure group and the non-Sun procedure group were 85.02% [95% confidence interval (CI): 80.3–90.03%] and 91.40% (95% CI: 85.90–97.28%), respectively (P=0.12).
• At the point of 3-year follow-up, the probability of no distal aortic events (DAEs) was 97.17% (95% CI: 94.76–99.65%) and 91.59% (95% CI: 85.80–97.78%) in the Sun procedure group and the non-Sun procedure group, respectively (P=0.02).
What is known and what is new?
• The incidence of DAE with the Sun procedure was lower than that without the Sun procedure.
• There was no significant difference in postoperative mortality between the groups.
What is the implication, and what should change now?
• When conditions permit, the Sun procedure should be actively considered in the treatment of acute type A aortic dissection.
Introduction
Aortic dissection represents an acute course of disease and is most commonly categorized via the Stanford classification, which defines aortic dissection as type A, if the lesion involves the ascending aorta, and type B if the lesion doesn’t involve the ascending aorta (1). Acute type A aortic dissection (ATAAD) is generally defined as the presence of type A aortic dissection occurring within 14 days of onset (2). The annual incidence of ATAAD is approximately 6–30 per 100,000, while the mortality rate ranges from 58% to 62% (1). Moreover, the incidence of type A dissection is likely to be underestimated, as a significant number of patients die before reaching the hospital or being diagnosed. The principle of treatment for type A aortic dissection is to eliminate the primary entry tear, and surgery remains the priority method for the treatment of ATAAD. The surgical procedure is usually selected according to the location of the primary entry tear and the extent of dissection involvement. The ascending aorta and aortic root can be treated with ascending aorta replacement or aortic root replacement (Bentall surgery). For the lesions of the aortic arch and descending aorta, total arch replacement (TAR) plus frozen elephant trunk (FET) stent implantation (the Sun procedure) and partial arch replacement are typically implemented. In some cases, hybrid surgery combined with open surgery and interventional stent implantation can be applied (3). However, the optimal surgical choice for the treatment of type A aortic dissection with regards to the distal extension of the disease remains controversial.
We thus compared the prognosis of patients who underwent the Sun procedure with that of those who were not treated with the Sun procedure, with both groups having similar preoperative conditions. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2048/rc).
Methods
Patients
The data of all patients with ATAAD admitted to Beijing Anzhen Hospital from August 1, 2020, to August 16, 2022, were collected. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Beijing Anzhen Hospital of Capital Medical University approved this retrospective study (No. 2024246x). The requirement for individual consent was waived due to the retrospective nature of the analysis. In this study, 452 patients were included, including 344 patients who received the Sun procedure and 108 patients who did not, irrespective of the surgical technique concerning the proximal part of the aorta including either a Bentall procedure or a tube graft prosthesis replacing the ascending aorta. Those who did not receive surgical treatment and those whose interval between operation and onset of dissection was more than 14 days were excluded.
Definitions
For this study, cardiogenic shock was defined as systolic blood pressure ≤90 mmHg, cardiac index ≤2.0 L/min/m2, or the need for intravenous inotropic drugs. Pneumonia was defined as fever or elevated white blood cell count in patients with inflammatory infiltration on chest radiography or lung computed tomography (CT). Respiratory failure was defined as a blood gas oxygen partial pressure ≤60 mmHg or an oxygenation index ≤300 mmHg. Sepsis was defined as fever or elevated white blood cell count with a positive blood culture. Prolonged mechanical ventilation (PMV) was defined as a postoperative mechanical ventilation time ≥48 hours. Malperfusion syndrome (MPS) for all organs was defined as preoperative aortic CT angiography (CTA) showing the involvement of the corresponding vessels by dissection with the following concomitant symptoms: cerebral MPS (stroke symptoms in the corresponding vascular area with positive head CT findings), spinal MPS (paraplegia), limb MPS (corresponding to sensory or motor deficits or loss of pulse in the limb), renal MPS (serum creatinine ≥442 µmoI/L, oliguria, or both), mesenteric MPS (abdominal pain, abdominal tenderness, or gastrointestinal bleeding), and myocardial MPS (myocardial infarction in the corresponding area with elevated troponin). Distal aortic events (DAEs) were defined as postoperative distal aortic dilation ≥50 mm in diameter or ≥10 mm/y in velocity, with or without surgical intervention (4,5).
Surgical indications and techniques
Patients in the Sun procedure group received proximal treatment and the Sun procedure; the specific details of the Sun procedure have been in previous studies from our center (6,7). The non-Sun procedure group received proximal treatment with or without partial arch replacement. The principle of surgical treatment for ATAAD was to eliminate the primary entry tear. The Sun procedure was considered for patients with the following conditions: (I) aortic arch dilation; (II) severe dissection of brachiocephalic artery; (III) complication with Marfan syndrome; (IV) a primary entry tear located at the aortic arch; and (V) younger age and good physical condition. The operation included proximal treatment, arch treatment, and distal treatment. In this study, patients underwent proximal management including simple ascending aorta replacement, aortic root replacement (Bentall surgery), and David surgery. Aortic valve replacement was considered when any of the following occurred: (I) patients with moderate or severe aortic regurgitation indicated by preoperative echocardiography; (II) patients with large aortic root aneurysm; (III) patients with severe damage on the sinus canal junction that made it difficult to proximal anastomosis when performing ascending aorta replacement. The treatment of the arch included partial arch replacement (the distal end of the artificial vessel replacement was located at the proximal end of the left common carotid artery opening, without replacing the brachiocephalic arteries) and TAR. The distal treatment method is FET technique. Right axillary artery intubation or innominate artery intubation was used for unilateral selective cerebral perfusion. For those who have not used the right axillary artery or the innominate artery as the inflow site for cardiopulmonary bypass (CPB) for various reasons, a left common carotid artery cannulation would be chosen to perform unilateral selective cerebral perfusion. In individual preoperative cases where there were evidences of higher risks of brain complications, left common carotid artery intubation was performed in conjunction with the right axillary artery or innominate artery cannula for bilateral selective brain perfusion. The flow rate of cerebral perfusion was 5 mL/kg/min.
Clinical and imaging follow-up
Follow-up began immediately after the operation. Follow-up information included postoperative all-cause death and its causes, secondary aortic surgery, and postoperative aortic CT. Aortic CT review was performed at discharge; at 3 months, 6 months, and 1 year after surgery; and annually thereafter. According to the partitioning method proposed by Fillinger et al., the aorta was divided into 11 zones (8). The maximum diameter (mm) was measured from the axial plane of the outer wall of the aorta in each zone, and the maximum aortic diameter among all zones was recorded. The aortic dilation rate (mm/y) was calculated by dividing the difference between the diameter of the last CT and that of the first CT by an interval of more than 6 months.
Statistical analysis
Data processing and analysis were performed using SPSS 26 (IBM Corp., Armonk, NY, USA) for Windows and R version 4.4.0 (2024-04-24; The R Foundation for Statistical Computing). Since the number of cases included in each group of this study was more than 30, the continuous variables were treated according to a normal distribution and are expressed as the mean ± standard deviation. The t-test was used to analyze continuous variables, while the chi-square test was used for categorical variables. The nearest matching method was used for PSM, and the caliper value was set to 0.1. The Sun procedure group was used as the experimental group, and the non-Sun procedure group was used as the control group in 1:3 matching. Preoperative factors that could potentially affect prognosis as determined by clinical experience were included in the matching. Common baseline data and preoperative factors that may affect postoperative prognosis reported by clinicians in our center and previous literature were comprehensively referred to as variables for PSM. The Kaplan-Meier (K-M) method was used to analyze postoperative midterm survival and DAEs, and the log-rank test was used to compare the results between groups. Risk factors for postoperative midterm death and DAEs were analyzed via Cox regression. Due to the possible competition between postoperative death and DAEs, sensitivity analysis of DAEs was performed using Fine-Gray analysis. Risk factors for postoperative 30-day death were analyzed by logistic regression. The variables included in the multivariate regression analysis were selected with comprehensive reference to the results of univariate analysis, clinical experience and previous literature reports. With the exception of Marfan syndrome, all variables included in the regression analysis were intraoperative or postoperative factors. P<0.05 was considered statistically significant. All data produced in the study are provided in the article.
