
Advanced thymic epithelial tumour resection: vascular resection and reconstruction strategy
Highlight box
Key findings
• Advanced thymic epithelial tumors (TETs) with vascular invasion can be surgically with successful outcomes from specialized centres.
What is known and what is new?
• Advanced TETs that invade vascular structures present significant surgical challenges. This study provides detailed insights into the techniques and outcomes of advanced TET resections involving vascular resection and reconstruction.
What is the implication, and what should change now?
• High-volume centres performing TET resections should integrate tailored vascular reconstruction protocols and adopt a multidisciplinary approach.
Introduction
Thymic epithelial tumours (TETs) are the most common primary malignancy of the anterior mediastinum (1). They have the potential to invade all major structures of the mediastinum, vascular and visceral. The key to long-term disease control and survival is en bloc resection with preservation of the thymic capsule and microscopically clear margins (R0) (2). In advanced thymic malignancies, surgical clearance often requires resection and reconstruction of major vascular structures such as the superior vena cava (SVC) and brachiocephalic veins and an extrapleural pneumonectomy (EPP) in selected cases. When there is widespread infiltration as can often occur in stage III/IV disease, systemic biological control is necessary prior to surgery through a multi-modality approach (2). Vascular involvement has not been shown to impact overall survival but does increase the risk of recurrence (3,4). Vascular resection increases the intra-operative complexity and the risk of postoperative complications. The use of cardiopulmonary bypass (CPB) can further increase these risks however this must be balanced against the goal of achieving a complete resection, which is paramount. Here, we report our technical strategy of vascular resection and reconstruction based on our experience of treating this group of patients. We present this article in accordance with the SUPER reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1395/rc).
Methods
We present the key aspects of our surgical technique based on our experience of patients with stage III/IV TETs that have undergone extensive resection post neoadjuvant chemotherapy. All patients undergo full pre-operative evaluation with computed tomography (CT), positron emission tomography (PET) (Figure 1), CT-guided biopsy, pre-habilitation, and exercise testing. Magnetic resonance imaging (MRI) is sometimes carried out to further delineate the vascular anatomy if there is any area for concern based on the CT (myocardial invasion, arch invasion, tracheal invasion). Anaesthetic assessment is carried out in all patients, including screening for myasthenia gravis.
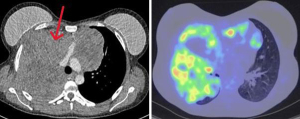
We interrogated our database over an 8-year period for all patients who had undergone resection for advanced stage III or higher TET based on the Tumour, Node, Metastasis (TNM) classification (5,6). This by definition on the T descriptor includes any patient with a thymic malignancy that radiologically demonstrates direct invasion into any one of the lung, innominate vein, SVC, phrenic nerve, chest wall or extra-pericardial pulmonary artery or vein (n=31). Those patients who had direct major vascular invasion on radiology pre-operatively were included as part of this case series (n=14). All cases were performed with a dedicated thoracic and a cardiac surgeon. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study did not involve direct patient contact, and therefore, ethical approval and informed consent were not required under UK regulations. The study was internally reviewed by the Guy’s and St. Thomas’ Trust Audit Department.
Surgical technique
The incision of choice was median sternotomy to gain access to the mass and the vascular structures. However, invasion of hilar structures or those in the posterior mediastinum, proximal SVC replacement or synchronous lung resection is better served through a combination of sternotomy and posterolateral thoracotomy. In case of bilateral extension, a clamshell incision or a sternotomy and clamshell or hemi clamshell incision was performed to have access to both hemi thoraces (Figure 2, Patient 1; Table 1).

