
Prognostic roles of hematological indicators in programmed cell death protein 1/programmed cell death ligand 1 inhibitors for small-cell lung cancer: a retrospective cohort study
Highlight box
Key findings
• This study aimed to explore the prognostic and predictive value of hematological indicators in patients with small-cell lung cancer (SCLC) before programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) inhibitor treatment.
What is known and what is new?
• Inflammation plays a pivotal role in tumorigenesis, tumor development, metastasis, and drug resistance. Multiple studies have established significant correlations between inflammatory factors and the prognosis of non-small cell lung cancer, malignant melanoma, and liver cancer.
• Monocyte:lymphocyte ratio and platelet:lymphocyte ratio were independent predictive factors for progression-free survival in patients with SCLC treated with PD-1/PD-L1 inhibitors. Meanwhile, the neutrophil:lymphocyte ratio and lactate dehydrogenase were independent prognostic factors for predicting overall survival in patients with SCLC treated with PD-1/PD-L1 inhibitors.
What are the implications, and what should change now?
• Our research showed that some hematological indicators might be associated with the efficacy of PD-1/PD-L1 inhibitors, as well as for survival, in patients with SCLC.
Introduction
Lung cancer remains the foremost cause of cancer-related mortality globally, with a staggering 2.481 million new cases and 1.82 million related deaths reported in 2022 (1). Among its subtypes, small-cell lung cancer (SCLC) represents approximately 10–15% of all lung cancer cases and is the most malignant and aggressive form, resulting in the worst prognosis (2). Although SCLC initially exhibits rapid responsiveness to chemotherapy and radiotherapy, the development of drug resistance is challenging, resulting in a dismal 5-year survival rate of less than 10% of patients (3).
In recent years, significant advancements have been made in the treatment of SCLC through the introduction of novel therapy regimens. Among these innovations, target drug delivery and combination regimens have shown great promise in improving treatment outcomes (4-16). Additionally, the introduction of immune checkpoint inhibitors (ICIs) has heralded a new era in the management of SCLC. Phase III clinical studies have demonstrated that combining first-line chemotherapy with ICIs in patients with extensive-stage SCLC (ES-SCLC) confers significant survival benefits (17-22). Despite the promising outcomes observed in some patients, not all regimens derive similar advantages from immunotherapy. Hence, there is a need to identify reliable biomarkers of immunotherapy response. Whilst programmed cell death ligand 1 (PD-L1) expression, tumor mutational burden, and microsatellite instability-high are used as predictive biomarkers for ICI treatment in patients with non-small cell lung cancer (NSCLC) and other tumors, the predictive value in patients with SCLC is unclear. The third luminal lobe (L3MI) is a prognostic factor for patients with ES-SCLC, therefore early maintenance of muscle mass can better achieve cancer management (23). Wu et al. have found that Hb is significantly correlated with overall survival (OS) in SCLC [hazard ratio (HR =0.163; P<0.001], and is an independent prognostic factor affecting OS. However, Hb is not significantly correlated with PFS in SCLC (24). The median OS (mOS) of SCLC patients with high procalcitonin (PCT) is shorter than that of SCLC patients with normal PCT. However, PCT may be difficult to distinguish bacterial infection in SCLC patients, because even in SCLC patients without infectious diseases, PCT will increase (25). But these studies did not involve immunotherapy. Consequently, there exists a pressing urgency to discover additional biomarkers for accurately evaluating the therapeutic efficacy of ICIs in patients with SCLC treated with ICI.
Inflammation plays a pivotal role in tumorigenesis, tumor development, metastasis, and drug resistance (26). The tumor immune microenvironment (TIME) is composed of various immune cell populations such as tumor infiltrating lymphocytes (TIL) and plays an important role in tumor immunotherapy (27). Systemic tumor immune environment (STIE) is composed of circulating blood immune cells and molecules. Various studies have shown a significant correlation between TIME determined by TIL status and prognostic nutritional index (PNI) scores (28,29). Previous studies have found that ES-SCLC with high PNI has better clinical response and survival rates when treated with immunotherapy. But this is a single center retrospective study, so further validation is needed (30). Hence investigations regarding the predictive value of these inflammatory indicators in SCLC are scarce. Thus, the primary aim of our study was to comprehensively examine the potential of hematological indicators as prognostic and predictive factors in immunotherapy for patients with SCLC. By elucidating the significance of inflammation-related markers, we aimed to refine patient stratification and foster the development of personalized therapeutic strategies in the realm of SCLC immunotherapy. We present this article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1826/rc).
Methods
Patients and study design
In this retrospective cohort study, 700 patients with SCLC treated with programmed cell death protein 1 (PD-1)/PD-L1 inhibitors in the Fourth Hospital of Hebei Medical University were investigated from January 2019 to January 2023. The inclusion criteria were as follows: (I) patients aged 18 years or over, (II) SCLC confirmed by cytology or histopathology, (III) completion of at least two cycles of PD-1/PD-L1 inhibitor treatment, and (IV) completion of pretreatment blood sampling. The exclusion criteria were as follows: (I) presence of a fever, systemic inflammation, blood disease, immune disease, cardiovascular or cerebrovascular events, or infection; (II) blood transfusions within 4 weeks prior to PD-1/PD-L1 inhibitors treatment; and (III) presence of other malignant tumors.
According to these inclusion and exclusion criteria, 246 patients were ultimately enrolled in the study. Assessment of baseline clinical factors included the patients’ clinical characteristics such as age, gender, stage, Eastern Cooperative Oncology Group Performance Score (ECOG-PS), and PD-1/PD-L1 status, among others (Table 1). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2023146). Individual consent for this retrospective analysis was waived.