Results
PSM analysis
A total of 300 patients were included after propensity score matching (PSM) between the two groups was completed, with 207 patients in the Sun procedure group and 93 patients in the non-Sun procedure group. The groups were well balanced, as shown in Table 1 and Figure 1. The results of PSM are shown in Table 1. Intraoperative data and postoperative complications after matching are shown in Tables 2,3, respectively. The comparison of postoperative results after PSM is shown in Table 3. The incidence of postoperative spinal MPS in the Sun procedure group was higher than that in the non-Sun procedure group (P=0.003).
Table 1
| Variable | Before PSM | After PSM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=452) | 0 (n=344) | 1 (n=108) | Statistic | P | SMD | Total (n=300) | 0 (n=207) | 1 (n=93) | Statistic | P | SMD | ||
| Age (years) | 49.46±11.67 | 48.62±10.86 | 52.12±13.67 | t=−2.432 | 0.02 | 0.256 | 50.72±11.73 | 50.31±10.73 | 51.63±13.71 | t=-0.825 | 0.41 | 0.097 | |
| Height (cm) | 171.78±7.94 | 172.17±7.62 | 170.52±8.80 | t=1.896 | 0.06 | −0.188 | 171.34±8.09 | 171.71±7.87 | 170.49±8.54 | t=1.210 | 0.23 | −0.143 | |
| Weight (kg) | 79.24±14.80 | 80.51±14.42 | 75.17±15.30 | t=3.311 | 0.001 | −0.349 | 77.64±13.88 | 78.55±13.61 | 75.60±14.33 | t=1.709 | 0.09 | −0.206 | |
| BSA (m2) | 1.92±0.21 | 1.93±0.20 | 1.86±0.22 | t=3.267 | 0.001 | −0.328 | 1.89±0.20 | 1.91±0.19 | 1.86±0.21 | t=1.878 | 0.06 | −0.224 | |
| BMI (kg/m2) | 26.79±4.31 | 27.12±4.30 | 25.75±4.18 | t=2.906 | 0.004 | −0.328 | 26.39±3.96 | 26.59±3.87 | 25.95±4.12 | t=1.295 | 0.20 | −0.155 | |
| SBP (mmHg) | 129.02±24.15 | 131.66±22.68 | 120.59±26.77 | t=3.882 | <0.001 | −0.413 | 127.25±24.94 | 128.55±24.89 | 124.37±24.95 | t=1.344 | 0.18 | −0.168 | |
| DBP (mmHg) | 74.12±15.09 | 75.13±14.39 | 70.90±16.79 | t=2.360 | 0.02 | −0.252 | 73.79±15.46 | 74.44±15.40 | 72.33±15.58 | t=1.094 | 0.28 | −0.135 | |
| Number of MPS | 0.35±0.65 | 0.37±0.69 | 0.31±0.52 | t=0.885 | 0.38 | −0.122 | 0.30±0.58 | 0.30±0.60 | 0.30±0.53 | t=0.045 | 0.96 | −0.006 | |
| Male | 349 (77.21) | 280 (81.40) | 69 (63.89) | χ²=14.317 | <0.001 | −0.364 | 220 (73.33) | 157 (75.85) | 63 (67.74) | χ²=2.155 | 0.14 | −0.173 | |
| Grade 2 or higher hypertension | 390 (86.28) | 302 (87.79) | 88 (81.48) | χ²=2.764 | 0.096 | −0.162 | 255 (85.00) | 178 (85.99) | 77 (82.80) | χ²=0.514 | 0.47 | −0.085 | |
| Stroke | 16 (3.54) | 9 (2.62) | 7 (6.48) | χ²=2.553 | 0.11 | 0.157 | 11 (3.67) | 8 (3.86) | 3 (3.23) | χ²=0.000 | >0.99 | −0.036 | |
| NYHA II and above | 47 (10.4) | 33 (9.59) | 14 (12.96) | χ²=1.002 | 0.32 | 0.100 | 33 (11.00) | 22 (10.63) | 11 (11.83) | χ²=0.094 | 0.76 | 0.037 | |
| Moderate or severe AI | 228 (50.44) | 175 (50.87) | 53 (49.07) | χ²=0.106 | 0.74 | −0.036 | 158 (52.67) | 111 (53.62) | 47 (50.54) | χ²=0.245 | 0.62 | −0.062 | |
| Marfan syndrome | 11 (2.43) | 9 (2.62) | 2 (1.85) | χ²=0.008 | 0.93 | −0.057 | 7 (2.33) | 5 (2.42) | 2 (2.15) | χ²=0.000 | >0.99 | −0.018 | |
| Alcohol abuse | 143 (31.64) | 121 (35.17) | 22 (20.37) | χ²=8.329 | 0.004 | −0.368 | 74 (24.67) | 54 (26.09) | 20 (21.51) | χ²=0.725 | 0.40 | −0.112 | |
| Smoking | 153 (33.85) | 124 (36.05) | 29 (26.85) | χ²=3.103 | 0.08 | −0.207 | 87 (29.00) | 61 (29.47) | 26 (27.96) | χ²=0.071 | 0.79 | −0.034 | |
| History of cardio-aortic surgery | 13 (2.88) | 10 (2.91) | 3 (2.78) | χ²=0.000 | >0.99 | −0.008 | 12 (4.00) | 9 (4.35) | 3 (3.23) | χ²=0.020 | 0.89 | −0.064 | |
| History of cardio-aortic intervention | 17 (3.76) | 16 (4.65) | 1 (0.93) | χ²=2.206 | 0.14 | −0.389 | 7 (2.33) | 6 (2.90) | 1 (1.08) | χ²=0.307 | 0.58 | −0.177 | |
| Cardiogenic shock | 51 (11.28) | 28 (8.14) | 23 (21.30) | χ²=14.214 | <0.001 | 0.321 | 40 (13.33) | 25 (12.08) | 15 (16.13) | χ²=0.912 | 0.34 | 0.110 | |
| Upper limb MPS | 19 (4.20) | 16 (4.65) | 3 (2.78) | χ²=0.327 | 0.57 | −0.114 | 7 (2.33) | 4 (1.93) | 3 (3.23) | χ²=0.074 | 0.79 | 0.073 | |
| Lower limb MPS | 60 (13.27) | 52 (15.12) | 8 (7.41) | χ²=4.243 | 0.04 | −0.294 | 30 (10.00) | 22 (10.63) | 8 (8.60) | χ²=0.293 | 0.59 | −0.072 | |
| Cerebral MPS | 15 (3.32) | 12 (3.49) | 3 (2.78) | χ²=0.003 | 0.96 | −0.043 | 11 (3.67) | 8 (3.86) | 3 (3.23) | χ²=0.000 | >0.99 | −0.036 | |
| Spinal MPS | 2 (0.44) | 2 (0.58) | 0 (0.00) | – | >0.99 | −0.088 | 2 (0.67) | 2 (0.97) | 0 (0.00) | – | >0.99 | −0.119 | |
| Renal MPS | 29 (6.42) | 22 (6.40) | 7 (6.48) | χ²=0.001 | 0.98 | 0.003 | 18 (6.00) | 11 (5.31) | 7 (7.53) | χ²=0.557 | 0.46 | 0.084 | |
| Mesenteric MPS | 2 (0.44) | 2 (0.58) | 0 (0.00) | – | >0.99 | −0.088 | 1 (0.33) | 1 (0.48) | 0 (0.00) | – | >0.99 | −0.084 | |
| Myocardial MPS | 33 (7.3) | 21 (6.10) | 12 (11.11) | χ²=3.044 | 0.08 | 0.159 | 22 (7.33) | 15 (7.25) | 7 (7.53) | χ²=0.007 | 0.93 | 0.011 | |
| CRRT | 1 (0.22) | 0 (0.00) | 1 (0.93) | – | 0.24 | 0.097 | 1 (0.33) | 0 (0.00) | 1 (1.08) | – | 0.31 | 0.104 | |
Data are presented as mean ± standard deviation or n (%). t, t-test; χ², chi-square test; –, Fisher exact test. 0, Sun procedure group; 1, non-Sun procedure group. PSM, propensity score matching; BSA, body surface area; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MPS, malperfusion syndrome; NYHA, New York Heart Association; AI, aortic valve insufficiency; CRRT, continuous renal replacement therapy.