Table 1
| Patient | Age (years) | Gender | Operation | Approach | Vascular element | Graft size | CPB use | RECIST criteria post neoadjuvant [cycles] | Histology | Length of stay (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | M | Thymectomy | Left thoracotomy (isolated pleural deposit) and sternotomy | SVC and innominate, and right atrium | PTFE 18/14 mm | Yes | Partial response [4] | B2 thymoma | 68 |
| 2 | 54 | F | Thymectomy | Sternotomy | Innominate vein | PTFE 14 mm | No | Partial response [6] | B2 thymoma | 9 |
| 3 | 52 | F | Thymectomy | Sternotomy | SVC | PTFE 18 mm | No | Partial response [6] | B2 thymoma | 11 |
| 4 | 24 | F | Extrapleural pneumonectomy + thymectomy | Sternotomy and right thoracotomy | SVC and innominate | PTFE 18/16 mm | No | Partial response [6] | B2 thymoma | 19 |
| 5 | 44 | F | Extrapleural pneumonectomy + thymectomy | Hemi clamshell | SVC | PTFE 18 mm | No | Partial response [6] | B2 thymoma | 44 |
| 6 | 37 | F | Thymectomy | Clamshell | SVC and innominate | PTFE 18/16 mm | No | Stable disease [4] | B2 thymoma | 10 |
| 7 | 34 | F | Thymectomy | Sternotomy | SVC | PTFE 18 mm | No | Partial response [6] | B3 thymoma | 11 |
| 8 | 75 | M | Thymectomy | Sternotomy | SVC | PTFE 18 mm | No | Partial response [6] | Thymic carcinoma | 22 |
| 9 | 41 | M | Thymectomy | Redo sternotomy | SVC | PTFE 18 mm | Yes | Partial response [6] | B1 thymoma | 12 |
| 10 | 49 | F | Thymectomy bilobectomy | Hemi clamshell | SVC | PTFE 18 mm | No | Partial response [6] | Thymic carcinoma | 7 |
| 11 | 55 | F | Thymectomy | Sternotomy | SVC and innominate | PTFE 18/16 mm | No | Stable disease [3] | Thymic carcinoma | 18 |
| 12 | 61 | F | Thymectomy | Sternotomy | SVC and innominate | PTFE 18/16 mm | No | Partial response [6] | B3 thymoma | 11 |
| 13 | 68 | F | Thymectomy | Sternotomy | SVC and innominate | PTFE 18/16 mm | No | Partial response [6] | AB thymoma | 25 |
| 14 | 59 | F | Thymectomy | Right thoracotomy and sternotomy | SVC and innominate—Y bifurcated graft, owing to short length of native vessels. Y graft allowed better run-off and less chance of kinking | PTFE 18/16 mm | No | Partial response [6] | Mixed B1/2/3 thymoma | 31 |
TET, thymic epithelial tumor; CPB, cardiopulmonary bypass; RECIST, Response Evaluation Criteria in Solid Tumors; SVC, superior vena cava; PTFE, polytetrafluoroethylene; M, male; F, female.
CPB is kept on standby in case of haemodynamic instability during tumour mobilisation and instituted if there is evidence of atrial infiltration.
Tumour mobilisation is achieved through the median sternotomy, freeing the mass from the pericardium, lung, pleura and innominate vein and SVC. As part of this dissection, dissection into the anterior (N1) and deep mediastinal (N2) nodal basins is carried out with harvesting of site-specific lymph nodes for staging.
Tumour resection is achieved with sequential resection of the left innominate vein (LIV) and SVC with extra-anatomical bypass to the right atrium as separate polytetrafluoroethylene (PTFE) tube grafts (Figures 3,4, Patient 1; Table 1). SVC replacement can be achieved with PTFE grafts (ideally 18 mm or above) or ringed PTFE graft (16 mm or above) (Figure 5, Patient 1; Table 1). Anastomoses are all performed with continuous 4-0 Prolene and 5,000 units of heparin is administered prior to clamping (if not on CPB). In order to reduce the chance of graft thrombosis, it is important to bevel the PTFE graft anastomoses at both ends to minimize flow impedance and turbulence as well as resecting the reinforcing ring from the distal end where necessary. In the setting of poor calibre vessels, bifurcated “Y” interposition configuration to the grafts is an option. The extra-anatomic bypass of the innominate to the RA, a continuous 4-0 prolene suture is used on the atrial side incorporating musculi pectinati into the distal end of the graft. Any areas of leak are reinforced with autologous pericardial pledgets in an interrupted manner. For the PTFE graft anastomosis, we generally begin with the distal anastomosis before proceeding centrally. Specifically, in cases involving the LIV reconstruction, we anastomose the LIV before moving to the SVC. Key roles for the first assistant during vascular reconstruction include paying attention to careful graft handling and the need for maintaining a haemostatic field, especially during vascular clamping.