Table 1
| Clinical characteristic | Baseline characteristics | Cases | Proportion (%) |
|---|---|---|---|
| Sex | Male | 192 | 78.1 |
| Female | 54 | 21.9 | |
| Age (years) | <63 | 115 | 46.7 |
| ≥63 | 131 | 53.3 | |
| BMI (kg/m2) | <24.8 | 126 | 51.2 |
| ≥24.8 | 120 | 48.8 | |
| Tumor site | Left | 116 | 47.2 |
| Right | 124 | 50.4 | |
| Others | 6 | 2.4 | |
| Stage | LS-SCLC | 61 | 24.7 |
| EC-SCLC | 185 | 75.2 | |
| PS | 0–1 | 233 | 94.7 |
| ≥2 | 13 | 5.30 | |
| Smoking | Yes | 157 | 63.8 |
| No | 89 | 36.2 | |
| Drinking | Yes | 128 | 52.0 |
| No | 118 | 48.0 | |
| Metastatic organs | <3 | 188 | 76.4 |
| ≥3 | 58 | 23.5 | |
| Treatment lines | First line | 186 | 75.6 |
| Second line | 41 | 16.7 | |
| Multiline | 19 | 7.7 | |
| Drugs | PD-1 inhibitor | 104 | 42.3 |
| PD-L1 inhibitor | 142 | 57.7 | |
| Treatment mode | Immunotherapy alone | 6 | 2.4 |
| Chemotherapy + PD-1/PD-L1 inhibitor | 209 | 85.0 | |
| Chemotherapy + PD-1/PD-L1 inhibitor+ radiotherapy | 13 | 5.3 | |
| Chemotherapy + PD-1/PD-L1 inhibitor + antiangiogenic drugs | 11 | 4.5 | |
| PD-1/PD-L1 inhibitor + antiangiogenic drugs | 7 | 2.8 | |
| Efficacy evaluation | PD | 25 | 10.1 |
| SD | 104 | 42.3 | |
| PR | 117 | 47.6 |
LS-SCLC, limited-stage small cell lung cancer; ES-SCLC, extensive small cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; PD, progressive disease; SD, stable disease; PR, partial response; PS, performance score.
Laboratory testing
A week before the initiation of PD-1/PD-L1 inhibitor treatment, blood samples were extracted for complete blood count and liver function tests. The following indexes were calculated as indicated: PNI, which is calculated as follows: PNI = serum albumin (ALB) level + 5 × lymphocyte count; neutrophil:lymphocyte ratio (NLR; neutrophil count/lymphocyte count); platelet:lymphocyte ratio (PLR; platelet count/lymphocyte count); systemic immune inflammation (SII; neutrophil count × platelet count/lymphocyte count), and monocyte:lymphocyte ratio (MLR; monocyte count/lymphocyte count).
Clinical evaluation and follow-up
Patients were subjected to dynamic computed tomography (CT) or nuclear magnetic resonance imaging (MRI) scan every two or three cycles of ICI treatment. The response to treatment was evaluated according to the criteria of Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, and were classified in complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Objective response was defined as CR or PR, while disease control was defined as CR, PR, or SD.
Objective response rate (ORR) was defined as the percentage of patients who achieved CR or PR. Disease control rate (DCR) was defined as the percentage of patients who achieved CR, PR or SD. Progression-free survival (PFS) was defined as interval between the first cycle of PD-1/PD-L1 inhibitors and tumor progression or death, while OS was defined as interval between the first cycle of PD-1/PD-L1 inhibitors and death or last follow-up. The patients were followed up by in-patient, out-patient reexamination and telephone contact, and the efficacy and survival status were recorded. As of 1st March 2023, all patients had received a post-diagnosis follow-up. The median follow-up time was 11.7 months.
Observation metrics
The observation metrics were the following: CT or MRI results prior to the initiation of PD-1/PD-L1 treatment; white blood cell count (WBC), platelet (PLT) count, absolute neutrophil count (ANC), lactate dehydrogenase level (LDH), absolute lymphocyte count (ALC), absolute monocyte count (AMC), ALB level, prealbumin level (PAB), PNI, PLR, SII, and MLR before PD-1/PD-L1 treatment; CT or MRI results after 2–3 cycles of PD-1/PD-L1 inhibitor treatment; and PFS and OS after PD-1/PD-L1 inhibitor treatment.
Statistical analysis
Statistical analyses were performed using SPSS 27 (IBM Corp., Armonk, NY, USA). Receiver operating characteristic (ROC) curves were designed. The test variable value corresponding to the maximum Youden index (YDI) was calculated to determine the best cutoff value of the peripheral blood inflammatory index. The continuous variable of inflammatory index was transformed into a binary variable as per the optimal cutoff value. Intergroup comparisons were conducted using the χ2 test. Univariate analyses were performed with Kaplan-Meier and log-rank analysis. Multivariate regression analyses were conducted with Cox regression analysis to identify the possible independent prognostic factors. P<0.05 was considered to be statistically significant.
Results
Clinicopathologic characteristics
This study included 246 patients with SCLC treated with PD-1/PD-L1 in combination with chemotherapy, including 192 males (78.1%) and 54 females (21.9%). The age range was 29–82 years, with a median age of 63 years. There were 61 cases (24.7%) of limited-stage and 185 cases (75.2%) of extensive-stage disease. The patients’ clinical characteristics are shown in Table 1.
Correlation between peripheral blood indicators and PD-1/PD-L1 inhibitor efficacy
Among the 246 patients with SCLC receiving PD-1/PD-L1 inhibitor treatment, none were evaluated as CR, 117 as PR, 104 as SD, and 25 as PD. The ORR and DCR of this cohort were 47.6% and 89.8%, respectively. Peripheral blood indicators were divided into high- and low-value groups according to the cutoff value determined by the Youden Index. Univariate analysis showed that ORR significantly differed between the high- and low-value groups of the following indicators, SII (P=0.01), WBC (P=0.001), ANC (P=0.006), ALC (P=0.01), RBC (P=0.03), and PLT (P<0.001) (Table 2). Significant differences in DCR were found between the high- and low-value groups of some indicators including NLR (P=0.001), PLR (P=0.01), PNI (P=0.004), MLR (P=0.01), WBC (P=0.001), ANC (P=0.01), ALC (P<0.001), RBC (P<0.001), hemoglobin (Hb) level (P<0.001), PLT (P<0.001), prealbumin (PAB) level (P=0.05), and LDH (P=0.03) (Table 2). Multivariate analysis showed that the above hematological indicators were negative predictors for ORR (Table 3). Multivariate analysis showed that PNI was independently associated with DCR (P=0.04) (Table 4).
