
Lobectomy vs. bisegmentectomy for lung cancer in the left upper lobe: a retrospective comparative cohort study
Highlight box
Key findings
• Left upper bisegmentectomy demonstrated comparable oncological outcomes to left upper lobectomy, even in the case of a larger tumor size (≥2 cm). However, the lingulectomy group exhibited a concerning inferiority in overall survival (OS), necessitating further evaluation.
What is known and what is new?
• Lobectomy has been established as the standard treatment for resectable non-small cell lung cancer (NSCLC) since the publication of a randomized prospective clinical trial conducted by the Lung Cancer Study Group in the 1990s.
• Our subgroup analysis examined the separate outcomes of lingulectomy and trisegmentectomy. Although no significant differences in disease-free survival were identified between different surgical procedures, the lingulectomy group appeared to have lower OS compared with the other two groups. To our knowledge, the findings of Japan Clinical Oncology Group 0802/West Japan Oncology Group 4607L indicate that segmentectomy should be the standard surgical procedure instead of lobectomy for patients with clinical stage IA, small-sized (≤2 cm, consolidation-to-tumor ratio >0.5) peripheral NSCLC.
What is the implication, and what should change now?
• Our study suggests that bisegmentectomy could be contemplated as an alternative to lobectomy in the left upper lobe, yielding similar oncological outcomes with the added advantage of parenchyma sparing. However, the potential lower OS in the lingulectomy group in our study raises considerations for decision-making and necessitates a larger patient cohort and further validation.
Introduction
Lobectomy has been established as the standard treatment for resectable non-small cell lung cancer (NSCLC) since the publication of a randomized prospective clinical trial conducted by the Lung Cancer Study Group (LCSG) in the 1990s (1). However, this study possesses certain drawbacks from a contemporary standpoint. The diagnostic tool used was chest radiography and not computed tomography (CT). Moreover, the tumor size was notably large, and no subgroup analysis was conducted for early-stage tumors. Finally, wedge resection, rather than segmentectomy, predominated in the limited resection group. Over the past decades, further studies have been conducted to comprehensively outline appropriate surgical procedures for NSCLC. Segmentectomy is considered a potential alternative for predominant ground-glass opacities (GGOs) or tumors smaller than 2 cm (2-5). And the improvement of diagnostic techniques that allow an earlier diagnosis and; the increasing interest for postoperative lung function and quality of life. Segmentectomy is the standard treatment in case of lesions smaller than 2 cm in patients with an impaired cardiopulmonary reserve who cannot be candidate for lobectomy. Yet, controversies persist regarding its local recurrence rate and associated long-term survival (6,7). Ongoing clinical trials, including Japan Clinical Oncology Group (JCOG) 0802 and Cancer and Leukemia Group B (CALGB) 1405038, hold promise in addressing these uncertainties in the near future.
Bisegmentectomy, which is more technically practicable and involves a larger parenchyma resection, may yield comparable or superior outcomes to those of segmentectomy, representing another viable option for treating resectable NSCLC. However, the relative efficacies of these approaches have not been extensively investigated.
The left upper lobe, being one of the largest lobes, shares anatomical similarities with the combination of the right upper lobe and right middle lobe (8). It can be divided into two separate units, namely the trisegment and lingular segment, both of which are composed of two segments (S1+2 + S3 and S4 + S5). Given the generally accepted sufficiency of right upper or middle lobectomy for NSCLC, it is conceivable that bisegmentectomy (trisegmentectomy or lingulectomy) is suitable for resectable NSCLC of the left upper lobe. However, several studies with very limited sample sizes, have been performed to confirm this (8-11).
Our retrospective study was designed to compare the overall survival (OS) and disease-free survival (DFS) between lobectomy and bisegmentectomy in patients with resectable left upper lobe NSCLC. Subgroup analyses were conducted for each type of resection (lobectomy vs. trisegmentectomy vs. lingulectomy) and tumor size (≥2 vs. <2 cm). We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2199/rc).
Methods
Study design
The study was conducted in accordance with the ethical standards of the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of the main study center at Zhongshan Hospital, Fudan University (Shanghai, China) (ethical approval No. B2023-017) and informed consent was obtained from all individual participants. All patients provided informed consent regarding the potential risks and benefits associated with different surgical procedures.
