
Postoperative day 1 discharge for segmentectomy using a minimally invasive approach after drain removal on the day of surgery
Highlight box
Key findings
• Of the 67 patients who underwent drain removal on the day of segmentectomy via minimally invasive approach (MIA), 31 (46.3%) were discharged on postoperative day (POD) 1.
What is known and what is new?
• Although several previous studies have shown successful discharge on POD1 after anatomical lung resections, the success rate is still low worldwide.
• POD1 discharge for segmentectomy using a MIA after drain removal on the day of surgery is considered feasible.
What is the implication, and what should change now?
• This study indicates the feasibility of POD1 discharge for segmentectomy via MIA, which can facilitate early reintegration of patients into society.
Introduction
Prolonged postoperative hospital stay reduces hospital capacity, increases costs, and increases patient exposure to potential nosocomial infections (1). Therefore, it is desirable to reduce the postoperative hospital stay to avoid them, which is also indicative of excellent postoperative outcomes.
Although several previous studies have shown successful discharge on postoperative day (POD) 1 after anatomical lung resections, the success rate is still low worldwide (2-6). We believe that there are two key factors, including early removal of chest tube and application of minimally invasive approach (MIA) to achieve it, because the former can contribute to early postoperative mobilization and the latter can provide less postoperative pain, which facilitates patient recovery. In addition, segmentectomy is considered a good candidate for POD1 discharge among anatomical lung resections if postoperative air leakage in intersegmental planes was well managed, because less volume of lung parenchyma was removed compared with lobectomy, indicating less invasiveness (7-9).
In this retrospective study, we investigated the feasibility of POD1 discharge for segmentectomy using a MIA after drain removal on the day of surgery (DOS). We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1372/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by institutional ethics board of Japanese Red Cross Maebashi Hospital (approval No. 2024-1, Date: April 19, 2024) and the need for individual consent for this retrospective analysis was waived.
Between July 2021 and September 2023, 108 patients underwent pulmonary segmentectomy via MIA at our institution. Among the patients, ninety patients who underwent segmentectomy via MIA between July 2021 and September 2023 were included in this retrospective study. Before conducting this retrospective study, we first investigated the safety and feasibility of early drain removal on DOS in uniportal thoracoscopic approach in a prospective nature (10). This study enrolled 20 of the 38 patients during the study period who were also enrolled in this retrospective study. After the successful results of the previous prospective study, 70 patients were consecutively enrolled in this study. The 90 patients were divided into those who underwent drain removal on DOS or after DOS. Clinical characteristics and perioperative outcomes were compared between the two groups. Clinical data analyzed for each case included operating surgeon, age, sex, lobe treated, smoking history, chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD) as underlying pulmonary disease, preoperative forced expiratory volume in one second (FEV1.0), preoperative FEV1.0%, disease, reason for segmentectomy, type of segmentectomy, surgical approach, operative time, blood loss, use of thrombin spray, postoperative drainage time, postoperative hospital stays, morbidity (Clavien-Dindo grade ≥ III), re-drainage after removal of postoperative drainage tube, readmission within 30 days after surgery, conversion to thoracotomy, 30-day postoperative mortality, and morning numerical rating scale (NRS) on POD1. All segmentectomies were categorized into two types: simple and complex (11). Simple pulmonary segmentectomy involved the lingual segment, basilar segment, superior segment of the lower lobe, or the upper division of the left upper lobe. Any pulmonary segmentectomy other than these was considered complex. In addition, factors associated with drain removal on DOS were identified using univariate and multivariate analyses. Finally, factors associated with discharge on POD1 among patients undergoing drain removal on DOS were also identified using univariable and multivariable analyses. Figure 1 shows the patient enrollment process.