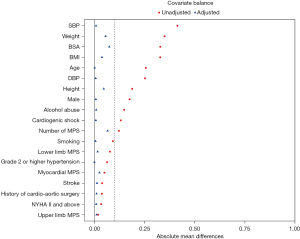
Table 2
| Variable | Before PSM | After PSM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=452) | 0 (n=344) | 1 (n=108) | Statistic | P | Total (n=300) | 0 (n=207) | 1 (n=93) | Statistic | P | ||
| SCP time (min) | 20.37±12.25 | 22.55±12.26 | 13.44±9.31 | t=8.18 | <0.001 | 19.49±12.03 | 22.34±12.14 | 13.16±9.03 | t=7.28 | <0.001 | |
| CPB time (min) | 189.99±47.57 | 195.09±45.27 | 173.76±51.18 | t=4.14 | <0.001 | 188.38±47.07 | 196.61±44.19 | 170.05±48.36 | t=4.67 | <0.001 | |
| Cardiac ischemic time (min) | 106.29±29.80 | 108.99±30.37 | 97.69±26.22 | t=3.48 | <0.001 | 104.91±28.49 | 108.71±29.26 | 96.44±24.80 | t=3.52 | <0.001 | |
| Pharyngeal temperature (℃) | 25.77±2.55 | 25.59±2.47 | 26.33±2.71 | t=−2.63 | 0.009 | 25.82±2.64 | 25.59±2.56 | 26.35±2.76 | t=−2.31 | 0.02 | |
| Anal temperature (℃) | 26.66±2.52 | 26.59±2.43 | 26.91±2.79 | t=−1.15 | 0.25 | 26.70±2.61 | 26.59±2.51 | 26.94±2.83 | t=−1.05 | 0.29 | |
| Bentall operation | 203 (44.91) | 142 (41.28) | 61 (56.48) | χ²=7.68 | 0.01 | 151 (50.33) | 96 (46.38) | 55 (59.14) | χ²=4.18 | 0.04 | |
| David operation | 2 (0.44) | 1 (0.29) | 1 (0.93) | – | 0.421 | 2 (0.67) | 1 (0.48) | 1 (1.08) | – | 0.53 | |
| Ascending aorta replacement | 244 (53.98) | 199 (57.85) | 45 (41.67) | χ²=8.66 | 0.003 | 144 (48.00) | 108 (52.17) | 36 (38.71) | χ²=4.66 | 0.03 | |
| Partial arch replacement | 90 (19.91) | 0 (0.00) | 90 (83.33) | χ²=357.94 | <0.001 | 77 (25.67) | 0 (0.00) | 77 (82.80) | χ²=230.57 | <0.001 | |
| Mitral valve surgery | 5 (1.11) | 3 (0.87) | 2 (1.85) | χ²=0.10 | 0.75 | 3 (1.00) | 1 (0.48) | 2 (2.15) | – | 0.23 | |
| Tricuspid valve surgery | 4 (0.88) | 4 (1.16) | 0 (0.00) | – | 0.58 | 3 (1.00) | 3 (1.45) | 0 (0.00) | – | 0.56 | |
| CABG | 35 (7.74) | 27 (7.85) | 8 (7.41) | χ²=0.02 | 0.88 | 21 (7.00) | 14 (6.76) | 7 (7.53) | χ²=0.06 | 0.81 | |
| Femoral artery cannulation | 65 (14.38) | 32 (9.30) | 33 (30.56) | χ²=30.15 | <0.001 | 47 (15.67) | 18 (8.70) | 29 (31.18) | χ²=24.56 | <0.001 | |
| Axillary artery cannulation | 246 (54.42) | 196 (56.98) | 50 (46.30) | χ²=3.78 | 0.052 | 158 (52.67) | 117 (56.52) | 41 (44.09) | χ²=3.98 | 0.046 | |
| Femoral and axillary artery cannulation | 129 (28.54) | 109 (31.69) | 20 (18.52) | χ²=6.99 | 0.01 | 83 (27.67) | 65 (31.40) | 18 (19.35) | χ²=4.65 | 0.03 | |
| Innominate artery cannulation | 23 (5.09) | 18 (5.23) | 5 (4.63) | χ²=0.06 | 0.80 | 17 (5.67) | 12 (5.80) | 5 (5.38) | χ²=0.02 | 0.88 | |
| Left common carotid artery cannulation | 84 (18.58) | 71 (20.64) | 13 (12.04) | χ²=4.02 | 0.045 | 53 (17.67) | 43 (20.77) | 10 (10.75) | χ²=4.43 | 0.04 | |
| DHCA | 427 (94.47) | 344 (100.00) | 83 (76.85) | χ²=84.29 | <0.001 | 277 (92.33) | 207 (100.00) | 70 (75.27) | χ²=55.44 | <0.001 | |
| SCP | 426 (94.25) | 344 (100.00) | 82 (75.93) | χ²=87.87 | <0.001 | 277 (92.33) | 207 (100.00) | 70 (75.27) | χ²=55.44 | <0.001 | |
Data are presented as mean ± standard deviation or n (%). t, t-test; χ², chi-square test; –, Fisher exact test. 0, Sun’s procedure group; 1, non-Sun’s procedure group. PSM, propensity score matching; SCP, selective cerebral perfusion; CPB, cardiopulmonary bypass; CABG, coronary artery bypass grafting; DHCA, deep hypothermia circulatory arrest.