Table 2
| Hematological index | Group | Efficacy evaluation | N | ORR (%) | P value for ORR | DCR (%) | P value for DCR | |||
|---|---|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | |||||||
| NLR | H | 0 | 25 | 18 | 12 | 55 | 45.5 | 0.72 | 78.2 | 0.001* |
| L | 0 | 92 | 86 | 13 | 191 | 48.2 | 93.2 | |||
| PLR | H | 0 | 23 | 19 | 10 | 52 | 44.2 | 0.59 | 80.8 | 0.02* |
| L | 0 | 94 | 85 | 15 | 194 | 48.5 | 92.3 | |||
| PNI | H | 0 | 75 | 77 | 10 | 162 | 46.3 | 0.58 | 93.8 | 0.004* |
| L | 0 | 42 | 27 | 15 | 84 | 50.0 | 82.1 | |||
| SII | H | 0 | 29 | 13 | 3 | 45 | 64.4 | 0.01* | 93.3 | 0.39 |
| L | 0 | 88 | 91 | 22 | 201 | 43.8 | 89.1 | |||
| MLR | H | 0 | 58 | 52 | 19 | 129 | 45.0 | 0.39 | 85.3 | 0.01* |
| L | 0 | 59 | 52 | 6 | 117 | 50.4 | 94.9 | |||
| WBC | H | 0 | 97 | 72 | 11 | 180 | 53.9 | 0.001* | 93.9 | 0.001* |
| L | 0 | 20 | 32 | 14 | 66 | 30.3 | 78.8 | |||
| ANC | H | 0 | 75 | 52 | 8 | 135 | 55.6 | 0.006* | 94.1 | 0.02* |
| L | 0 | 42 | 52 | 17 | 111 | 37.8 | 84.7 | |||
| ALC | H | 0 | 88 | 70 | 8 | 166 | 53.0 | 0.01* | 95.2 | <0.001* |
| L | 0 | 29 | 34 | 17 | 80 | 36.3 | 78.8 | |||
| AMC | H | 0 | 57 | 46 | 8 | 111 | 51.4 | 0.28 | 92.8 | 0.16 |
| L | 0 | 60 | 58 | 17 | 135 | 44.4 | 87.4 | |||
| RBC | H | 0 | 101 | 88 | 10 | 199 | 50.8 | 0.04* | 95.0 | <0.001* |
| L | 0 | 16 | 16 | 15 | 47 | 34.0 | 68.1 | |||
| Hb | H | 0 | 79 | 68 | 6 | 153 | 51.6 | 0.10 | 96.1 | <0.001* |
| L | 0 | 38 | 36 | 19 | 93 | 40.9 | 79.6 | |||
| PLT | H | 0 | 96 | 69 | 8 | 173 | 55.5 | <0.001* | 95.4 | <0.001* |
| L | 0 | 21 | 35 | 17 | 73 | 28.8 | 76.7 | |||
| PAB | H | 0 | 73 | 78 | 13 | 164 | 44.5 | 0.52 | 92.1 | 0.045* |
| L | 0 | 32 | 22 | 11 | 65 | 49.2 | 83.1 | |||
| ALB | H | 0 | 89 | 90 | 16 | 195 | 45.6 | 0.18 | 91.8 | 0.10 |
| L | 0 | 26 | 12 | 8 | 46 | 56.5 | 82.6 | |||
| LDH | H | 0 | 22 | 18 | 8 | 48 | 45.8 | 0.61 | 83.3 | 0.03* |
| L | 0 | 80 | 70 | 10 | 160 | 50.0 | 94.9 | |||
*, P<0.05 is statistically significant, conforming to a normal distribution. PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; N, number; ORR, overall response rate; DCR, disease control rate; H, high; L, low; NLR, neutrophil:lymphocyte ratio; PLR, platelet:lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune inflammation; MLR, monocyte:lymphocyte ratio; WBC, white blood cell count; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; AMC, absolute monocyte count; RBC, red blood cell; Hb hemoglobin; PLT, platelet; PAB, prealbumin; ALB, albumin; LDH, lactate dehydrogenase.
Table 3
| Group | B | SE | Wald | df | Sig. | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| SII | −0.385 | 0.356 | 1.169 | 1 | 0.28 | 0.681 | 0.339 | 1.367 |
| WBC | 0.430 | 0.462 | 0.865 | 1 | 0.35 | 1.537 | 0.621 | 3.800 |
| ANC | 0.326 | 0.393 | 0.686 | 1 | 0.41 | 1.385 | 0.641 | 2.992 |
| ALC | −0.16 | 0.371 | 0.198 | 1 | 0.66 | 0.848 | 0.409 | 1.756 |
| RBC | 0.213 | 0.381 | 0.313 | 1 | 0.58 | 1.238 | 0.586 | 2.613 |
ORR, overall response rate; SCLC, small-cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; SE, standard error; 95% CI, 95% confidence interval; SII, systemic immune inflammation; WBC, white blood cell count; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; RBC, red blood cell count.