Preoperative treatment planning
Lung segmentectomy via MIA was planned to determine whether a tumor was malignant or to remove a confirmed malignant tumor. In our department, intentional segmentectomy with lymph node sampling was performed for cTis-1aN0M0 primary lung cancer, following patient consent. For patients with compromised health conditions, unintentional segmentectomy was conducted for primary lung cancer. In cases of pulmonary metastasis, segmentectomy was chosen over wedge resection if the tumor was difficult to resect due to its deep location.
Multidetector row computed tomography (CT) was used to localize the tumor. In addition, three-dimensional CT bronchoangiography was performed in all patients except those who were allergic to the contrast agent to confirm the branching pattern of the pulmonary vessels and bronchi. In addition, the virtual intersegmental plane was simulated preoperatively to ensure sufficient surgical margin using Ziostation2 software (12).
Surgical procedures
All procedures were carried out with the patient positioned in the lateral decubitus position, under general anesthesia and single-lung ventilation.
In the uniportal thoracoscopic approach, a single 3.5–4 cm skin incision was made along the anterior axillary line at the 4th or 5th intercostal space (Figure 2A). All surgical instruments, along with a 10-mm, 30-degree angled thoracoscope, were inserted simultaneously through the incision.

In the multiportal thoracoscopic approach, 3 or 4 ports were created (Figure 2B).
The robotic approach (using the DaVinci XI robotic system; Intuitive, Sunnyvale, USA) involved five ports, including an assistant port (Figure 2C). A 0-degree angled camera was used to evaluate the cavity. Three 8 mm robotic trocars were placed in the 6th or 7th intercostal spaces, centered on the scapular line. For retraction or stapler insertion, a 12-mm robotic trocar was positioned at the anterior axillary line in the 7th or 8th intercostal space. The assistant port was placed at the scapular line in the 9th intercostal space. CO2 insufflation was maintained at a pressure of 8 mmHg.
The surgical techniques were consistent across all approaches. Major vessels, such as the pulmonary artery and vein, were carefully exposed and then divided, primarily using endovascular staplers. In the RATS approach, robotic endostaplers were always used. Smaller branches of these vessels were divided using an energy device, following proximal ligation with silk sutures. The dominant bronchus was also divided using a stapler. Intersegmental planes were identified by infrared thoracoscopy with intravenous indocyanine green administration. For patients with iodine allergies, the inflation-deflation method was employed instead. All intersegmental planes were divided using staplers. At the conclusion of the surgery, a seal test (pressure: 15 cmH2O) was performed to check for any pulmonary air leaks. If no leaks were found, no further treatment was applied to the remaining lung. However, if a leak was detected, the site was sutured using absorbable monofilament sutures, followed by an additional seal test. If this second test showed no leak, polyglycolic acid (PGA) felt (Neoveil, sheet type; Igaki Medical Planning Co., Ltd., Kyoto, Japan) and fibrin glue (Beriplast P; CSL Behring, King of Prussia, PA, USA) were applied to the leak site. If leaks persisted, the surgeon either re-sutured the area or applied these materials as necessary. A 24-Fr trocar (Argyle thoracic catheter; Medtronic, Dublin, Ireland) was then placed in the thorax to complete the procedure.
For patients with primary lung cancer undergoing segmentectomy, interlobar and hilar lymph node sampling was conducted to confirm the pathologic stage. If a positive lymph node was found in intentional segmentectomies, a follow-up lobectomy was planned. Intraoperative frozen sections of lymph nodes were not examined, as cTis-1aN0M0 was the criterion for intentional segmentectomy in our department. In cases of unintentional segmentectomies, no additional lobectomy was planned because these patients were unable to tolerate the procedure. Lymphadenectomy was not performed for patients with metastatic or benign disease. A chest tube was placed at the end of the surgery.
Postoperative management
Indications for drain removal on DOS were absence of air leak during an intraoperative seal test, radiographic evidence of lung expansion, and continuous absence of air leak without active bleeding through a drainage bottle for 2–4 hours after surgery. If not, the tube was left in place until at least the next day. The volume of postoperative pleural effusion was not considered in the criteria for drain removal. Patients could be discharged if the chest X-ray taken the day after chest tube removal showed no problems. The criteria for re-drainage were mentioned in our previous article (10).