Patient selection and surgical procedure
From January 2009 to December 2016, patients undergoing lobectomy or bisegmentectomy for stage I–IIIA NSCLC in the left upper lobe were screened. Subgroup analyses were performed for each type of bisegmentectomy and different tumor sizes. The exclusion criteria were as follows: (I) simultaneous or metachronous (within the past 5 years) double cancers; (II) interstitial pneumonitis, pulmonary fibrosis, or severe pulmonary emphysema; (III) patients with missing follow-up data; (IV) neoadjuvant therapy; (V) incomplete resection, or lacking systematic mediastinal lymphadenectomy.
After screening, 997 patients were enrolled, comprising 888 lobectomy cases, 33 lingulectomy cases, and 76 trisegmentectomy cases. All patients underwent routine preoperative examinations and staging. Qualified surgeons from the Department of Thoracic Surgery, Zhongshan Hospital of Fudan University performed the surgeries, and the choice between lobectomy or bisegmentectomy was determined by the surgeon based on patient’s condition, tumor location, and other factors.
Follow-up and data collection
Comprehensive details of patients, from preoperative preparation to postoperative evaluation, were documented in our internal database for thoracic surgery. Patients were assessed by physical examinations, hematologic, and biochemical analysis including tumor markers and contrast-enhanced chest CT and superior abdomen ultrasonography every 3–4 months for the first 2 years after surgery and then every 6 months. Follow-up methods included clinic visit, telephone, video conference, and mail or email. The endpoint in our study was the date of patient death or August 30, 2018.
Statistical analysis
Data analyses were performed with SPSS 20.0 software (IBM Corporation, Armonk, NY, USA) and R software version 3.0.0 (The R Foundation for Statistical Computing, Vienna, Austria). Propensity score matching (PSM) was carried out at a 1:1 ratio for the following parameters: age, gender, video-assisted thoracic surgery (VATS), smoking status, tumor size, histology, T stage, N stage, and days of hospital stay.
Continuous variables are presented as the mean with standard deviation (SD), while categorical variables are presented as case number. Group comparisons were conducted via the two-tailed Pearson’ Chi-squared test for categorical variables and the Student t-test for continuous variables. Survival curves for OS and DFS were depicted using the Kaplan-Meier method and analyzed via log-rank analysis. A Cox proportional hazards regression model was adopted for univariate and multivariate analyses, and only variables with P<0.10 in the univariate analyses were further assessed in the multivariate analyses, with a significance level set at P<0.05.
Results
Between January 2009 and December 2016, a total of 997 patients were enrolled in the study, comprising 888 lobectomy patients and 109 bisegmentectomy patients (Figure 1). Baseline clinical characteristics were initially not comparable between the two groups, with significant differences in gender, smoking status, tumor size, surgical procedure, histology, stage, and posthospital stay before matching. PSM generated 109 pairs, resulting in no significant differences in the baseline characteristics between the two groups (Table 1). In the lobectomy group and bisegmentectomy group, 99 and 103 patients underwent VATS, the mean tumor size was 1.638 and 1.545 cm, and there were six N1 and two N2 patients, respectively.