Table 4
| Group | B | SE | Wald | df | Sig. | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| NLR | −8.229 | 4.323 | 3.623 | 1 | 0.06 | 0 | 0 | 1.277 |
| PLR | −4.607 | 3.228 | 2.037 | 1 | 0.15 | 0.01 | 0 | 5.586 |
| PNI | −8.176 | 4.059 | 4.057 | 1 | 0.04* | 0 | 0 | 0.802 |
| MLR | −7.18 | 3.911 | 3.37 | 1 | 0.07 | 0.001 | 0 | 1.626 |
| WBC | 1.337 | 3.09 | 0.187 | 1 | 0.67 | 3.806 | 0.009 | 1,623.512 |
| ANC | 6.758 | 3.951 | 2.926 | 1 | 0.09 | 861.284 | 0.373 | 1,986,763.651 |
| ALC | −2.424 | 2.81 | 0.744 | 1 | 0.39 | 0.089 | 0 | 21.819 |
| RBC | 2.916 | 2.133 | 1.869 | 1 | 0.17 | 18.46 | 0.282 | 1,206.549 |
| Hb | 3.017 | 2.6 | 1.346 | 1 | 0.25 | 20.422 | 0.125 | 3,336.469 |
| PLT | 4.994 | 3.94 | 1.607 | 1 | 0.21 | 147.576 | 0.065 | 333,115.844 |
| PAB | 7.088 | 4.097 | 2.992 | 1 | 0.08 | 1,197.13 | 0.389 | 3,679,907.598 |
| LDH | −4.44 | 2.931 | 2.294 | 1 | 0.13 | 0.012 | 0 | 3.688 |
*, P<0.05 is statistically significant, conforming to a normal distribution. DCR, disease control rate; SCLC, small-cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; SE, standard error; 95% CI, 95% confidence interval; NLR, neutrophil:lymphocyte ratio; PLR, platelet:lymphocyte ratio; PNI, prognostic nutritional index; MLR, monocyte:lymphocyte ratio; WBC, white blood cell count; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; RBC, red blood cell count; Hb, hemoglobin; PLT, platelet; PAB, prealbumin; LDH, lactate dehydrogenase.
Correlation between peripheral blood indicators and PFS with PD-1/PD-L1 inhibitor treatment
Up to March 1, 2023, 153 patients had progressed, of whom 91 died, with a median PFS (mPFS) of 9.0 months [95% confidence interval (CI): 7.8–10.2 months] (Figure 1). PFS was evaluated after stratifying the patients in high- and low-value groups of NLR, PLR, PNI, SII, MLR, and other blood indicators. The differences between groups were compared via the log-rank test. According to the univariate analysis, blood indicators including NLR (P=0.03), PNI (P=0.02), MLR (P=0.003), RBC (P=0.001), Hb (P=0.003), PLT (P=0.002), and PAB (P=0.009) were correlated with PFS with PD-1/PD-L1 inhibitor treatment (Table 5). Multivariate analysis showed that MLR [hazard ratio (HR) =0.631, P=0.01] and PLT (HR =1.641, P=0.009) were factors independently associated with PFS (Table 6). Therefore, patients treated with PD-1/PD-L1 inhibitors and with high MLR (Figure 2) or low PLT (Figure 3) had a shorter PFS (Table 6).

Table 5
| Index | Group | Cases | PFS | OS | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median (months) | 95% CI | P | Median (months) | 95% CI | P | ||||
| NLR | H | 55 | 7.53 | 4.41–10.66 | 0.03* | 14.17 | 10.36–17.97 | 0.01* | |
| L | 191 | 9.60 | 7.77–11.43 | 24.23 | 17.62–30.85 | ||||
| PLR | H | 52 | 7.90 | 5.50–10.30 | 0.31 | 24.21 | 20.17–28.25 | 0.34 | |
| L | 194 | 9.47 | 7.81–11.12 | 19.37 | 14.94–23.80 | ||||
| PNI | H | 162 | 9.73 | 7.83–11.64 | 0.02* | 22.93 | 17.97–27.90 | 0.53 | |
| L | 84 | 7.40 | 6.04–8.76 | 18.37 | 11.78–24.96 | ||||
| SII | H | 133 | 8.60 | 7.08–10.12 | 0.56 | 24.23 | 17.44–31.03 | 0.09 | |
| L | 113 | 9.53 | 7.48–11.59 | 16.53 | 13.02–20.05 | ||||
| MLR | H | 129 | 7.40 | 6.11–8.69 | 0.003* | 18.50 | 13.19–23.82 | 0.19 | |
| L | 117 | 11.23 | 5.79–16.68 | 22.93 | 14.25–31.62 | ||||
| WBC | H | 180 | 9.57 | 8.12–11.01 | 0.10 | 22.30 | 15.39–29.21 | 0.15 | |
| L | 66 | 6.13 | 4.18–8.09 | 16.30 | 12.65–20.02 | ||||
| ANC | H | 135 | 9.57 | 7.95–11.18 | 0.06 | 22.30 | 14.40–30.20 | 0.18 | |
| L | 111 | 6.97 | 5.04–8.90 | 18.37 | 10.33–26.41 | ||||
| ALC | H | 166 | 9.57 | 7.80–11.34 | 0.22 | 20.47 | 13.41–27.52 | 0.93 | |
| L | 80 | 7.83 | 6.12–9.55 | 22.30 | 16.04–28.56 | ||||
| AMC | H | 111 | 9.37 | 7.05–11.68 | 0.69 | 21.36 | 10.36–32.37 | 0.96 | |
| L | 135 | 9.03 | 7.50–10.56 | 20.47 | 14.02–26.92 | ||||
| RBC | H | 199 | 9.73 | 8.03–11.43 | 0.001* | 24.23 | 16.75–31.72 | 0.001* | |
| L | 47 | 4.50 | 3.14–5.86 | 14.03 | 9.76–18.31 | ||||
| Hb | H | 153 | 10.53 | 8.60–12.46 | 0.003* | 27.70 | 20.76–34.64 | <0.001* | |
| L | 93 | 6.83 | 5.15–8.52 | 14.50 | 12.21–16.79 | ||||
| PLT | H | 173 | 10.53 | 7.88–13.19 | 0.002* | 24.23 | 16.10–32.36 | 0.03* | |
| L | 73 | 7.47 | 5.18–9.76 | 15.93 | 10.10–21.77 | ||||
| PAB | H | 164 | 10.50 | 8.69–12.31 | 0.009* | 22.30 | 18.07–26.53 | 0.46 | |
| L | 65 | 6.40 | 4.52–8.28 | 18.50 | 2.13–34.88 | ||||
| ALB | H | 195 | 9.57 | 8.08–11.06 | 0.09 | 24.30 | 17.47–29.33 | 0.08 | |
| L | 46 | 7.40 | 5.48–9.32 | 16.43 | 8.12–24.75 | ||||
| LDH | H | 48 | 7.40 | 4.44–10.36 | 0.18 | 13.57 | 11.14–16.00 | 0.008* | |
*, P<0.05 is statistically significant, conforming to a normal distribution. PFS, progression-free survival; OS, overall survival; SCLC, small-cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; H, high; L, low; 95% CI, 95% confidence interval; NLR, neutrophil:lymphocyte ratio; PLR, platelet:lymphocyte ratio; PNI, prognostic nutritional index; MLR, monocyte:lymphocyte ratio; WBC, white blood cell count; ANC, absolute neutrophil count; AMC, absolute monocyte count; ALC, absolute lymphocyte count; RBC, red blood cell count; Hb, hemoglobin; PLT, platelet; PAB, prealbumin; ALB, albumin; LDH, lactate dehydrogenase.