Intra- and postoperative pain control
Intercostal nerve block with levobupivacaine hydrochloride was performed in the affected intercostal space at the beginning of surgery. Immediately after surgery, patient-controlled analgesia (PCA) was administered with fentanyl at 0.006 µg/kg/min, followed by intravenous paracetamol. When oral administration became feasible, oral non-steroidal anti-inflammatory drugs (NSAIDs) were started, and PCA was discontinued in the morning of POD1 prior to postoperative pain assessment.
Statistical analysis
The Mann-Whitney U test for continuous variables or Fisher’s exact test for categorical variables was used to assess patient characteristics and perioperative outcomes between the two groups. Multivariable analyses were performed using a logistic regression model to identify factors associated with drain removal on DOS in all patients and POD1 discharge in patients who received drain removal on DOS. Differences were considered significant at P<0.05. All calculations and statistical analyses were performed using the EZR graphical user interface for R (Saitama Medical Centre, Jichi Medical University, Saitama, Japan).
Results
Comparison of the distribution of segmentectomies performed between patients who received drain removal on DOS and POD1 or later is shown in Table S1.
Drains were removed on DOS in 67 patients (74.4%). Therefore, the 90 patients were divided into those who underwent drain removal on DOS (n=67) or after DOS (n=23). Table 1 shows the comparison of patient characteristics and perioperative outcomes between patients who received drain removal on DOS (n=67) and later (n=23). Among patient characteristics, patients who received drain removal on DOS had significantly higher forced expiratory volume in 1 second (FEV1.0)% (P=0.03). Among perioperative outcomes, patients who received drain removal on DOS had significantly shorter operative time (P<0.001), less blood loss (P=0.02), lower rate of patients receiving thrombin spray (P<0.001), shorter postoperative drainage time (P<0.001), and shorter postoperative hospital stay (P<0.001). In addition, no patient in either group required re-drainage after chest tube removal. Figure 3A shows the comparison of the median morning NRS score on POD1 between patients who received drain removal on DOS and later, which showed no significant differences between the two groups (P=0.97). In addition, Figure 3B shows that the rate of patients with an NRS score of 3 or less was 76.1% in patients who received drain removal on DOS and 87% in patients who received drain removal on POD1 or later, which was also not significantly different (P=0.38).
Table 1
| Variables | Patients receiving drain removal on the day of surgery (n=67) | Patients receiving drain removal on postoperative day 1 or later (n=23) | P value |
|---|---|---|---|
| Operating surgeon | >0.99 | ||
| Chief surgeon | 35 (52.2) | 12 (52.2) | |
| Other staff surgeons | 32 (47.8) | 11 (47.8) | |
| Age (years) | 73 [64–78] | 70 [63–77.5] | 0.63 |
| Sex | 0.15 | ||
| Female | 41 (61.2) | 13 (56.5) | |
| Male | 26 (38.8) | 10 (43.5) | |
| Lobe treated | 0.48 | ||
| LUL | 29 (43.3) | 7 (30.4) | |
| LLL | 7 (10.4) | 5 (21.7) | |
| RUL | 13 (19.4) | 4 (17.4) | |