Table 1
| Variables | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| Lobectomy (n=888) | Bisegmentectomy (n=109) | P | Lobectomy (n=109) | Bisegmentectomy (n=109) | P | ||
| Age (years) | 60.64±9.144 | 59.05±12.825 | 0.21 | 58.88±9.007 | 59.05±12.825 | 0.91 | |
| Gender | <0.001 | 0.67 | |||||
| Male | 512 | 40 | 37 | 40 | |||
| Female | 376 | 69 | 72 | 69 | |||
| Smoking status | <0.001 | >0.99 | |||||
| Never | 635 | 95 | 94 | 95 | |||
| Ever | 253 | 14 | 15 | 14 | |||
| Tumor size (cm) | 2.857±1.592 | 1.545±0.999 | <0.001 | 1.638±0.826 | 1.545±0.999 | 0.46 | |
| Surgical procedure | <0.001 | 0.30 | |||||
| VATS | 612 | 103 | 99 | 103 | |||
| Open | 276 | 6 | 10 | 6 | |||
| Histology | 0.002 | >0.99 | |||||
| SCC | 149 | 7 | 7 | 7 | |||
| AD | 683 | 100 | 99 | 100 | |||
| Others | 56 | 2 | 3 | 2 | |||
| T stage | <0.001 | 0.28 | |||||
| Tis | 5 | 8 | 2 | 8 | |||
| T1a | 52 | 36 | 26 | 36 | |||
| T1b | 184 | 32 | 38 | 32 | |||
| T1c | 137 | 8 | 9 | 8 | |||
| T2a | 365 | 21 | 29 | 21 | |||
| T2b | 55 | 1 | 1 | 1 | |||
| T3 | 67 | 3 | 4 | 3 | |||
| T4 | 23 | 0 | 0 | 0 | |||
| N stage | <0.001 | >0.99 | |||||
| N0 | 668 | 101 | 101 | 101 | |||
| N1 | 104 | 6 | 6 | 6 | |||
| N2 | 116 | 2 | 2 | 2 | |||
| Hospital stay (days) | 7.37±4.516 | 5.47±2.332 | <0.001 | 5.46±1.793 | 5.47±2.332 | 0.97 | |
| Lung function (L) | |||||||
| FVC | 3.138±0.734 | 3.009±0.773 | 0.12 | 3.046±0.711 | 3.009±0.773 | 0.74 | |
| FEV1 | 2.433±0.609 | 2.365±0.810 | 0.35 | 2.423±0.560 | 2.365±0.810 | 0.58 | |
| DLCO | 18.265±6.249 | 17.768±8.423 | 0.50 | 19.011±6.967 | 17.768±8.423 | 0.28 | |
Data are presented as mean ± SD or number. PSM, propensity score matching; VATS, video-assisted thoracoscopic surgery; SCC, squamous cell carcinoma; AD, adenocarcinoma; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; DLCO, diffusion capacity for carbon monoxide; SD, standard deviation.
The OS and DFS curves are depicted in Figure 2. There was no significant difference (P=0.49) in OS between the lobectomy group [101.481±2.646 months; 95% confidence interval (CI): 96.295–106.668] and the bisegmentectomy group (101.756±4.799 months; 95% CI: 92.350–111.162). Similarly, the DFS between the lobectomy group (95.941±3.186 months; 95% CI: 89.697–102.185) and bisegmentectomy group (96.460±4.358 months; 95% CI: 87.920–105.001) was not significantly different (P=0.62).
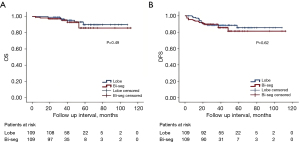
Univariate analysis indicated that age, tumor size, histology, N stage, and T stage may be associated with OS. Subsequent multivariate analysis incorporating these parameters revealed that age (P=0.01), histology (squamous cell carcinoma: P=0.007; others: P=0.03), N stage (N1: P=0.004; N2: P=0.01), and T stage (T2: P=0.11; T3: P<0.001) were associated with OS in these patients (Table 2). In terms of DFS, both univariate and multivariate analyses indicated that only smoking status (P=0.056), N stage (N1: P=0.03; N2: P<0.001), and T stage (T2, P=0.047; T3, P=0.003) as the potential indicators (Table 3).
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (years) | |||||||
| ≥60 | Reference | ||||||
| <60 | 0.088 | 0.011–0.684 | 0.02 | 0.056 | 0.006–0.533 | 0.01 | |
| Gender | |||||||
| Male | Reference | ||||||
| Female | 0.430 | 0.136–1.358 | 0.15 | ||||
| Smoking status | |||||||
| Never | Reference | ||||||
| Ever | 0.502 | 0.136–1.858 | 0.30 | ||||
| Surgical type | |||||||
| Open | Reference | ||||||
| VATS | 0.675 | 0.144–3.165 | 0.62 | ||||
| Tumor size (cm) | |||||||
| ≥2 | Reference | ||||||
| <2 | 0.196 | 0.053–0.729 | 0.02 | 1.882 | 0.334–10.595 | 0.47 | |
| Histology | |||||||
| AD | Reference | ||||||
| SCC | 5.749 | 1.652–20.010 | 0.006 | 9.570 | 1.830–50.057 | 0.007 | |
| Others | 4.171 | 0.509–34.183 | 0.18 | 15.075 | 1.233–184.364 | 0.03 | |
| Surgical procedure | |||||||
| Lobectomy | Reference | ||||||
| Bisegmentectomy | 1.488 | 0.473–4.685 | 0.50 | ||||
| N stage | |||||||
| N0 | Reference | ||||||
| N1 | 11.452 | 3.348–39.179 | <0.001 | 10.060 | 2.135–47.389 | 0.004 | |
| N2 | 14.983 | 1.755–127.940 | 0.01 | 24.962 | 2.137–291.653 | 0.01 | |
| T stage | |||||||
| T1 | Reference | ||||||
| T2 | 3.857 | 1.087–13.687 | 0.04 | 3.816 | 0.746–19.512 | 0.11 | |
| T3 | 11.652 | 2.123–63.948 | 0.005 | 76.827 | 7.911–746.130 | <0.001 | |
OS, overall survival; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; VATS, video-assisted thoracoscopic surgery; AD, adenocarcinoma; SCC, squamous cell carcinoma.