Table 6
| Group | B | SE | Wald | df | Sig. | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| NLR | −0.326 | 0.203 | 2.574 | 1 | 0.11 | 0.722 | 0.485 | 1.075 |
| PNI | 0.315 | 0.183 | 2.958 | 1 | 0.09 | 1.370 | 0.957 | 1.962 |
| MLR | −0.461 | 0.182 | 6.424 | 1 | 0.01* | 0.631 | 0.442 | 0.901 |
| RBC | 0.453 | 0.269 | 2.830 | 1 | 0.09 | 1.573 | 0.928 | 2.665 |
| Hb | 0.148 | 0.234 | 0.403 | 1 | 0.53 | 1.160 | 0.733 | 1.835 |
| PLT | 0.495 | 0.189 | 6.901 | 1 | 0.009* | 1.641 | 1.134 | 2.375 |
| PAB | 0.320 | 0.201 | 2.543 | 1 | 0.11 | 1.378 | 0.929 | 2.042 |
*, P<0.05 is statistically significant, conforming to a normal distribution. PFS, progression-free survival; SCLC, small-cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; SE, standard error; 95% CI, 95% confidence interval; NLR, neutrophil:lymphocyte ratio; PNI, prognostic nutritional index; MLR, monocyte:lymphocyte ratio; RBC, red blood cell count; Hb, hemoglobin; PLT, platelet; PAB, prealbumin.


Correlation between peripheral blood indicators and mOS after PD-1/PD-L1 inhibitor treatment
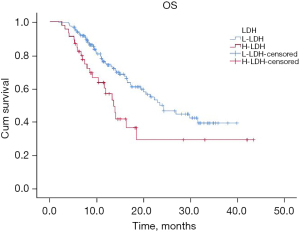
The mOS of this cohort was 21.4 months (95% CI: 17.1–25.6 months) (Figure 4). Univariate analysis showed that peripheral blood indicators including NLR (P=0.01), RBC (P=0.001), Hb (P=0.000), PLT (P=0.03), and LDH (P=0.008) were associated with mOS of patients with SCLC receiving PD-1/PD-L1 inhibitor treatment (Table 5). Multivariate analysis indicated that NLR (HR =0.57; P=0.01) and LDH (HR =0.45; P=0.002) were independently associated with mOS (Table 7). Patients high NLR (Figure 5) and LDH (Figure 6) had shorter mOS.

Table 7
| Group | B | SE | Wald | df | Sig. | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| NLR | −0.569 | 0.231 | 6.086 | 1 | 0.01* | 0.566 | 0.360 | 0.890 |
| RBC | 0.609 | 0.353 | 2.973 | 1 | 0.09 | 1.839 | 0.920 | 3.677 |
| Hb | 0.371 | 0.305 | 1.475 | 1 | 0.23 | 1.449 | 0.797 | 2.635 |
| PLT | 0.134 | 0.248 | 0.292 | 1 | 0.59 | 1.143 | 0.704 | 1.858 |
| LDH | −0.807 | 0.261 | 9.575 | 1 | 0.002* | 0.446 | 0.267 | 0.744 |
*, P<0.05 is statistically significant, conforming to a normal distribution. OS, overall survival; SCLC, small-cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; SE, standard error; 95% CI, 95% confidence interval; NLR, neutrophil:lymphocyte ratio; RBC, red blood cell count; Hb, hemoglobin; PLT, platelet; LDH, lactate dehydrogenase.

Discussion
To data, predictive biomarkers for ICI therapy in combination with chemotherapy have not yet been identified and which is still a high unmet medical need. NLR, MLR, and PLR are readily available inflammatory biomarkers, and the test is clinically convenient and almost noninvasive.
Neutrophils are the most abundant myeloid cells in the peripheral blood and are regarded to be an important component of the tumor microenvironment (TME). Furthermore, neutrophils and so-called tumor-associated neutrophils (TANs) have been found to play a significant role for tumor prognosis and resistance to ICIs (31). In this regard, several parameters linked to immune cells, tumor cells, and the general condition of patients have been identified to be associated with intratumoral immune cell infiltration, and, in turn, with inhibition of the recruitment and activation of T cells (32). TANs usually negatively regulate T cells, inhibiting the immune functions of T cells and accelerating tumor progression (33).
NLR has emerged as a potential systemic inflammation marker with significant implications for predicting survival and prognosis of patients across a variety of tumor types (34,35). Recently, a study with more than 500 patients with NSCLCs, melanoma, renal cell carcinoma, head and neck cancer, and sarcoma demonstrated that patients with a baseline NLR <5 had a significantly longer mOS after ICI therapies (36). Moreover, a bundle of preclinical and clinical data has demonstrated that a higher NLR is significantly is significantly associated with a decreased mOS and mPFS in many cancers, and also with lower ORR and worse clinical outcomes after ICI therapy (37).
Several lines of preclinical and clinical research have provided considerable evidence that neutrophils play a significant role for ICI responses in many tumor models (37). TANs have been found to switch between pro- and anti-tumor phenotypes and the pro-tumor type TANs are able to cause immune suppression within the TME, thereby causing resistance to ICI therapies (33). A study has also investigated the association between NLR and the prognosis of patients with SCLC. Xie et al. reported that high baseline NLR levels were significantly associated with poor prognosis in patients with SCLC (38), and Suzuki et al. observed a correlation between high baseline NLR levels and shorter mOS and the 2-year OS rate (39). Chen et al. established a nomogram model, confirming that an increase in preoperative NLR was associated with shorter OS in postoperative patients with limited-stage SCLC (LS-SCLC) (40).