| RLL | 18 (26.9) | 7 (30.4) | |
| Smoking history | 32 (47.8) | 13 (56.5) | 0.63 |
| COPD as underlying pulmonary disease | 6 (9.0) | 4 (17.4) | 0.27 |
| ILD as underlying pulmonary disease | 4 (6.0) | 0 (0.0) | 0.57 |
| Preoperative FEV1.0 (mL) | 2,000 [1,600–2,648] | 2,130 [1,590–2,490] | 0.98 |
| Preoperative FEV1.0% (%) | 77.1 [71.5–82.8] | 70.7 [62.2–77.4] | 0.03 |
| Disease | 0.93 | ||
| Primary lung cancer | 44 (65.7) | 15 (65.2) | |
| Pulmonary metastasis | 15 (22.4) | 5 (21.7) | |
| Other benign | 8 (12.0) | 3 (13.0) | |
| Reason for segmentectomy | 1 | ||
| Intentional | 37 (55.2) | 13 (56.5) | |
| Unintentional | 15 (22.4) | 5 (21.7) | |
| Others | 15 (22.4) | 5 (21.7) | |
| Type of segmentectomy | 0.22 | ||
| Simple | 39 (58.2) | 17 (73.9) | |
| Complicated | 28 (41.8) | 6 (26.1) | |
| Surgical approach | >0.99 | ||
| Uniport | 42 (62.7) | 14 (60.9) | |
| Multiport | 2 (3.0) | 1 (4.3) | |
| Robot | 23 (34.3) | 8 (34.8) | |
| Operative time (minutes) | 115 [92–136] | 145 [120–176] | <0.001 |
| Blood loss (g) | Unmeasurable (unmeasurable–unmeasurable) | Unmeasurable (unmeasurable–30) | 0.02 |
| Use of thrombin spray | 14 (20.9) | 17 (73.9) | <0.001 |
| Postoperative drainage time (days) | 0 [0–0] | 1 [1–1] | <0.001 |
| Postoperative hospital stay (days) | 2 [1–2] | 2 [2–3] | <0.001 |
| Morbidity (Clavien-Dindo classification grade ≥ III) | 1 (1.5) | 0 (0.0) | >0.99 |
| Re-drainage after removal of postoperative drainage tube | 0 (0.0) | 0 (0.0) | – |
| Readmission within 30 days after surgery | 0 (0.0) | 0 (0.0) | – |
| Conversion to thoracotomy | 0 (0.0) | 0 (0.0) | – |
| 30-day postoperative mortality | 0 (0.0) | 0 (0.0) | – |
Data are presented as n (%) or median [IQR]. LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RLL, right lower lobe; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; FEV, forced expiratory volume; IQR, interquartile range.
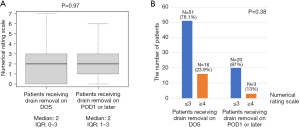
Table 2 lists univariable and multivariable analyses to identify factors associated with drain removal on DOS. Among the variables, FEV1.0% was the only factor significantly associated with drain removal on DOS (odds ratio: 0.934, 95% confidence interval: 0.880–0.993, P=0.03).
Table 2
| Variables | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Operating surgeon, chief surgeon | 0.9970 (0.3860–2.5700) | >0.99 | 0.7340 (0.1950–2.7700) | 0.65 | |
| Age | 0.9940 (0.9540–1.0400) | 0.77 | 0.9760 (0.9280–1.0300) | 0.34 | |
| Sex, male | 2.0500 (0.7850–5.350) | 0.14 | 2.5300 (0.6280–10.2000) | 0.19 | |
| Treated lobe, upper lobe | 0.5810 (0.2240–1.5100) | 0.27 | 0.4650 (0.1480–1.4700) | 0.19 | |
| History of smoking | 1.4200 (0.5480–3.6900) | 0.47 | 0.7000 (0.1610–3.0400) | 0.63 | |
| FEV1.0% | 0.9550 (0.9150–0.9970) | 0.04 | 0.9340 (0.880–0.9930) | 0.03 | |
| Disease, primary lung cancer | 0.9800 (0.3620–2.6500) | 0.97 | 0.5030 (0.1310–1.9300) | 0.32 | |
| Reason for segmentectomy, intentional | 1.0500 (0.4060–2.7400) | 0.91 | 3.1000 (0.7500–12.8000) | 0.12 | |
| Type of segmentectomy, complicated | 2.0300 (0.7120–5.8100) | 0.19 | 3.1100 (0.8800–11.0000) | 0.08 | |
| Surgical approach, uniport | 0.9260 (0.3500–2.4500) | 0.88 | 0.6350 (0.1700–2.3700) | 0.50 | |
FEV, forced expiratory volume; CI, confidence interval.