Table 3
| Variable | Before PSM | After PSM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=452) | 0 (n=344) | 1 (n=108) | Statistic | P | Total (n=300) | 0 (n=207) | 1 (n=93) | Statistic | P | ||
| Number of MPS | 0.60±0.93 | 0.61±0.94 | 0.54±0.88 | t=0.75 | 0.46 | 0.60±0.90 | 0.65±0.93 | 0.51±0.83 | t=1.27 | 0.21 | |
| Pneumonia | 57 (12.61) | 47 (13.66) | 10 (9.26) | χ²=1.45 | 0.23 | 41 (13.67) | 32 (15.46) | 9 (9.68) | χ²=1.82 | 0.18 | |
| Exploratory thoracotomy | 20 (4.42) | 17 (4.94) | 3 (2.78) | χ²=0.47 | 0.49 | 10 (3.33) | 8 (3.86) | 2 (2.15) | χ²=0.17 | 0.68 | |
| Cardiogenic shock | 18 (3.98) | 13 (3.78) | 5 (4.63) | χ²=0.01 | 0.91 | 12 (4.00) | 9 (4.35) | 3 (3.23) | χ²=0.02 | 0.89 | |
| Tracheotomy | 23 (5.09) | 16 (4.65) | 7 (6.48) | χ²=0.57 | 0.45 | 18 (6.00) | 12 (5.80) | 6 (6.45) | χ²=0.05 | 0.83 | |
| Respiratory failure | 38 (8.41) | 30 (8.72) | 8 (7.41) | χ²=0.18 | 0.67 | 28 (9.33) | 21 (10.14) | 7 (7.53) | χ²=0.52 | 0.47 | |
| Sepsis | 15 (3.32) | 12 (3.49) | 3 (2.78) | χ²=0.00 | 0.96 | 11 (3.67) | 9 (4.35) | 2 (2.15) | χ²=0.37 | 0.55 | |
| ECMO | 4 (0.88) | 3 (0.87) | 1 (0.93) | – | >0.99 | 2 (0.67) | 1 (0.48) | 1 (1.08) | – | 0.53 | |
| PMV | 124 (27.43) | 96 (27.91) | 28 (25.93) | χ²=0.16 | 0.69 | 86 (28.67) | 62 (29.95) | 24 (25.81) | χ²=0.54 | 0.46 | |
| Upper limb MPS | 4 (0.88) | 4 (1.16) | 0 (0.00) | – | 0.58 | 1 (0.33) | 1 (0.48) | 0 (0.00) | – | >0.99 | |
| Lower limb MPS | 14 (3.10) | 13 (3.78) | 1 (0.93) | χ²=1.38 | 0.24 | 6 (2.00) | 5 (2.42) | 1 (1.08) | χ²=0.10 | 0.75 | |
| Cerebral MPS | 61 (13.50) | 45 (13.08) | 16 (14.81) | χ²=0.21 | 0.65 | 47 (15.67) | 32 (15.46) | 15 (16.13) | χ²=0.02 | 0.88 | |
| Spinal MPS | 25 (5.53) | 25 (7.27) | 0 (0.00) | χ²=8.31 | 0.004 | 19 (6.33) | 19 (9.18) | 0 (0.00) | χ²=9.11 | 0.003 | |
| Renal MPS | 129 (28.54) | 98 (28.49) | 31 (28.70) | χ²=0.00 | 0.97 | 87 (29.00) | 61 (29.47) | 26 (27.96) | χ²=0.07 | 0.79 | |
| Mesenteric MPS | 23 (5.09) | 18 (5.23) | 5 (4.63) | χ²=0.06 | 0.80 | 13 (4.33) | 10 (4.83) | 3 (3.23) | χ²=0.11 | 0.75 | |
| Myocardial MPS | 13 (2.88) | 8 (2.33) | 5 (4.63) | χ²=0.85 | 0.36 | 8 (2.67) | 6 (2.90) | 2 (2.15) | χ²=0.00 | >0.99 | |
| CRRT | 68 (15.04) | 57 (16.57) | 11 (10.19) | χ²=2.62 | 0.11 | 41 (13.67) | 32 (15.46) | 9 (9.68) | χ²=1.82 | 0.18 | |
| 30-day death | 48 (10.62) | 40 (11.63) | 8 (7.41) | χ²=1.54 | 0.21 | 31 (10.33) | 26 (12.56) | 5 (5.38) | χ²=3.57 | 0.06 | |
Data are presented as mean ± standard deviation or n (%). t, t-test; χ², chi-square test; –, Fisher exact test. 0, Sun procedure group; 1, non-Sun procedure group. PSM, propensity score matching; MPS, malperfusion syndrome; ECMO, extracorporeal membrane oxygenation; PMV, prolonged mechanical ventilation; CRRT, continuous renal replacement therapy.
Analysis of postoperative 30-day death
A total of 26 deaths occurred in the Sun procedure group while 5 occurred in the non-Sun procedure group at the point of 30-day after the operation. The postoperative 30-day mortality rates were 12.56% and 5.38% in the Sun procedure group and the non-Sun procedure group after PSM, respectively (P=0.06) (Table 3). Although there was no statistical difference in postoperative 30-day mortality rates between the two groups, Sun procedure group did have a higher death rate than the other group. Multivariate logistic regression (Table 4) showed that the risk factors for postoperative 30-day death were postoperative cardiogenic shock [odds ratio (OR) =5.16, 95% confidence interval (CI): 1.16–22.95; P=0.03], postoperative cerebral MPS (OR =7.01, 95% CI: 2.57–19.08; P<0.001), postoperative spinal MPS (OR =4.35, 95% CI: 1.20–15.81; P=0.03), and need for CRRT (OR =8.09, 95% CI: 3.05–21.45; P<0.001). According to Table 3, although there was no significant difference, the probability of postoperative cardiogenic shock and need for CRRT was slightly higher in Sun procedure group. While the incidence of postoperative cerebral MPS was very similar between the two groups.
Table 4
| Variables | Univariate | Multivariate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | S.E | Z | P | OR (95% CI) | β | S.E | Z | P | OR (95% CI) | ||
| Non-Sun procedure | −17.27 | 1,115.14 | −0.02 | 0.99 | 0.00 (0.00–Inf) | −0.79 | 0.61 | −1.29 | 0.2 | 0.45 (0.14–1.51) | |
| DHCA time | 0.03 | 0.02 | 1.75 | 0.08 | 1.03 (1.00–1.06) | ||||||
| CPB time | 0.01 | 0.00 | 3.77 | <0.001 | 1.01 (1.01–1.02) | ||||||
| Cardiac ischemic time | 0.02 | 0.01 | 3.48 | <0.001 | 1.02 (1.01–1.03) | ||||||
| Marfan syndrome | 0.38 | 1.10 | 0.35 | 0.73 | 1.46 (0.17–12.55) | ||||||
| Mitral valve surgery | 1.49 | 1.24 | 1.20 | 0.23 | 4.45 (0.39–50.54) | ||||||
| Tricuspid valve surgery | −13.42 | 840.27 | −0.02 | 0.99 | 0.00 (0.00–Inf) | ||||||
| CABG | −0.10 | 0.77 | −0.13 | 0.90 | 0.91 (0.20–4.09) | ||||||
| Femoral artery cannulation | 0.51 | 0.46 | 1.11 | 0.27 | 1.67 (0.67–4.13) | ||||||
| Axillary artery cannulation | 0.24 | 0.38 | 0.63 | 0.53 | 1.28 (0.60–2.71) | ||||||
| Femoral and axillary artery cannulation | −0.51 | 0.47 | −1.08 | 0.28 | 0.60 (0.24–1.52) | ||||||
| Innominate artery cannulation | 0.67 | 0.67 | 1.00 | 0.32 | 1.95 (0.53–7.21) | ||||||
| Left common carotid artery cannulation | 0.35 | 0.46 | 0.75 | 0.45 | 1.41 (0.58–3.48) | 0.86 | 0.53 | 1.63 | 0.10 | 2.37 (0.84–6.71) | |
| DHCA | 15.49 | 824.92 | 0.02 | 0.99 | 5,361,338.12 (0.00–Inf) | ||||||
| SCP | 15.49 | 824.92 | 0.02 | 0.99 | 5,361,338.12 (0.00–Inf) | ||||||
| Pneumonia | 1.30 | 0.43 | 3.02 | 0.003 | 3.66 (1.58–8.48) | ||||||
| Exploratory thoracotomy | 0.81 | 0.81 | 1.00 | 0.32 | 2.25 (0.46–11.10) | ||||||
| Cardiogenic shock | 2.35 | 0.61 | 3.83 | <0.001 | 10.52 (3.16–35.06) | 1.64 | 0.76 | 2.16 | 0.03 | 5.16 (1.16–22.95) | |