Our study we investigated the predictive value of NLR in the prognosis of patients with SCLC treated with PD-1/PD-L1 inhibitors and our results reported here were found to be consistent with the aforementioned studies (38-40). This adds weight to the proposal that NLR holds promise as a predictive factor for the prognosis of patients with SCLC undergoing PD-1/PD-L1 inhibitor treatment.
The prognostic relevance of PLRs in tumour undergoing ICI-based therapies still remains unclear. Some evidence has been provided that PLR might be associated in NSCLCs and breast cancer, however, not in others (41). A most recently published meta-analysis provided the first evidence that elevated PLR levels in gastric cancer patients is significantly associated with worse mOS and mPFS (41). A meta-analysis of nine studies with 949 patients revealed a significant correlation between elevated PRL and poorer mOS and mPFS (HR =1.67 and HR =1.51, respectively) suggesting that PLR may be a suitable biomarker to predict outcome of ICI-based therapies in gastric cancers (41).
Some studies exploring the relationship between PLR and SCLC prognosis have shown conflicting results. For instance, a study of patients with ES-SCLC treated with atezolizumab in combination with chemotherapy demonstrated that high PLR (>119.23) was significantly associated with shorter PFS and OS (42). Another study of patients with LS-SCLC found that PLR (≥53) was associated with longer PFS and OS (43).
However, our study found no significant correlation of PLR with PFS or OS in patients with SCLC treated with PD-1/PD-L1 inhibitors, in contrast to previous findings (38,42). The discrepancy in results may be attributed to differences in the cutoff values used to classify patients into high- or low-PLR groups. Furthermore, our study used ROC curves to determine an optimal cutoff value for PLR (275.89), further highlighting the need for standardized cutoff values in future investigations.
Monocytes are known to affect the TME by inducing angiogenesis and immune resistance (44). A previous study has shown that a decrease in the MLR was significantly associated with beneficial effects of nivolumab in NSCLC suggesting that MLR might be an attractive biomarker to predict the outcome of ICI therapies in lung cancer patients (45). However, the role of MLR to predict the treatment outcome of ICI therapies in SCLC has not yet been investigated, and, in addition, cutoff values of MLR are still discussed controversial in the literature (44). In our study, MLR emerged as an independent predictor of mPFS in patients with SCLC.
Similarly, PNI, an indicator of the host’s nutritional and immune status, has shown varying associations with SCLC prognosis in different studies. Although some investigations reported a significant association between high PNI (≥53) and improved PFS and OS (43,46), our study found no significant correlation of PNI with PFS or OS in patients with SCLC treated with PD-1/PD-L1 inhibitors. These differences may be due to differences in sample size and the choice of cutoff values, warranting further prospective studies for validation. Furthermore, SII has emerged as potential prognostic factors in SCLC. A meta-analysis found that high SII was significantly associated with shorter OS in patients with SCLC (47). In our study, SII did not exhibit predictive value for prognosis in patients receiving immunotherapy.
RBC and Hb have been linked to tumor progression, and studies have reported associations between RBC or Hb levels and prognosis in patients with SCLC (24,48). Our study found an association between RBC and Hb with PFS and OS in patients with SCLC undergoing immunotherapy; but after multivariate analysis were not found to be independent prognostic factors. Thrombocytosis, reflected by elevated PLT counts, has been linked to tumor development, growth, and metastasis (49). Our study showed that low PLT levels are inversely associated with survival in patients with SCLC undergoing immunotherapy. A bigger cohort is required for confirmation.
Finally, LDH has also been studied as an indicator of tumor activity and prognosis in SCLC (50-52). Our study found LDH to be an independent factor for OS but not PFS in patients with SCLC treated with PD-1/PD-L1 inhibitors.
Our study had several important limitations, including the inherent biases associated with a retrospective study, small sample size and lack of a validation set. And our study did not predict immune related adverse reactions, but previous studies have reported a higher frequency of adverse events in combination anti-tumor therapy, but only appetite loss, hyponatremia, and any level of hypothyroidism are significant, and immunotherapy seems to reduce mortality caused by toxicity (53).
Conclusions
Our study yielded significant findings regarding the prognostic value of certain hematological indicators in patients with SCLC receiving PD-1/PD-L1 inhibitor treatment. Specifically, we identified MLR and PLT as independent prognostic factors for PFS in patients with SCLC, while NLR and LDH emerged as independent prognostic factors for OS.
Patients with high MLR and elevated PLT showed a more favorable PFS outcome, indicating that these factors may serve as potential biomarkers to predict the PFS of patients with SCLC undergoing PD-1/PD-L1 inhibitor treatment.
Meanwhile, patients with high NLR and LDH values demonstrated improved OS, suggesting that NLR and LDH could be valuable biomarkers for assessing the overall prognosis of patients with SCLC receiving PD-1/PD-L1 inhibitor treatment.
These findings provide valuable insights into the use of hematological indicators as prognostic markers for patients with SCLC undergoing PD-1/PD-L1 inhibitor treatment. The identification of these independent prognostic factors may, if confirmed in large prospective clinical trials, guidance in patient stratification, treatment planning, and the development of personalized therapeutic approaches to improving outcomes in SCLC management. Further validation studies and larger clinical trials are warranted to confirm the clinical utility of NLR, LDH, MLR, and PLT as prognostic biomarkers in this context. Overall, these hematological indicators hold promise for enhancing prognostic assessments and patient care in the realm of SCLC treatment.