Of the 67 patients who underwent drain removal on DOS, 31 (46.3%) were discharged on POD1 and 26 (38.8%) on POD2. Figure 4 shows the percentage of patients discharged on POD1 and later. No patients were unexpectedly readmitted within 30 days of surgery. Table 3 shows univariable and multivariable analyses to identify factors associated with discharge on POD1 among patients undergoing drain removal on the DOS. Among the variables, chief surgeon was the only factor significantly associated with discharge on POD1 (vs. others, odds ratio: 0.117, 95% confidence interval: 0.019–0.730, P=0.02). Reasons for delayed discharge (POD2 or later) in patients who successfully underwent drain removal on the DOS (n=37) are listed in Table S2. In nineteen of the 37 patients (52.8%), the reason was a personal matter not related to the medical problem.

Table 3
| Variables | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Operating surgeon, chief surgeon | 0.3030 (0.1110–0.8300) | 0.02 | 0.1170 (0.0188–0.7300) | 0.02 | |
| Age | 1.0300 (0.9983–1.0700) | 0.24 | 1.0300 (0.9720–1.1000) | 0.29 | |
| Sex, male | 0.4690 (0.1730–1.280) | 0.14 | 0.2750 (0.0482–1.5700) | 0.15 | |
| Treated lobe, upper lobe | 0.5595 (0.2190–1.6200) | 0.31 | 1.6900 (0.3950–7.2300) | 0.48 | |
| History of smoking | 0.7500 (0.2860–1.9700) | 0.56 | 0.4490 (0.0861–2.3400) | 0.34 | |
| FEV1.0% | 0.9640 (0.9140–1.020) | 0.19 | 0.9560 (0.8870–1.0300) | 0.24 | |
| Disease, primary lung cancer | 1.8800 (0.6770–5.2100) | 0.23 | 4.2100 (0.7720–23.0000) | 0.10 | |
| Reason for segmentectomy, intentional | 0.8070 (0.3070–2.1300) | 0.67 | 0.3230 (0.0557–1.8700) | 0.21 | |
| Type of segmentectomy, complicated | 0.4760 (0.1760–1.2900) | 0.15 | 0.9230 (0.1940–4.3900) | 0.92 | |
| Surgical approach, uniport | 1.8700 (0.6870–5.1000) | 0.22 | 1.6600 (0.3590–7.6400) | 0.52 | |
| Operative time | 1.0100 (0.9940–1.0300) | 0.22 | 1.0100 (0.9900–1.0300) | 0.32 | |
| Blood loss | 1.0300 (0.9900–1.0700) | 0.15 | 1.0400 (0.9990–1.0800) | 0.055 | |
| NRS, 4 or over | 2.2900 (0.6950–7.5300) | 0.17 | 1.0700 (0.2030–5.6400) | 0.94 | |
FEV, forced expiratory volume; NRS, numerical rating scale; CI, confidence interval.
Discussion
In this retrospective study, of the ninety patients who underwent segmentectomy via MIA, 67 patients (74.4%) had drains removed on DOS. In addition, no patient required re-drainage after chest tube removal. Subsequently, 31 (46.2%) of the 67 patients who underwent drain removal on DOS were discharged on POD1. In addition, no patients were unexpectedly readmitted within 30 days of surgery. These results indicated that POD1 discharge for segmentectomy using a MIA after drain removal on DOS is considered feasible.