| Tracheotomy | 1.33 | 0.57 | 2.36 | 0.02 | 3.79 (1.25–11.46) | −1.24 | 0.79 | −1.57 | 0.12 | 0.29 (0.06–1.36) | |
| Respiratory failure | 2.10 | 0.45 | 4.65 | <0.001 | 8.15 (3.37–19.75) | ||||||
| Sepsis | 1.25 | 0.71 | 1.77 | 0.08 | 3.50 (0.88–13.94) | ||||||
| ECMO | 2.19 | 1.43 | 1.53 | 0.13 | 8.93 (0.54–146.52) | ||||||
| PMV | 2.06 | 0.42 | 4.89 | <0.001 | 7.83 (3.43–17.86) | ||||||
| Upper limb MPS | −12.41 | 882.74 | −0.01 | 0.99 | 0.00 (0.00–Inf) | ||||||
| Lower limb MPS | 2.25 | 0.84 | 2.68 | 0.007 | 9.50 (1.83–49.32) | ||||||
| Cerebral MPS | 2.10 | 0.41 | 5.17 | <0.001 | 8.19 (3.69–18.18) | 1.95 | 0.51 | 3.81 | <0.001 | 7.01 (2.57–19.08) | |
| Spinal MPS | 1.55 | 0.54 | 2.90 | 0.004 | 4.73 (1.65–13.52) | 1.47 | 0.66 | 2.24 | 0.03 | 4.35 (1.20–15.81) | |
| Renal MPS | 2.63 | 0.48 | 5.52 | <0.001 | 13.91 (5.46–35.44) | ||||||
| Mesenteric MPS | 2.20 | 0.59 | 3.69 | <0.001 | 8.98 (2.80–28.80) | ||||||
| Myocardial MPS | 1.73 | 0.76 | 2.29 | 0.02 | 5.66 (1.28–24.94) | ||||||
| CRRT | 2.70 | 0.42 | 6.35 | <0.001 | 14.81 (6.45–34.02) | 2.09 | 0.50 | 4.20 | <0.001 | 8.09 (3.05–21.45) | |
| Number of MPS | 1.38 | 0.21 | 6.46 | <0.001 | 3.99 (2.62–6.06) | ||||||
DHCA, deep hypothermia circulatory arrest; CPB, cardiopulmonary bypass; CABG, coronary artery bypass grafting; SCP, selective cerebral perfusion; ECMO, extracorporeal membrane oxygenation; PMV, prolonged mechanical ventilation; MPS, malperfusion syndrome; CRRT, continuous renal replacement therapy; S.E, standard error; OR, odds ratio; CI, confidence interval.
Analysis of postoperative midterm death
At the end of follow-up, a total of 31 deaths occurred in the Sun procedure group while 8 occurred in the non-Sun procedure group. A K-M analysis was performed for postoperative death, as shown in Figure 2. The 3-year survival rates were 85.02% (95% CI: 80.30–90.03%) and 91.40% (95% CI: 85.87–97.28%) in the Sun procedure group and the non-Sun procedure group after PSM, respectively (P=0.12) (Tables 5,6). Multivariate Cox regression (Table 7) showed that the risk factors for postoperative death were postoperative cardiogenic shock [hazard ratio (HR) =2.98, 95% CI: 1.29–6.92; P=0.01], postoperative cerebral MPS (HR =5.34, 95% CI: 2.56–11.16; P<0.001), postoperative spinal MPS (HR =2.82, 95% CI: 1.11–7.16; P=0.03), and need for CRRT (HR =4.55, 95% CI: 2.22–9.32; P<0.001).
Table 5
| Group | Time (month) | Number at risk | Number of events | Survival (95% CI), % |
|---|---|---|---|---|
| 0 | 1 | 181 | 26 | 87.44 (83.04–92.07) |
| 12 | 176 | 5 | 85.02 (80.30–90.03) | |
| 24 | 176 | 0 | 85.02 (80.30–90.03) | |
| 36 | 92 | 0 | 85.02 (80.30–90.03) | |
| 1 | 1 | 88 | 5 | 94.62 (90.15–99.32) |
| 12 | 86 | 2 | 92.47 (87.26–97.99) | |
| 24 | 85 | 1 | 91.40 (85.87–97.28) | |
| 36 | 32 | 0 | 91.40 (85.87–97.28) |
0, Sun procedure group; 1, non-Sun procedure group. CI, confidence interval.
Table 6
| Survival | 0 | 1 | P value |
|---|---|---|---|
| Survival at 36-month (%) | 85.02 | 91.40 | 0.12 |
0, Sun procedure group; 1, non-Sun procedure group.
Table 7
| Variable | Univariate | Multivariate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | S.E | Z | P | HR (95% CI) | β | S.E | Z | P | HR (95% CI) | ||
| Non-Sun procedure | −0.60 | 0.40 | −1.52 | 0.13 | 0.55 (0.25–1.19) | −0.27 | 0.42 | −0.64 | 0.52 | 0.76 (0.34–1.73) | |
| Marfan syndrome | 0.90 | 0.73 | 1.24 | 0.21 | 2.47 (0.60–10.24) | ||||||
| Mitral valve surgery | 1.04 | 1.01 | 1.02 | 0.31 | 2.82 (0.39–20.56) | ||||||
| Tricuspid valve surgery | −15.02 | 2,810.83 | −0.01 | >0.99 | 0.00 (0.00–Inf) | ||||||
| CABG | 0.09 | 0.60 | 0.15 | 0.88 | 1.10 (0.34–3.56) | ||||||
| Femoral artery cannulation | 0.50 | 0.38 | 1.31 | 0.19 | 1.65 (0.78–3.47) | ||||||
| Axillary artery cannulation | 0.17 | 0.32 | 0.54 | 0.59 | 1.19 (0.63–2.24) | ||||||
| Femoral and axillary artery cannulation | −0.42 | 0.40 | −1.06 | 0.29 | 0.66 (0.30–1.43) | ||||||
| Innominate artery cannulation | 0.70 | 0.53 | 1.32 | 0.19 | 2.01 (0.71–5.65) | ||||||
| Left common carotid artery cannulation | 0.35 | 0.38 | 0.93 | 0.35 | 1.42 (0.68–3.00) | ||||||
| DHCA | 17.14 | 2,821.46 | 0.01 | >0.99 | 27,681,265.88 (0.00–Inf) | ||||||
| SCP | 17.14 | 2,821.46 | 0.01 | >0.99 | 27,681,265.88 (0.00–Inf) | ||||||
| Pneumonia | 1.12 | 0.35 | 3.21 | 0.001 | 3.05 (1.55–6.03) | ||||||
| Exploratory thoracotomy | 0.90 | 0.60 | 1.50 | 0.13 | 2.46 (0.76–8.00) | ||||||
| Cardiogenic shock | 2.17 | 0.40 | 5.44 | <0.001 | 8.77 (4.01–19.18) | 1.09 | 0.43 | 2.55 | 0.01 | 2.98 (1.29–6.92) | |
| Tracheotomy | 1.61 | 0.38 | 4.22 | <0.001 | 5.00 (2.37–10.55) | -0.62 | 0.47 | −1.34 | 0.18 | 0.54 (0.22–1.34) | |
| Respiratory failure | 1.89 | 0.34 | 5.64 | <0.001 | 6.62 (3.43–12.77) | ||||||
| Sepsis | 0.80 | 0.60 | 1.34 | 0.18 | 2.23 (0.69–7.26) | ||||||
| ECMO | 2.43 | 0.73 | 3.31 | <0.001 | 11.31 (2.70–47.47) | ||||||
| PMV | 1.97 | 0.36 | 5.53 | <0.001 | 7.18 (3.57–14.43) | ||||||
| Upper limb MPS | −14.01 | 2,946.71 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| Lower limb MPS | 1.55 | 0.60 | 2.57 | 0.01 | 4.71 (1.45–15.33) | ||||||
| Cerebral MPS | 2.14 | 0.32 | 6.60 | <0.001 | 8.50 (4.50–16.03) | 1.68 | 0.38 | 4.46 | <0.001 | 5.34 (2.56–11.16) | |
| Spinal MPS | 1.28 | 0.42 | 3.07 | 0.002 | 3.61 (1.59–8.19) | 1.04 | 0.47 | 2.19 | 0.03 | 2.82 (1.11–7.16) | |
| Renal MPS | 2.43 | 0.40 | 6.13 | <0.001 | 11.40 (5.23–24.82) | ||||||
| Mesenteric MPS | 2.29 | 0.38 | 5.99 | <0.001 | 9.90 (4.68–20.97) | ||||||
| Myocardial MPS | 1.90 | 0.48 | 3.95 | <0.001 | 6.70 (2.61–17.19) | ||||||
| CRRT | 2.29 | 0.32 | 7.08 | <0.001 | 9.85 (5.23–18.54) | 1.51 | 0.37 | 4.14 | <0.001 | 4.55 (2.22–9.32) | |
| Number of MPS | 1.03 | 0.11 | 9.24 | <0.001 | 2.81 (2.25–3.49) | ||||||
| DHCA time | 0.02 | 0.01 | 1.61 | 0.11 | 1.02 (1.00–1.05) | ||||||