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (years) | |||||||
| ≥60 | Reference | ||||||
| <60 | 0.472 | 0.212–1.050 | 0.07 | 0.651 | 0.283–1.498 | 0.31 | |
| Gender | |||||||
| Male | Reference | ||||||
| Female | 0.422 | 0.197–0.903 | 0.03 | 1.476 | 0.481–4.530 | 0.50 | |
| Smoking status | |||||||
| Never | Reference | ||||||
| Ever | 2.858 | 1.249–6.539 | 0.01 | 3.018 | 0.971–9.379 | 0.056 | |
| Surgical type | |||||||
| Open | Reference | ||||||
| VATS | 0.737 | 0.220–2.473 | 0.62 | ||||
| Tumor size (cm) | |||||||
| ≥2 | Reference | ||||||
| <2 | 0.201 | 0.088–0.460 | <0.001 | 0.615 | 0.212–1.783 | 0.38 | |
| Histology | |||||||
| AD | Reference | ||||||
| SCC | 2.429 | 0.832–7.089 | 0.10 | 1.300 | 0.367–4.604 | 0.69 | |
| Others | 1.569 | 0.211–11.682 | 0.66 | 1.850 | 0.225–15.242 | 0.57 | |
| Surgical procedure | |||||||
| Lobectomy | Reference | ||||||
| Bisegmentectomy | 1.207 | 0.564–2.583 | 0.63 | ||||
| N stage | |||||||
| N0 | Reference | ||||||
| N1 | 5.300 | 1.975–14.223 | 0.001 | 3.492 | 1.119–10.895 | 0.03 | |
| N2 | 12.882 | 3.724–44.559 | <0.001 | 12.978 | 3.178–53.002 | <0.001 | |
| T stage | |||||||
| T1 | Reference | ||||||
| T2 | 4.130 | 1.837–9.301 | 0.001 | 2.713 | 1.015–7.255 | 0.047 | |
| T3 | 7.755 | 2.131–28.216 | 0.002 | 8.370 | 2.020–34.676 | 0.003 | |
DFS, disease-free survival; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; VATS, video-assisted thoracoscopic surgery; AD, adenocarcinoma; SCC, squamous cell carcinoma.
Subgroup analysis was conducted according to the type of bisegmentectomy (Figure 3A,3B). In the comparison of DFS, no significant difference (P=0.40) was found between the lobectomy group, lingulectomy group, and trisegmentectomy group. However, the lingulectomy group appeared to have a lower OS (57.204±3.848 months; 95% CI: 49.661–64.747; P=0.049) as compared to the other two groups.
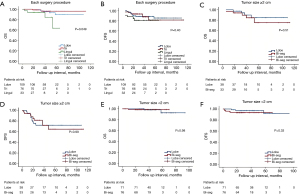
Another subgroup analysis was performed between the lobectomy group and bisegmentectomy group for tumor size ≥2 cm (Figure 3C,3D) or <2 cm (Figure 3E,3F). For tumor sizes <2 cm, no significant difference was found between the two groups in terms of OS (P=0.98) or DFS (P=0.33). When the tumor size was equal or greater than 2 cm, the two groups still exhibited a comparable OS (P=0.51) and DFS (P=0.99), with a similar distribution of patient characteristics (Table 4).