In nationwide data from the United States, only 3.9% of patients undergoing anatomic pulmonary resections were discharged on POD1, which is still considered low (13). In contrast, previous single institution studies focusing on POD1 discharge after anatomic pulmonary resections showed excellent results (3,8,9). Geraci et al. showed that, apparently better than the national results in the United States, 53% of the included patients undergoing robotic-assisted anatomic pulmonary resections were discharged on POD1 (8). In particular, on multivariate analysis, segmentectomy was associated with POD1 discharge, suggesting that patients undergoing segmentectomy may be easier to discharge than those undergoing lobectomy due to its less invasive nature. In addition, Dolan et al. showed that 35% of the included patients who underwent pulmonary resections via MIA were discharged on POD1, although only early stage (stage I–II) NSCLC were candidates (9). This study also showed that segmentectomy had better POD1 discharge outcomes compared to lobectomy.
To achieve rapid discharge among the patients who received pulmonary segmentectomy, there are several key factors, including ERAS program, adoption of MIA, and so on (14-16). Especially, the management of postoperative air leak on the divided intersegmental planes, which may lead to early removal of postoperative drainage tube, is very important because pulmonary segmentectomy tends to have it compared with lobectomy (7). Our group has adopted the fissureless technique without dissecting a fissure not only for lobectomy but also for segmentectomy when a fissure was fused (17,18). In addition, a stapler is always used to divide the intersegmental plane because Chen et al. prospectively reported higher complication rates, including postoperative prolonged air leak, in an electrocautery group than in a stapler group for dividing the intersegmental plane in pulmonary segmentectomy (19). Finally, we successfully achieved drain removal on DOS in 74.4% of patients who underwent segmentectomy via MIS. As a caveat, in this study, multivariable analyses revealed that low FEV1.0%, indicating the coexistence of underlying pulmonary disease including emphysema or fibrosis, was a significantly negative associated factor with drain removal on DOS. These patients should be considered for additional procedures, including the attachment of reinforced materials to the staple line, to achieve drain removal on DOS.
In our study, 53.8% of patients were discharged on POD2 or later despite drain removal on DOS. Furthermore, in 52.8% of the patients, the reason was a personal matter unrelated to the medical problem, indicating that we still have room for improvement. We believe it is necessary to adequately explain the benefits of POD1 discharge preoperatively and ensure that patients’ concerns about postoperative care are addressed. In the multivariate analysis, surgery performed by the chief surgeon (H.I.) was the only factor significantly associated with discharge on POD1. HI implemented this POD1 discharge protocol with more clinical experience compared to other surgeons. In our department, an operating surgeon usually explains the surgical procedure and postoperative course. This result indicated that the precise explanation of this POD1 discharge protocol by H.I. may contribute to better outcomes.
Most previous studies have found that tubeless management significantly reduces postoperative pain on POD1 compared to management with drainage tubes (20,21). However, in this study, the median NRS scores were equivalent between the two groups. In addition, the rate of patients with an NRS score of 3 or less was also statistically similar between the two groups. This result indicated that the presence of chest tube did not contribute to postoperative pain, which was not our expectation, although the surgical approaches including uVATS, mVATS and RATS were different in the respective patients. Intra- or postoperative pain management may be more important and effective because 87% of patients with chest tube on POD1 had NRS score of 3 or less.
Limitations
The present study had several limitations. First, it was single institution and retrospective in nature with a small number of patients enrolled. Second, long-term outcomes, including recurrence, were not evaluated. Third, it did not consider the final administration time of the analgesic. Fourth, several surgeons performed segmentectomy using different types of approaches, although each approach was MIA. Fifth, 53.8% of patients were discharged on POD2 or later despite drain removal on DOS. In most patients, the delay in discharge was not related to medical reasons.
Conclusions
POD1 discharge for segmentectomy using a MIA after drain removal on DOS is considered feasible. However, we still have room for improvement as 53.7% of patients were discharged on POD2 or later despite drain removal on DOS.