| CPB time | 0.01 | 0.00 | 4.74 | <0.001 | 1.01 (1.01–1.02) | ||||||
| Cardiac ischemic time | 0.01 | 0.00 | 3.69 | <0.001 | 1.02 (1.01–1.02) | ||||||
| Pharyngeal temperature | −0.13 | 0.07 | −1.88 | 0.06 | 0.88 (0.77–1.01) | ||||||
| Anal temperature | −0.16 | 0.06 | −2.78 | 0.005 | 0.85 (0.76–0.95) | ||||||
CABG, coronary artery bypass grafting; DHCA, deep hypothermia circulatory arrest; SCP, selective cerebral perfusion; ECMO, extracorporeal membrane oxygenation; PMV, prolonged mechanical ventilation; MPS, malperfusion syndrome; CRRT, continuous renal replacement therapy; CPB, cardiopulmonary bypass; S.E, standard error; OR, odds ratio; CI, confidence interval.
Analysis of postoperative DAEs
At the end point of follow-up, five DAEs occurred in the Sun procedure group while eight occurred in the non-Sun procedure group. K-M analysis was performed for postoperative DAEs, as shown in the Figure 3. At 3-year follow-up, the probability of no DAEs occurring was 97.17% (95% CI: 94.76–99.65%) and 91.59% (95% CI: 85.80–97.78%) (P=0.02) in the Sun procedure group and non-Sun procedure group, respectively, representing a significant difference (Tables 8,9). Multivariate Cox regression (Table 10) showed that the risk factors for DAE were Marfan syndrome (HR =20.36, 95% CI: 5.51–75.20; P<0.001) and non-Sun procedure (HR =3.42, 95% CI: 1.11–10.56; P=0.03). Considering the possibility of competition between death and DAEs, we proceeded the Fine-Gray analysis (Table 11), and the results were similar to those of cox regression, which showed that the risk factors for DAEs were Marfan syndrome (HR =19.02, 95% CI: 4.40–82.23; P<0.001) and non-Sun procedure (HR =3.38, 95% CI: 1.04–11.02; P=0.04).
Table 8
| Group | Time (month) | Number at risk | Number of events | Survival (95% CI), % |
|---|---|---|---|---|
| 0 | 1 | 181 | 0 | 100.00 (100.00–100.00) |
| 12 | 174 | 2 | 98.88 (97.34–100.00) | |
| 24 | 172 | 2 | 97.74 (95.57–99.95) | |
| 36 | 88 | 1 | 97.17 (94.76–99.65) | |
| 1 | 1 | 88 | 0 | 100.00 (100.00–100.00) |
| 12 | 84 | 2 | 97.67 (94.54–100.00) | |
| 24 | 80 | 4 | 93.02 (87.79–98.57) | |
| 36 | 31 | 1 | 91.59 (85.80–97.78) |
DAEs, distal aortic events; 0, Sun procedure group; 1, non-Sun procedure group. CI, confidence interval.
Table 9
| Survival | 0 | 1 | P value |
|---|---|---|---|
| Survival at 36-month (%) | 97.17 | 91.59 | 0.02 |
DAEs, distal aortic events; 0, Sun procedure group; 1, Non-Sun procedure group.
Table 10
| Variable | Univariate | Multivariate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | S.E | Z | P | HR (95% CI) | β | S.E | Z | P | HR (95% CI) | ||
| Non-Sun procedure | 1.24 | 0.57 | 2.17 | 0.03 | 3.46 (1.13–10.59) | 1.19 | 0.57 | 2.07 | 0.04 | 3.29 (1.07–10.14) | |
| Marfan syndrome | 3.05 | 0.66 | 4.60 | <0.001 | 21.18 (5.77–77.69) | 3.00 | 0.68 | 4.43 | <0.001 | 20.03 (5.32–75.37) | |
| Mitral valve surgery | −15.02 | 5,210.48 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| Tricuspid valve surgery | −16.02 | 7,926.96 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| CABG | 0.08 | 1.04 | 0.07 | 0.94 | 1.08 (0.14–8.30) | ||||||
| Femoral artery cannulation | 0.62 | 0.66 | 0.94 | 0.35 | 1.86 (0.51–6.78) | ||||||
| Axillary artery cannulation | 0.70 | 0.60 | 1.17 | 0.24 | 2.02 (0.62–6.58) | ||||||
| Femoral and axillary artery cannulation | −1.58 | 1.04 | −1.52 | 0.13 | 0.21 (0.03–1.59) | ||||||
| Innominate artery cannulation | −17.08 | 6,212.02 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| Left common carotid artery cannulation | −0.13 | 0.77 | −0.17 | 0.87 | 0.88 (0.19–3.97) | ||||||
| DHCA | 18.15 | 7,731.94 | 0.00 | >0.99 | 76,249,818.93 (0.00–Inf) | ||||||
| SCP | 18.15 | 7,731.94 | 0.00 | >0.99 | 76,249,818.93 (0.00–Inf) | ||||||
| Pneumonia | −0.40 | 1.04 | −0.38 | 0.70 | 0.67 (0.09–5.17) | ||||||
| Exploratory thoracotomy | −17.05 | 7,919.34 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| Cardiogenic shock | −16.03 | 6,443.60 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| Tracheotomy | 0.74 | 1.04 | 0.71 | 0.48 | 2.09 (0.27–16.21) | ||||||
| Respiratory failure | −17.09 | 5,925.20 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| Sepsis | −17.05 | 7,664.45 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| PMV | −0.46 | 0.77 | −0.59 | 0.55 | 0.63 (0.14–2.86) | ||||||
| Upper limb MPS | −14.01 | 5,312.53 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| Lower limb MPS | −16.02 | 7,391.87 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| Cerebral MPS | −0.27 | 1.04 | −0.26 | 0.80 | 0.77 (0.10–5.89) | ||||||
| Spinal MPS | −17.08 | 6,411.46 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| Renal MPS | 0.49 | 0.60 | 0.82 | 0.41 | 1.64 (0.50–5.31) | 0.59 | 0.61 | 0.98 | 0.33 | 1.81 (0.55–5.99) | |
| Mesenteric MPS | −16.03 | 6,261.22 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| Myocardial MPS | −16.02 | 7,287.42 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| CRRT | −18.14 | 8,009.94 | −0.00 | >0.99 | 0.00 (0.00–Inf) | ||||||
| Number of MPS | −0.05 | 0.42 | −0.11 | 0.91 | 0.96 (0.42–2.18) | ||||||
| DHCA time | −0.00 | 0.02 | −0.07 | 0.95 | 1.00 (0.95–1.04) | ||||||
| CPB time | 0.00 | 0.01 | 0.02 | 0.98 | 1.00 (0.99–1.01) | ||||||
| Cardiac ischemic time | −0.00 | 0.01 | −0.33 | 0.74 | 1.00 (0.98–1.02) | ||||||
| Pharyngeal temperature | −0.09 | 0.11 | −0.79 | 0.43 | 0.92 (0.73–1.14) | ||||||
| Anal temperature | −0.20 | 0.10 | −2.13 | 0.03 | 0.82 (0.68–0.98) | ||||||
CABG, coronary artery bypass grafting; DHCA, deep hypothermia circulatory arrest; SCP, selective cerebral perfusion; PMV, prolonged mechanical ventilation; MPS, malperfusion syndrome; CRRT, continuous renal replacement therapy; CPB, cardiopulmonary bypass; S.E, standard error; HR, hazard ratio; CI, confidence interval.