Acknowledgments
The authors would like to thank the Qiang Li and Ming Zhang for their contributions to this study.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1826/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1826/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1826/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1826/coif). H.A. receives consulting fees from Astra Zeneca; travel and accommodation from MSD, BMS, Roche, Takeda. R.A.S. serves as the advisory board of Abbvie, Amgen, AnHeart, Astra-Zeneca, Bayer, BMS, Boehringer Ingelheim, Daiichi Sankyo, GSK, J INTS BIO, Janssen, Lily, Merck, Merck Serono, Novartis, Pfizer, Puma, Roche, Sanofi, Taiho, Takeda, Thermo Fisher, Yuhan Corporation; receives research grant from Astra-Zeneca, Boehringer Ingelheim, Pfizer; and honorarium from Abbvie, Amgen, AnHeart, Astra-Zeneca, Bayer, BMS, Boehringer Ingelheim, Chugai, Daiichi Sankyo, GSK, J INTS BIO, Janssen, Lily, Merck, Merck Serono, Novartis, Pfizer, Puma, Roche, Sanofi, Taiho, Takeda, Thermo Fisher, Yuhan Corporation. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of the Fourth Hospital of Hebei Medical University (No. 2023146). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent 2024;4:47-53. [Crossref] [PubMed]
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Stinchcombe TE, Gore EM. Limited-stage small cell lung cancer: current chemoradiotherapy treatment paradigms. Oncologist 2010;15:187-95. [Crossref] [PubMed]
- Xiao Y, Li X, Mao J, et al. Reverse anti-breast cancer drug resistance effects by a novel two-step assembled nano-celastrol medicine. Nanoscale 2022;14:7856-63. [Crossref] [PubMed]
- Li X, Zhang Z, Harris A, et al. Bridging the gap between fundamental research and product development of long acting injectable PLGA microspheres. Expert Opin Drug Deliv 2022;19:1247-64. [Crossref] [PubMed]
- Qiu X, Li S, Li X, et al. Experimental study of β-TCP scaffold loaded with VAN/PLGA microspheres in the treatment of infectious bone defects. Colloids Surf B Biointerfaces 2022;213:112424. [Crossref] [PubMed]
- Li X, Wei Y, Wen K, et al. Novel insights on the encapsulation mechanism of PLGA terminal groups on ropivacaine. Eur J Pharm Biopharm 2021;160:143-51. [Crossref] [PubMed]
- Li X, Wei Y, Lv P, et al. Preparation of ropivacaine loaded PLGA microspheres as controlled-release system with narrow size distribution and high loading efficiency. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2019;562:237-46.
- Jin H, Chong H, Zhu Y, et al. Preparation and evaluation of amphipathic lipopeptide-loaded PLGA microspheres as sustained-release system for AIDS prevention. Eng Life Sci 2020;20:476-84. [Crossref] [PubMed]
- Wen K, Na X, Yuan M, et al. Preparation of novel ropivacaine hydrochloride-loaded PLGA microspheres based on post-loading mode and efficacy evaluation. Colloids Surf B Biointerfaces 2022;210:112215. [Crossref] [PubMed]
- Zhang GQ, Feng W, Gao Z, et al. A NIR ratiometric fluorescent biosensor for sensitive detection and imaging of alpha-L-fucosidase in living cells and HCC tumor-bearing mice. Aggregate 2023;4:e286.
- Zeng H, Zeng X, Wang C, et al. Combination therapy using Cel-CSO/Taxol NPs for reversing drug resistance in breast cancer through inhibiting PI3K/AKT/NF-κB/HIF-1α pathway. Drug Deliv Transl Res 2024; Epub ahead of print. [Crossref]
- Guo JC, An Q, Guo MY, et al. Oxygen-independent free radical generation mediated by core-shell magnetic nanocomposites synergizes with immune checkpoint blockade for effective primary and metastatic tumor treatment. Nano Today 2021;36:101024.
- Tang J, Guo M, Wang P, et al. Gd-Metallofullerenol nanoparticles cause intracellular accumulation of PDGFR-α and morphology alteration of fibroblasts. Nanoscale 2019;11:4743-50. [Crossref] [PubMed]
- Xiao Y, Liu J, Guo M, et al. Synergistic combination chemotherapy using carrier-free celastrol and doxorubicin nanocrystals for overcoming drug resistance. Nanoscale 2018;10:12639-49. [Crossref] [PubMed]
- Yang J, Fang L, Jiang R, et al. RuCu Nanosheets with Ultrahigh Nanozyme Activity for Chemodynamic Therapy. Adv Healthc Mater 2023;12:e2300490. [Crossref] [PubMed]
- Liu SV, Reck M, Mansfield AS, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol 2021;39:619-30. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Wang J, Zhou C, Yao W, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:739-47. [Crossref] [PubMed]
- Cheng Y, Han L, Wu L, et al. LBA9 Updated results of first-line serplulimab versus placebo combined with chemotherapy in extensive-stage small cell lung cancer: An international multicentre phase III study (ASTRUM-005). Ann Oncol 2022;33:S1562.
- Cheng Y, Fan Y, Zhao Y, et al. OA01. 06 First-Line Chemotherapy with or without tislelizumab for extensive-stage small cell lung cancer: RATIONALE-312 phase 3 study. J Thorac Oncol 2023;18:S46.
- Cheng Y, Liu Y, Zhang W, et al. LBA93 EXTENTORCH: A randomized, phase III trial of toripalimab versus placebo, in combination with chemotherapy as a first-line therapy for patients with extensive stage small cell lung cancer (ES-SCLC). Ann Oncol 2023;34:S1334.