Acknowledgments
The authors thank the involved surgeons and their teams, the editors, and the reviewers for their assistance with the manuscript. The abstract was presented at the 104th Annual Meeting of the American Association for Thoracic Surgery in Toronto, April 27–30, 2024.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1372/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1372/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1372/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1372/coif). H.I. serves as an unpaid editorial board member of Journal of Thoracic Disease from August 2024 to July 2026. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Japanese Red Cross Maebashi Hospital (approval No. 2024-1) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giambrone GP, Smith MC, Wu X, et al. Variability in length of stay after uncomplicated pulmonary lobectomy: is length of stay a quality metric or a patient metric?. Eur J Cardiothorac Surg 2016;49:e65-e71. [Crossref] [PubMed]
- Freeman RK, Dilts JR, Ascioti AJ, et al. A comparison of length of stay, readmission rate, and facility reimbursement after lobectomy of the lung. Ann Thorac Surg 2013;96:1740-6. [Crossref] [PubMed]
- Greer S, Miller AD, Smith JS, et al. Safety of Next Day Discharge After Lobectomy: Have We Broken the Speed Limit? Ann Thorac Surg 2018;106:998-1001. [Crossref] [PubMed]
- Fiore JF Jr, Bejjani J, Conrad K, et al. Systematic review of the influence of enhanced recovery pathways in elective lung resection. J Thorac Cardiovasc Surg 2016;151:708-715.e6. [Crossref] [PubMed]
- Madani A, Fiore JF Jr, Wang Y, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery 2015;158:899-910. [Crossref] [PubMed]
- Handy JR Jr, Child AI, Grunkemeier GL, et al. Hospital readmission after pulmonary resection: prevalence, patterns, and predisposing characteristics. Ann Thorac Surg 2001;72:1855-9; discussion 1859-60. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Geraci TC, Chang SH, Chen S, et al. Discharging Patients by Postoperative Day One After Robotic Anatomic Pulmonary Resection. Ann Thorac Surg 2022;114:234-40. [Crossref] [PubMed]
- Dolan DP, Visa M, Lee D, et al. Rapid Discharge After Anatomic Lung Resection: Is Ambulatory Surgery for Early Lung Cancer Possible? Ann Thorac Surg 2024;117:297-303. [Crossref] [PubMed]
- Igai H, Matsuura N, Numajiri K, et al. Early chest drain removal on the day of uniportal thoracoscopic segmentectomy. Gen Thorac Cardiovasc Surg 2023;71:700-7. [Crossref] [PubMed]
- Brunelli A. Uncommon pulmonary anatomic segmentectomies: state of the art and technical aspects. J Vis Surg 2018;4:175.
- Matsuura N, Igai H, Ohsawa F, et al. Novel thoracoscopic segmentectomy combining preoperative three-dimensional image simulation and intravenous administration of indocyanine green. Interact Cardiovasc Thorac Surg 2022;35:ivac064. [Crossref] [PubMed]
- Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ 2020;371:m4087. [Crossref] [PubMed]
- Haro GJ, Sheu B, Marcus SG, et al. Perioperative Lung Resection Outcomes After Implementation of a Multidisciplinary, Evidence-based Thoracic ERAS Program. Ann Surg 2021;274:e1008-13. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1843-52. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Yoshikawa R, et al. The efficacy of thoracoscopic fissureless lobectomy in patients with dense fissures. J Thorac Dis 2016;8:3691-6. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M. A uniportal thoracoscopic fissureless lingual segmentectomy for a patient with a dense fissure. Multimed Man Cardiothorac Surg 2023;
- Chen X, Jin R, Xiang J, et al. Methods for Dissecting Intersegmental Planes in Segmentectomy: A Randomized Controlled Trial. Ann Thorac Surg 2020;110:258-64. [Crossref] [PubMed]
- Murakami J, Ueda K, Tanaka T, et al. The Validation of a No-Drain Policy After Thoracoscopic Major Lung Resection. Ann Thorac Surg 2017;104:1005-11. [Crossref] [PubMed]
- Pfeuty K, Lenot B. Early postoperative day 0 chest tube removal using a digital drainage device protocol after thoracoscopic major pulmonary resection. Interact Cardiovasc Thorac Surg 2020;31:657-63. [Crossref] [PubMed]