Table 11
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Non-Sun procedure | 3.38 | 1.04, 11.02 | 0.04 |
| Marfan syndrome | 19.02 | 4.40, 82.23 | <0.001 |
| Renal MPS | 1.03 | 0.28, 3.85 | 0.96 |
DAEs, distal aortic events; HR, hazard ratio; CI, confidence interval; MPS, malperfusion syndrome.
Discussion
This study shows that the Sun procedure is a feasible option in many patients with ATAAD. The Sun procedure is generally considered to have better long-term treatment effect for ATAAD because it can rapidly dilate the true lumen, activate positive vascular remodeling, provide early restoration of blood perfusion to the affected organs, and reduce the risk of secondary surgical intervention (9). However, the Sun procedure poses certain perils to patients, such as greater surgical risk, especially in the elderly patients with organ malperfusion (10), and thus is generally recommended to be performed in a surgical center with experienced specialists (11). A meta-analysis of 7,967 cases of the Sun procedure (12) indicated that the incidence of postoperative cerebrovascular complications, paraplegia, renal failure, and in-hospital mortality were 7.104%, 3.465%, 14.969%, and 8.933%, respectively. In contrast, simultaneous simple ascending aorta replacement, aortic root replacement, and partial arch replacement, provides a shorter operation time and less trauma and thus has been considered more suitable for older adult or frail patients who cannot tolerate the Sun procedure (13). In our study, after we obtained cases with similar preoperative conditions using PSM, the incidence of postoperative complications in the Sun procedure group, except for that of postoperative spinal MPS, was not found to be significantly different from that in the non-Sun procedure group. Although there was no statistical difference, postoperative 30-day mortality was higher in Sun procedure group than that in the other group. This discrepancy may be due to the difference in population selection of different studies and the improvement of surgical techniques in our center in recent years.
With regard to risk factors associated with postoperative 30-day death, the incidence of postoperative cardiogenic shock and need for CRRT remained higher in the Sun procedure group, although there was no significant difference. This may indicate that compared with simple proximal operation, Sun’s procedure is more likely to cause potential myocardial and renal injury, resulting in slightly higher early postoperative mortality. There was little difference in the incidence of postoperative cerebral MPS between the two groups. This indicates that under reasonable cerebral protection measures (deep hypothermia circulatory arrest and selective cerebral perfusion), there is no difference in the risk of cerebral MPS between Sun’s procedure and simple proximal surgery.
Takagi et al. (14) compared the prognosis of patients who underwent simple ascending aorta replacement and TAR + FET surgery and found that there was no significant difference in early and middle postoperative mortality between the two groups, while the incidence of postoperative aortic events was higher in the simple ascending aorta replacement group, which was similar to the conclusion reached in our study. Omura et al. (15) also reported that TAR + FET surgery may provide a better long-term prognosis. The study by Biancari et al. (16), however, arrived at an opposite conclusion after comparing the prognosis of patients who underwent type A aortic dissection repair, which may be due to the fact that the study population was European and younger than 60 years old. Many studies have shown that advanced age is a risk factor for poor prognosis after type A dissection (17,18). Therefore, simple proximal surgery should be considered for older adult patients. Some studies have suggested that the 5-year survival rate after type A dissection may range from 70% to 80%, and about 12% to 24% of late deaths may due to distal aneurysm rupture (19-21). Moreover, patients who undergo non-TAR surgery have a higher incidence of postoperative DAEs (21,22). In this study, the average age of all patients was 49.46±11.67 years, and the average age after PSM was 50.72±11.73 years. Thus, there exists a difference of more than 15 years between the onset age and the life expectancy of the population. The age of onset of aortic dissection is generally young, which is associated with poor control of hypertension. Since most of the patients admitted to our center were brought in by ambulance, most of these patients were treated with antihypertensive drugs on the way to the emergency department of our center, so the preoperative blood pressure was close to normal.
In this study, PSM was used to control preoperative confounders. Multivariate logistic regression and multivariate cox regression were used to analyze the risk factors related to postoperative death and DAEs. Fine-Gray analysis was used for sensitivity analysis, thus the conclusion was relatively reliable. However, because the variables included in the regression were selected based on clinical experience and results reported in previous studies (19,23,24), there may be some bias.
Marfan syndrome is currently a recognized high-risk factor for aortic dissection, which causes the degeneration of the medial aortic wall, leading to a high incidence of dissection and aneurysm (19). In our study, patients with type A aortic dissection combined with Marfan syndrome had a higher incidence of postoperative long-term events. There were seven patients with Marfan syndrome after PSM, two of whom died before the occurrence of aortic events, and three of the remaining five experienced DAEs. Rylski et al. (25) found that for patients with type A aortic dissection complicated with Marfan syndrome, a primary surgical replacement range not reaching the aortic arch may lead to poor prognosis. The indications for reintervention after first-stage dissection repair in Marfan syndrome patients are also more stringent than are those in the general population, and the Sun procedure is more likely to be selected in first-stage surgery to obtain a better long-term prognosis. The study of Chen et al. (26) showed that the Sun procedure had a relatively obvious effect on postoperative positive remodeling of the aorta in patients with ATAAD complicated with Marfan syndrome. Overall, although the postoperative 30-day and midterm mortality was slightly higher in the Sun procedure group which might due to the possibility for Sun’s procedure causing a relatively high incidence of postoperative spinal MPS, there was no significant difference between patients treated with the Sun procedure and those treated with other operations involving only proximal and partial arch treatment, but the Sun procedure can achieve a lower long-term reintervention rate. Therefore, for patients with Marfan syndrome, younger patients, and most patients who can tolerate it, the Sun procedure with a wider range of operations should optimally be considered as the first-stage of treatment. On the other hand, the higher incidence of spinal MPS after the Sun procedure is concerning. In our study, 19 patients developed paraplegia after the Sun procedure, 7 of whom died during hospitalization. Among the 12 survivors, 3 had the paraplegia symptoms basically disappear at the time of discharge, and 5 had symptoms improved to varying degrees during follow-up. Based on our previous experience, the reason for Sun procedure increasing the risk of paraplegia depends more on the number and condition of the patient’s intercostal artery opening into the false lumen. Because the insertion of the elephant trunk stent can cause rapid closure and intraluminal thrombosis of the false lumen, this can lead to acute interruption of blood flow in the intercostal artery opening in the false lumen, resulting in acute spinal ischemia. In fact, the largest vessel that supplies blood to the spinal cord is the Adamkiewicz artery (27). The length of the trunk stent is far from reaching the level of the Adamkiewicz artery, so in most patients, Sun procedure is safe. Also, there is study has shown that residual tear at the distal end of elephant stent is also one of the risk factors for paraplegia. Therefore, more adequate and timely preoperative and postoperative aortic CTA assessment may reduce the incidence of paraplegia after Sun procedure (28). For patients with a high proportion of the intercostal artery located in the false lumen preoperatively, patients with severe Adamkiewicz arteries dissection, and patients with naturally thin Adamkiewicz arteries, it may be necessary to consider avoiding Sun procedure or using a shorter elephant trunk stent.