- Wang K, Long W, Sima X, et al. Sarcopenia defined by skeletal muscle mass index at the third lumbar vertebra is a prognostic factor for extensive-stage small cell lung cancer patients: a retrospective study. J Thorac Dis 2022;14:2645-51. [Crossref] [PubMed]
- Wu F, Yang S, Tang X, et al. Prognostic value of baseline hemoglobin-to-red blood cell distribution width ratio in small cell lung cancer: A retrospective analysis. Thorac Cancer 2020;11:888-97. [Crossref] [PubMed]
- Ichikawa K, Watanabe S, Miura S, et al. Prognostic significance of procalcitonin in small cell lung cancer. Transl Lung Cancer Res 2022;11:43-52. [Crossref] [PubMed]
- Kiriu T, Yamamoto M, Nagano T, et al. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS One 2018;13:e0193018. [Crossref] [PubMed]
- Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24:541-50. [Crossref] [PubMed]
- Okadome K, Baba Y, Yagi T, et al. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann Surg 2020;271:693-700. [Crossref] [PubMed]
- Choi Y, Kim JW, Nam KH, et al. Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer 2017;20:602-11. [Crossref] [PubMed]
- Deng C, Liao J, Fu Z, et al. Systemic immune index predicts tumor-infiltrating lymphocyte intensity and immunotherapy response in small cell lung cancer. Transl Lung Cancer Res 2024;13:292-306. [Crossref] [PubMed]
- Gibellini L, Borella R, Santacroce E, et al. Circulating and Tumor-Associated Neutrophils in the Era of Immune Checkpoint Inhibitors: Dynamics, Phenotypes, Metabolism, and Functions. Cancers (Basel) 2023;15:3327. [Crossref] [PubMed]
- Tung I, Sahu A. Immune checkpoint inhibitor in first-line treatment of metastatic renal carcinoma: a review of current evidence and future directions. Front Oncol 2021;11:707214. [Crossref] [PubMed]
- Xie P, Yu M, Zhang B, et al. CRKL dictates anti-PD-1 resistance by mediating tumor-associated neutrophil infiltration in hepatocellular carcinoma. J Hepatol 2024;81:93-107. [Crossref] [PubMed]
- Kemal Y, Yucel I, Ekiz K, et al. Elevated serum neutrophil to lymphocyte and platelet to lymphocyte ratios could be useful in lung cancer diagnosis. Asian Pac J Cancer Prev 2014;15:2651-4. [Crossref] [PubMed]
- Yodying H, Matsuda A, Miyashita M, et al. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann Surg Oncol 2016;23:646-54. [Crossref] [PubMed]
- Valero C, Lee M, Hoen D, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun 2021;12:729. [Crossref] [PubMed]
- Que H, Fu Q, Lan T, et al. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim Biophys Acta Rev Cancer 2022;1877:188762. [Crossref] [PubMed]
- Xie D, Marks R, Zhang M, et al. Nomograms Predict Overall Survival for Patients with Small-Cell Lung Cancer Incorporating Pretreatment Peripheral Blood Markers. J Thorac Oncol 2015;10:1213-20. [Crossref] [PubMed]
- Suzuki R, Wei X, Allen PK, et al. Prognostic Significance of Total Lymphocyte Count, Neutrophil-to-lymphocyte Ratio, and Platelet-to-lymphocyte Ratio in Limited-stage Small-cell Lung Cancer. Clin Lung Cancer 2019;20:117-23. [Crossref] [PubMed]
- Chen C, Yang H, Cai D, et al. Preoperative peripheral blood neutrophil-to-lymphocyte ratios (NLR) and platelet-to-lymphocyte ratio (PLR) related nomograms predict the survival of patients with limited-stage small-cell lung cancer. Transl Lung Cancer Res 2021;10:866-77. [Crossref] [PubMed]
- Hou S, Song D, Zang Y, et al. Prognostic relevance of platelet lymphocyte ratio (PLR) in gastric cancer patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Front Oncol 2024;14:1367990. [Crossref] [PubMed]
- Qi WX, Xiang Y, Zhao S, et al. Assessment of systematic inflammatory and nutritional indexes in extensive-stage small-cell lung cancer treated with first-line chemotherapy and atezolizumab. Cancer Immunol Immunother 2021;70:3199-206. [Crossref] [PubMed]
- Zhang JQ, Wang YY, Xu KP, et al. Prognostic evaluation of nutritional indicators in patients with limited-stage small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2019;41:937-42. [Crossref] [PubMed]
- Zheng L, Xiong A, Wang S, et al. Decreased monocyte-to-lymphocyte ratio was associated with satisfied outcome of first-line PD-1 inhibitors plus chemotherapy in strage IIIB-IV non-small cell lung cancer. Front Oncol 2023;14:1094378. [Crossref] [PubMed]
- Sekine K, Kanda S, Goto Y, et al. Chnage in the lymphocyte-to-monocyte ratio in an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non-small-cell lung cancer. Lung Cancer 2018;124:179-88. [Crossref] [PubMed]
- Minami S, Ogata Y, Ihara S, et al. Pretreatment Glasgow prognostic score and prognostic nutritional index predict overall survival of patients with advanced small cell lung cancer. Lung Cancer (Auckl) 2017;8:249-57. [Crossref] [PubMed]
- Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget 2017;8:75381-8. [Crossref] [PubMed]
- Bremnes RM, Sundstrom S, Aasebø U, et al. The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer 2003;39:303-13. [Crossref] [PubMed]
- Sagman U, Maki E, Evans WK, et al. Small-cell carcinoma of the lung: derivation of a prognostic staging system. J Clin Oncol 1991;9:1639-49. [Crossref] [PubMed]
- Hsieh AH, Tahkar H, Koczwara B, et al. Pre-treatment serum lactate dehydrogenase as a biomarker in small cell lung cancer. Asia Pac J Clin Oncol 2018;14:e64-70. [Crossref] [PubMed]
- Chen C, Zhu YH, Huang JA. Clinical evaluation of potential usefulness of serum lactate dehydrogenase level in follow-up of small cell lung cancer. J Cancer Res Ther 2018;14:S336-40. [Crossref] [PubMed]
- Lim JU, Kang HS, Shin AY, et al. Investigation of poor predictive factors in extensive stage small cell lung cancer under etoposide-platinum-atezolizumab treatment. Thorac Cancer 2022;13:3384-92. [Crossref] [PubMed]
- Moraes FCA, Lôbo AOM, Sano VKT, et al. Treatment-related Adverse Events, Including Fatal Toxicities, in Patients With Extensive-stage Small-cell Lung Cancer Receiving Adjuvant Programmed Cell Death 1/Programmed Cell Death Ligand 1 Inhibitors: A Meta-analysis and Trial Sequential Analysis of Randomized Controlled Trials. Clin Oncol (R Coll Radiol) 2024;36:e408-19. [Crossref] [PubMed]