This staged reconstruction off CPB also helps to reduce the cerebral pressure gradient when clamping the SVC prior to resection and reconstruction. Intravenous volume expanders and vasoconstrictors are used to offset the initial drop in cardiac output when clamping the SVC if not on CPB at this point. Cerebral pressure can be monitored by insertion of a catheter into the right brachiocephalic vein but is not routinely done. Mediastinal lymph node dissection is routinely performed in all cases. Pericardial defects are reconstructed with thin Gortex or PTFE membranes to prevent cardiac herniation especially if an extra-pleural pneumonectomy is performed. The patch is secured to the remaining pericardium using a locking suture, to reduce the risks of future pericardial constriction. A minimum of three thoracic drains (28 F) are placed in all patients.
Patients who undergo vascular reconstruction using a PTFE graft are commenced on warfarin, provided there are no contraindications, for a period of 6 months following surgery to reduce the risk of graft thrombosis.
Results
Between 2015 and 2023, 31 patients with advanced stage TET (stage III/IV) underwent surgery at our centre. All of these underwent open resection and major vessel reconstruction was required in 14 cases (45%). Extra-pleural pneumonectomy was required in 7 patients, pleurectomy-decortication in 14 patients, diaphragmatic resection in 10, and concomitant chest wall and lung resection in 4 patients. For the purposes of this manuscript, we will present our data pertaining only to those patients who required major vascular reconstruction.
Table 1 below summarises the key operative and disease-specific characteristics of the 14 patients who underwent resection with vascular reconstruction (non-salvage cases, all MDT recommendations). All patients (n=14) underwent image-led staging with CT-PET and vascular invasion of the SVC/innominate/SVC-RA junction was seen pre-operatively in all cases. In cases of known vascular invasion, all patients undergo induction chemotherapy alone with 3 cycles of neoadjuvant carboplatin/paclitaxel (CAP) with a “response to treatment” scan thereafter. If there is stable disease (n=2) or partial response (n=12), a further 3 cycles are given unless the patient does not tolerate. All patients in this subgroup underwent a pre-operative biopsy to determine the nature of the disease and hence guide neoadjuvant therapy. Eleven out of fourteen patients had the full six cycles of induction treatment; 2 patients completed 4 cycles of treatment, and 1 patient completed three cycles of treatment. None of these patients had concomitant autoimmune related disorders such as myasthenia gravis or otherwise. Patients requiring adjuvant treatment are managed in accordance with the ESMO guidelines (2) with a usual post-operative radiotherapy dose of 45–50 Gy and boost to areas of concern.
All the vascular reconstructions as described above, required full segmental resection with end-to-end anastomoses. In order to offset the risk of cerebral venous hypertension with prolonged SVC clamping, an end-to-side anastomosis of the innominate vein to right atrial appendage was carried out to allow unrestricted drainage from the head and neck. This also obviated the need for aggressive CVP monitoring. This was followed by SVC resection and reconstruction where clamp time was not so much of an issue. In 6/14 patients, only an SVC reconstruction was required, and SVC clamp time was kept to a maximum of 20 minutes, all of these cases only required partial resection with patch placement.