Table 4
| Variables | Tumor size ≥2 cm | Tumor size <2 cm | |||||
|---|---|---|---|---|---|---|---|
| Lobectomy (n=38) | Bisegmentectomy (n=33) | P | Lobectomy (n=71) | Bisegmentectomy (n=76) | P | ||
| Age (years) | 0.03 | 0.21 | |||||
| Mean | 61.26 | 67.21 | 57.61 | 55.50 | |||
| SD | 8.006 | 13.610 | 9.304 | 10.757 | |||
| Gender | 0.98 | 0.36 | |||||
| Male | 22 | 19 | 15 | 21 | |||
| Female | 16 | 14 | 56 | 55 | |||
| Smoking status | 0.62 | 0.63 | |||||
| Never | 28 | 26 | 5 | 69 | |||
| Ever | 10 | 7 | 66 | 7 | |||
| Tumor size (cm) | 0.31 | 0.02 | |||||
| Mean | 2.571 | 2.767 | 1.138 | 1.015 | |||
| SD | 0.684 | 0.930 | 0.279 | 0.354 | |||
| Surgical type | 0.37 | 0.96 | |||||
| VATS | 29 | 28 | 70 | 75 | |||
| Open | 9 | 5 | 1 | 1 | |||
| Histology | 0.77 | 0.42 | |||||
| SCC | 31 | 26 | 1 | 2 | |||
| AD | 6 | 5 | 68 | 74 | |||
| Others | 1 | 2 | 2 | 0 | |||
| T stage | >0.99 | 0.12 | |||||
| Tis | 0 | 0 | 2 | 8 | |||
| T1a | 0 | 0 | 26 | 36 | |||
| T1b | 6 | 6 | 32 | 26 | |||
| T1c | 9 | 8 | 0 | 0 | |||
| T2a | 20 | 16 | 9 | 5 | |||
| T2b | 1 | 1 | 0 | 0 | |||
| T3 | 2 | 2 | 2 | 1 | |||
| N stage | 0.88 | >0.99 | |||||
| N0 | 31 | 27 | 70 | 74 | |||
| N1 | 5 | 5 | 1 | 1 | |||
| N2 | 2 | 1 | 0 | 1 | |||
| Hospital stay (days) | 0.83 | 0.92 | |||||
| Mean | 6.13 | 6.24 | 5.10 | 5.13 | |||
| SD | 2.095 | 2.236 | 1.504 | 2.306 | |||
Data are presented as number, unless otherwise stated. PSM, propensity score matching; SD, standard deviation; VATS, video-assisted thoracoscopic surgery; SCC, squamous cell carcinoma; AD, adenocarcinoma.
Discussion
Since the 1990s, lobectomy has the prevailing treatment for resectable NSCLC, as supported by compelling evidence from Ginsberg and Rubinstein’s randomized prospective study (1). Their research revealed a notable disparity in survival outcomes between lobectomy and limited lung resection. However, it is worth noting that segmentectomy was not a primary procedure in the limited lung resection group, and bisegmentectomy was not considered at that time.
Advancements in technology have transformed the landscape of cancer treatment, as NSCLC is now typically discovered in patients at an early-stage of disease, characterized by significantly smaller tumor sizes compared to those in Ginsberg and Rubinstein’s study. The application of high-resolution CT enables the early detection of lung nodules (12), while positron emission tomography-CT aids in excluding patients with unresectable or late-stage NSCLC before surgery (13,14). These methods allow for limited lung resection to once again be a potentially curative strategy. Recent research into limited lung resection has primarily focused on pure GGOs, for which both wedge resection and segmentectomy demonstrate comparable oncological outcomes to those of lobectomy (3,15). Controversy persists regarding the applicability of limited lung resection to solid or part-solid early-stage NSCLC as a curative surgery option (6,7,16). Prospective studies on the indication for segmentectomy have been carried out for several years, with the results being imminent. The field of thoracic surgery has witnessed significant improvement in surgical techniques in recent decades. VATS and robot-assisted thoracic surgical (RATS) procedures have rapidly evolved (10), gradually emerging as the preferable choice due to smaller incisions and reduced pain compared to thoracotomy (17-21). Study has confirmed that segmentectomy could be successfully performed via VATS without compromising perioperative outcomes (22). Data from prominent centers also suggest that RATS is a safe and valuable approach for lung surgery, including wedge resection, segmentectomy, and lobectomy (23,24).