Study limitations
The follow-up time of this study was relatively short, and the long-term prognosis may not have been accurately described and compared. Assessment of life quality during follow-up was inadequate. The involvement of the proximal part of the aorta including the surgical decision may have had an impact on the outcome after surgery for ATAAD. A large-sample, multicenter study is warranted.
Conclusions
This retrospective study shows that the Sun procedure is a suitable option for many patients with ATAAD.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2048/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2048/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2048/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2048/coif). A.M. serves as Editor for EJCTS, Secretary General for SATS, and a member for AATS, EACTS, ST, TT and SATS. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Beijing Anzhen Hospital of Capital Medical University approved this retrospective study (No: 2024246x). The requirement for individual consent was waived due to the retrospective nature of the analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gudbjartsson T, Ahlsson A, Geirsson A, et al. Acute type A aortic dissection - a review. Scand Cardiovasc J 2020;54:1-13. [Crossref] [PubMed]
- Rylski B, Milewski RK, Bavaria JE, et al. Outcomes of surgery for chronic type A aortic dissection. Ann Thorac Surg 2015;99:88-93. [Crossref] [PubMed]
- El-Hamamsy I, Ouzounian M, Demers P, et al. State-of-the-Art Surgical Management of Acute Type A Aortic Dissection. Can J Cardiol 2016;32:100-9. [Crossref] [PubMed]
- Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022;146:e334-482. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Ma WG, Zhu JM, Zheng J, et al. Sun's procedure for complex aortic arch repair: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:642-8. [Crossref] [PubMed]
- Ma WG, Zheng J, Zhang W, et al. Frozen elephant trunk with total arch replacement for type A aortic dissections: Does acuity affect operative mortality? J Thorac Cardiovasc Surg 2014;148:963-70; discussion 970-2. [Crossref] [PubMed]
- Fillinger MF, Greenberg RK, McKinsey JF, et al. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg 2010;52:1022-33, 1033.e15.
- Ibrahim A, Motekallemi A, Yahia A, et al. Volume Changes in the Descending Aorta after Frozen Elephant Trunk and Conventional Hemi-Arch Repair after Acute Type A Aortic Dissection. Diagnostics (Basel) 2022;12:2524. [Crossref] [PubMed]
- Beckmann E, Martens A, Kaufeld T, et al. Is total aortic arch replacement with the frozen elephant trunk procedure reasonable in elderly patients? Eur J Cardiothorac Surg 2021;60:131-7. [Crossref] [PubMed]
- Hagl C, Pichlmaier M, Khaladj N. Elephant trunks in aortic surgery: fresh and frozen. J Thorac Cardiovasc Surg 2013;145:S98-102. [Crossref] [PubMed]
- Rezaei Y, Bashir M, Mousavizadeh M, et al. Frozen elephant trunk in total arch replacement: A systematic review and meta-analysis of outcomes and aortic proximalization. J Card Surg 2021;36:1922-34. [Crossref] [PubMed]
- Rathore K, Newman M. Aortic Root and Distal Arch Management During Type A Aortic Dissection Repair: Expanding Horizons. Braz J Cardiovasc Surg 2022;37:37-6. [Crossref] [PubMed]
- Takagi S, Goto Y, Yanagisawa J, et al. Strategy for acute DeBakey type I aortic dissection considering midterm results: a retrospective cohort study comparing ascending aortic replacement and total arch replacement with frozen elephant trunk technique. J Cardiothorac Surg 2024;19:15. [Crossref] [PubMed]
- Omura A, Miyahara S, Yamanaka K, et al. Early and late outcomes of repaired acute DeBakey type I aortic dissection after graft replacement. J Thorac Cardiovasc Surg 2016;151:341-8. [Crossref] [PubMed]
- Biancari F, Lega JR, Mariscalco G, et al. Aortic arch surgery for DeBakey type 1 aortic dissection in patients aged 60 years or younger. BJS Open 2024;8:zrae047. [Crossref] [PubMed]
- Lin H, Chang Y, Zhou H, et al. Early results of frozen elephant trunk in acute type-A dissection in 1445 patients. Int J Cardiol 2023;389:131213. [Crossref] [PubMed]
- Chen P, Wei J, Ding R, et al. Major adverse outcomes in patients with acute type A aortic dissection undergoing total arch replacement with frozen elephant trunk procedure. Int J Cardiol 2024;415:132254. [Crossref] [PubMed]
- Fattouch K, Sampognaro R, Navarra E, et al. Long-term results after repair of type a acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244-50. [Crossref] [PubMed]
- Geirsson A, Bavaria JE, Swarr D, et al. Fate of the residual distal and proximal aorta after acute type a dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg 2007;84:1955-64; discussion 1955-64. [Crossref] [PubMed]
- Ikeno Y, Yokawa K, Koda Y, et al. The fate of the downstream aorta after open aortic repair for acute DeBakey type I aortic dissection: total arch replacement with elephant trunk technique versus non-total arch replacement†. Eur J Cardiothorac Surg 2019;55:966-74. [Crossref] [PubMed]
- Kimura N, Tanaka M, Kawahito K, et al. Influence of patent false lumen on long-term outcome after surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2008;136:1160-6, 1166.e1-3.
- Patel PM, Dong A, Chiou E, et al. Aortic Arch Management During Acute and Subacute Type A Aortic Syndromes. Ann Thorac Surg 2022;114:694-701. [Crossref] [PubMed]
- Berger T, Kreibich M, Rylski B, et al. Composition of the surgical team in aortic arch surgery-a risk factor analysis. Eur J Cardiothorac Surg 2022;62:ezac171. [Crossref] [PubMed]
- Rylski B, Bavaria JE, Beyersdorf F, et al. Type A aortic dissection in Marfan syndrome: extent of initial surgery determines long-term outcome. Circulation 2014;129:1381-6. [Crossref] [PubMed]
- Chen Y, Ma WG, Zhi AH, et al. Fate of distal aorta after frozen elephant trunk and total arch replacement for type A aortic dissection in Marfan syndrome. J Thorac Cardiovasc Surg 2019;157:835-49. [Crossref] [PubMed]
- Martirosyan NL, Feuerstein JS, Theodore N, et al. Blood supply and vascular reactivity of the spinal cord under normal and pathological conditions. J Neurosurg Spine 2011;15:238-51. [Crossref] [PubMed]
- Melissano G, Bertoglio L, Rinaldi E, et al. An anatomical review of spinal cord blood supply. J Cardiovasc Surg (Torino) 2015;56:699-706.
(English Language Editor: J. Gray)