All the SVC reconstructions were carried out with 18 mm PTFE ringed grafts, and the innominate reconstructions were 16 mm (n=6) and 14 mm (n=2) PTFE ringed grafts. All cases which required redo sternotomy had their femoral vessels prepared in case of the need for peripheral arterial CPB. CPB was employed in 2/14 cases, one for right atrial involvement and one for bleeding encountered during redo sternotomy. The average duration of bypass in both these cases was 119 minutes. Phrenic nerve sacrifice was required in one case (patient 4), where EPP was performed with diaphragmatic reconstruction. Median operating time was 350 minutes (180–540 minutes) and median blood loss was 1,300 mL (400–5,000 mL).
The median duration of chest tube drainage was 6 days (4–17 days), with a median length of hospital stay of 15 days (11–68 days). The rate of R0 resection was 78.6% (11/14), and the recurrence rate was 14.3% (2/14) at a median follow-up of 57 months. There has been no evidence of conduit occlusion at median follow-up. Three patients had an R1 resection and received post-operative chemoradiation.
The overall complication rate in this subgroup was 42.9% (6/14) as detailed in Table 2. These are graded according to the Clavien-Dindo classification, with the majority of these scoring at grade III (21.4%). The in-hospital mortality rate was 7.1% (1/14) which was as a result of severe haemoptysis post-operatively (bronchial vascular invasion with breach into the airway) and concurrent chest sepsis.
Table 2
| Patient | Pathological resection | Post-operative complications (Clavien-Dindo grade) | In-hospital mortality | Recurrence | Disease-free survival (months) | Overall survival (months) |
|---|---|---|---|---|---|---|
| 1 | R0 | No | No | No | 103 | 103 |
| 2 | R1 | Yes, severe post-operative haemoptysis (V) | Yes | No | 1 | 1 |
| 3 | R1 | No | No | No | 96.8 | 96.8 |
| 4 | R0 | Yes, hospital acquired pneumonia requiring mechanical ventilation and tracheostomy for weaning (IV) | No | No | 19.3 | 19.3 |
| 5 | R0 | Yes, bleeding with cardiac tamponade requiring percutaneous drainage (III) | No | No | 18.2 | 18.2 |
| 6 | R0 | Yes, cardiac herniation requiring refashioning of Gortex membrane (III) | No | Yes | 3.6 | 12.6 |
| 7 | R0 | No | No | No | 51.5 | 51.5 |
| 8 | R0 | Yes, hospital acquired pneumonia requiring intravenous antibiotics (II) | No | No | 40.9 | 40.9 |
| 9 | R0 | No | No | No | 55.6 | 55.6 |
| 10 | R0 | No | No | No | 63 | 63 |
| 11 | R0 | No | No | Yes (T1 vertebral) | 7.2 | 19.2 |
| 12 | R0 | Yes, haemothorax requiring drainage (III) | No | No | 4.9 | 4.9 |
| 13 | R0 | No | No | No | 3.3 | 3.3 |
| 14 | R1 | No | No | No | 2.1 | 2.1 |
TET, thymic epithelial tumor; T1, thoracic vertebra 1; R0, microscopically clear resection; R1, microscopic involvement.
Discussion
SVC resection and reconstruction
SVC resection and reconstruction has been described using two main techniques: cross-clamping or SVC bypass. The former was demonstrated to be a safe and durable technique from a series of 27 patients who underwent radical resection for stage III/IV thymic malignancies (7). Here, authors described use of a bovine pericardial conduit (n=12) or PTFE (n=13) for venous reconstruction. The heterologous pericardial conduit offered the most robust long-term patency rates. Considerations with this technique is primarily the clamp time; In a study of 28 patients with non-small cell lung cancer, median clamp times were 40 minutes. Clamping the SVC can result in systemic hypotension and cerebral venous hypertension. The resultant post-operative mortality rate from this series was 14% (8).
The CPB technique is an option to avoid haemodynamic instability in patients. This technique can be employed to temporarily bypass the blood flow between the innominate vein and right atrium to allow for complete tumour resection (8-10). Alternatively, a permanent bypass can be performed which has also been described by Asadi et al. (11), grafting the LIV directly onto the right atrium can facilitate SVC resection without incurring gross perturbations in haemodynamics by allowing sufficient return from the head and neck. Patency of both draining veins is important in order for this technique to work properly.