Bisegmentectomy, while entailing a larger resection than segmentectomy, still preserves more of the lung parenchyma than does lobectomy. However, limited evidence exists regarding bi- and multisegmentectomy as a curative strategy for resectable NSCLC. The prevailing belief remains that lobectomy is necessary for patients with NSCLC to achieve radical resection. Nonetheless, lobectomy involves the resection of substantial lung parenchyma, potentially worsening patients’ quality of life and reducing their likelihood of future lung surgeries. The crucial question at hand is whether bisegmentectomy can also achieve radical resection while offering the advantage of parenchymal sparing.
Several studies have addressed trisegmentectomy and lingulectomy in the left upper lobe (8-11). Houck et al. introduced a stable VATS procedure for trisegmentectomy in 2004 (25). Subsequently, in 2012, RATS was proposed as a feasible alternative by Pardolesi et al. (26). The initial comparison of oncological outcomes between trisegmentectomy and left upper lobectomy was conducted in 2007 by Iwasaki et al. (10). Their findings indicated no significant difference in oncological outcome for patients with stage I NSCLC. However, it is crucial to note that this study exclusively focused on patients with small tumors, with a limited case number, with 55 cases in the lobectomy group and 31 cases in the trisegmentectomy group; meanwhile, the postoperative morbidity rates were notably high, at 19.3% and 10.9%, respectively, in the two groups, potentially attributable to the still-evolving surgical techniques.
In 2012, Soukiasian et al. suggested that trisegmentectomy and left upper lobectomy might exhibit equivalent survival rates for stage IA and IB NSCLC (9). Their study enrolled 73 patients in trisegmentectomy group, comprising 62 stage IA and IB patients. However, the omission of baseline patient characteristics in their study introduced a potential bias in the comparison. In 2014, Witte et al. published a study involving 22 cases of split-lobe resection (8). Their preliminary results aligned with the findings of the previous two studies (8,10). In 2018, Aprile et al. compared the OS and DFS of 54 patients undergoing trisegmentectomy/lingulectomy with those of 105 patients undergoing left upper lobectomy, concluding that the two groups exhibited similar oncological outcomes (11). It is worth noting the study’s limitations, including the enrollment of only patients with T1N0 and T2N0 NSCLC and the significant differences in patient characteristics between the two groups, such as tumor size, lymph node dissection, and days of hospital stay.
Our study enrolled 109 patients who underwent bisegmentectomy in our hospital from January 2009 to December 2016, representing potentially the largest case number of its kind examined to date. PSM was employed to ensure similar baseline patient characteristics, and 109 patients with lobectomy were also enrolled for further comparison, and the cases other than early-stage cancer were included. Among them, 71 patients had tumors ≥2 cm (38 and 33, respectively), and 16 patients exhibited lymph node metastasis. Preliminary comparisons of OS and DFS between the two groups suggested that bisegmentectomy yielded similar oncological outcomes to those of lobectomy.
Further subgroup analyses were conducted based on tumor size. When the tumor size was smaller than 2 cm, both groups demonstrated comparable OS and DFS. In cases of tumors ≥2 cm, no significant difference in OS or DFS was observed between the two groups. Hence, bisegmentectomy emerged as a viable option for large-tumor NSCLC and was not limited to tumors <2 cm.
Another subgroup analysis examined the outcomes of lingulectomy and trisegmentectomy separately. Although no significant differences in DFS were identified between different surgical procedures, the lingulectomy group appeared to have a lower OS than as compared to the other two groups (57.204±3.848 months; 95% CI: 49.661–64.747; P=0.049). This observation contrasts with other similar studies (8,9,11) but aligns with Ginsberg and Rubinstein’s conclusions (1). The tumor sizes of the deceased patients in the lingulectomy group were 3.0, 3.0, 2.5, and 0.6 cm, with N stages of N2, N1, N0, and N0. However, this finding necessitates further confirmation, as an increased sample size number and extended follow-up may yield different results.