Innominate vein resection and reconstruction
Deciding which vessels to resect and reconstruct depends upon the degree of tumour invasion. With LIV invasion, different approaches have been described for vessel reconstruction, grafting directly to the right atrial body, or to the appendage or as a side graft to the SVC. Right innominate vein (RIV) reconstruction has invariably been performed on to the SVC body (12). In the setting of bilateral innominate vein invasion, LIV resection and reconstruction is preferred.
Our experience has taught us that reconstructing both vessels using conduits that are separately implanted directed into the right atrium prevents any risk of cerebral hypertension and reduces the risk of graft occlusion. Other important technical considerations include early and full mobilisation and preparation of the right atrial appendage with dissection and division of the apical pectinate muscles to avoid turbulence within the conduits and this can be further reduced by using adequately sized grafts (16 mm internal diameter or greater) that are not unnecessarily long. Using this technique, we only used bypass in two cases: redo sternotomy after incomplete resection and radiotherapy (n=1) and disease involvement of right atrium which required clamping and reconstruction of the right atrium (Figure 6, Patient 1; Table 1).

CPB
Invasion of the myocardium or epi-aortic vessels and arch necessitates the use of CPB to achieve complete resection. CPB may also be required in cases of redo sternotomy, where there is potential risk of injury to the heart or great vessels. In cases where clamping of the SVC near the cavo-atrial junction is necessary, impingement of the sinoatrial node may result in bradycardia, making temporary bypass difficult. Temporary pacing or use of CPB may also be necessary to prepare for such a situation. Ried et al. (13) have described their series of 6 patients who required cardioplegic arrest or unloading with CPB to achieve tumour resection. Of these, 5 had an R0 resection. Deep hypothermic circulatory arrest has been described for aortic arch resection and reconstruction in a patient with invasive stage IV thymoma (14). The use of CPB must be carefully considered given the massive post-operative burden of morbidity, bleeding risk and dissemination of tumour cells which may compromise the integrity of the cancer resection.
Overall, we feel a key aspect to our vascular reconstruction strategy is to avoid the use of SVC clamping during reconstruction and to first create an innominate-RA extra-anatomic bypass to allow unrestricted drainage of the head and neck which allows more time to safely perform a competent and adequate SVC reconstruction. Zhang et al. (15) have demonstrated similar outcomes with comparable complication rates in 22 patients who underwent SVC reconstruction only. They adopted a pure cross-clamping technique which is a feasible approach. Data from another group highlighted the importance of induction treatment in advanced stage disease (16). The median overall survival and DFS in patients with Masaoka stage IVa disease was 67 and 21 months, respectively but undergoing only preoperative chemotherapy (P=0.007) or receiving no chemotherapy (P=0.009) had a DFS that was significantly higher than receiving both preoperative and postoperative chemotherapy. This may be due to the added physiological insult of increased chemotherapy, but nonetheless multi-modality treatment is the key management principle of these conditions. The European Society of Thoracic Surgeons database published their series on Masaoka-Koga Stage III TET resections with vascular involvement (17). They found that thymic carcinoma was a significant independent predictor for recurrence (hazard ratio 3.59; 95% confidence interval: 1.66–7.78; P=0.001). Resection status and histological subtype did not impact overall survival, however.
Surgical strategy in these cases requires careful assessment of the imaging and patient characteristics. An open approach post induction treatment is always required. If there is extension into one of the hemithoraces or presence of pleural disease, an initial thoracotomy is required to resect pleural deposits and start the dissection of the caval structures. When the chest is opened from the front, if there is dual involvement of the SVC and innominate vein, extra-anatomic bypass of innominate to RA is the preferred initial strategy to avoid SVC clamping. In cases of isolated SVC involvement, clamping is unavoidable and if this is likely to disrupt the sino-atrial node, use of CPB/pacing should be considered. CPB use should be instituted for RA involvement.
Conclusions
Advanced stage TET represents a complex and rare group of pathologies which require multi-disciplinary input, careful pre-operative planning, and systemic biological control with chemoradiation. Complex vascular resection and reconstruction is safe and allows for complete en bloc resection but should be performed at high volume centres by high volume surgeons.
Acknowledgments
None.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1395/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1395/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1395/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1395/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study did not involve direct patient contact, and therefore, ethical approval and informed consent were not required under UK regulations. The study was internally reviewed by the Guy’s and St. Thomas’ Trust Audit Department.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Comacchio GM, Dell'Amore A, Marino MC, et al. Vascular Involvement in Thymic Epithelial Tumors: Surgical and Oncological Outcomes. Cancers (Basel) 2021;13:3355. [Crossref] [PubMed]
- Kaba E, Ozkan B, Erus S, et al. Role of Surgery in the Treatment of Masaoka Stage IVa Thymoma. Ann Thorac Cardiovasc Surg 2018;24:6-12. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Smith A, Cavalli C, Harling L, et al. Impact of the TNM staging system for thymoma. Mediastinum 2021;5:32. [Crossref] [PubMed]
- Maurizi G, Poggi C, D'Andrilli A, et al. Superior Vena Cava Replacement for Thymic Malignancies. Ann Thorac Surg 2019;107:386-92. [Crossref] [PubMed]
- Spaggiari L, Thomas P, Magdeleinat P, et al. Superior vena cava resection with prosthetic replacement for non-small cell lung cancer: long-term results of a multicentric study. Eur J Cardiothorac Surg 2002;21:1080-6. [Crossref] [PubMed]
- Matsumoto K, Yamasaki N, Tsuchiya T, et al. Temporary bypass for superior vena cava reconstruction with Anthron bypass tube(TM). J Thorac Dis 2017;9:E614-8. [Crossref] [PubMed]
- Wright CD. Extended resections for thymic malignancies. J Thorac Oncol 2010;5:S344-7. [Crossref] [PubMed]
- Asadi N, Barr J, Perikleous P, et al. Complete superior vena cava resection for invasive thymoma. Curr Chall Thorac Surg 2019;1:2.
- Liu L, Zhang J, Wang G, et al. Extended thymectomy with blood vessel resection and reconstruction improves therapeutic outcome for clinical stage III thymic carcinoma patients: a real-world research. J Cardiothorac Surg 2020;15:267. [Crossref] [PubMed]
- Ried M, Neu R, Schalke B, et al. Radical surgical resection of advanced thymoma and thymic carcinoma infiltrating the heart or great vessels with cardiopulmonary bypass support. J Cardiothorac Surg 2015;10:137. [Crossref] [PubMed]
- Marcano JE, Salas de Armas IA, Gregoric ID, et al. En-bloc thymectomy with aortic arch reconstruction under circulatory arrest for invasive malignant thymoma. Interact Cardiovasc Thorac Surg 2020;31:282. [Crossref] [PubMed]
- Zhang Z, Huang M, Pan X. Prosthetic Reconstruction of Superior Vena Cava System for Thymic Tumor: A Retrospective Analysis of 22 Cases. Thorac Cardiovasc Surg 2021;69:165-72. [Crossref] [PubMed]
- Toker A, Hayanga JWA, Dhamija A, et al. Superior Vena Cava Reconstruction in Masaoka Stage III and IVa Thymic Epithelial Tumors. Ann Thorac Surg 2022;113:1882-90. [Crossref] [PubMed]
- Mendogni P, Toker A, Moser B, et al. Surgical resection of Masaoka stage III thymic epithelial tumours with great vessels involvement: a retrospective multicentric analysis from the European Society of Thoracic Surgeons thymic database. Eur J Cardiothorac Surg 2022;62:ezac021. [Crossref] [PubMed]