Several limitations to this study should be acknowledged. Its retrospective nature diminishes its evidential strength compared to a randomized controlled trial. Moreover, the number of patients with stage II–IIIA was relatively small, and postoperative pulmonary function test results were lacking, precluding the assessment of parenchyma-sparing benefit. The follow-up time, particularly in the lingulectomy group, was not sufficiently long. Furthermore, there is a possibility that the tumor stage of the bisegmentectomy group was underestimated. Incorrect staging during sublobar resection has been raised as a concern in the literature (27-30). Nevertheless, if our data included more late-stage patients than anticipated, the oncological outcomes of the bisegmentectomy group would only appear improved and the current conclusions unchanged. Additionally, decisions regarding bisegmentectomy were at the discretion of the performing surgeons rather than a multidisciplinary team, introducing a potential bias.
Conclusions
Our study suggests that bisegmentectomy could be considered as an alternative to lobectomy in the left upper lobe, yielding similar oncological outcomes, but with the added advantage of parenchymal sparing. However, the potential lower OS in the lingulectomy group raises important considerations in decision-making, necessitating larger-sample and randomized controlled trial.
Acknowledgments
The article was presented at the 60th Annual Meeting of Japan Lung Cancer Society held at the Osaka International Convention Center on December 7, 2019.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2199/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2199/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2199/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2199/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the ethical standards of the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of the main study center at Zhongshan Hospital, Fudan University (Shanghai, China) (ethical approval No. B2023-017) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Zhong C, Sakurai H, Wei S, et al. Sublobar resections for small-sized stage Ia lung adenocarcinoma: a Sino-Japanese multicenter study. J Thorac Dis 2018;10:991-8. [Crossref] [PubMed]
- Sagawa M, Oizumi H, Suzuki H, et al. A prospective 5-year follow-up study after limited resection for lung cancer with ground-glass opacity. Eur J Cardiothorac Surg 2018;53:849-56. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66-71. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [Crossref] [PubMed]
- Potter AL, Kim J, McCarthy ML, et al. Segmentectomy versus lobectomy in the United States: Outcomes after resection for first primary lung cancer and treatment patterns for second primary lung cancers. J Thorac Cardiovasc Surg 2024;167:350-364.e17. [Crossref] [PubMed]
- Subramanian M, McMurry T, Meyers BF, et al. Long-Term Results for Clinical Stage IA Lung Cancer: Comparing Lobectomy and Sublobar Resection. Ann Thorac Surg 2018;106:375-81. [Crossref] [PubMed]
- Witte B, Wolf M, Hillebrand H, et al. Split-lobe resections versus lobectomy for lung carcinoma of the left upper lobe: a pair-matched case-control study of clinical and oncological outcomes. Eur J Cardiothorac Surg 2014;45:1034-9. [Crossref] [PubMed]
- Soukiasian HJ, Hong E, McKenna RJ Jr. Video-assisted thoracoscopic trisegmentectomy and left upper lobectomy provide equivalent survivals for stage IA and IB lung cancer. J Thorac Cardiovasc Surg 2012;144:S23-6. [Crossref] [PubMed]
- Iwasaki A, Hamanaka W, Hamada T, et al. Comparison between a case-matched analysis of left upper lobe trisegmentectomy and left upper lobectomy for small size lung cancer located in the upper division. Thorac Cardiovasc Surg 2007;55:454-7. [Crossref] [PubMed]
- Aprile V, Bertoglio P, Dini P, et al. Is left upper lobectomy always worthwhile for early stage lung cancer? A comparison between left upper lobectomy, trisegmentectomy, and lingulectomy. J Surg Oncol 2018;117:618-24. [Crossref] [PubMed]
- Rasmussen K, Madsen HH, Rasmussen F, et al. The value of HRCT and Tc-depreotide in the evaluation of pulmonary lesions. J Thorac Oncol 2006;1:296-301.
- Cerfolio RJ, Ojha B, Bryant AS, et al. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg 2004;78:1017-23; discussion 1017-23. [Crossref] [PubMed]
- Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med 2009;361:32-9. [Crossref] [PubMed]
- Cho JH, Choi YS, Kim J, et al. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg 2015;99:218-22. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:504-11. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc 2008;22:298-310. [Crossref] [PubMed]
- Swanson SJ. Video-assisted thoracic surgery segmentectomy: the future of surgery for lung cancer? Ann Thorac Surg 2010;89:S2096-7. [Crossref] [PubMed]
- Li C, Han Y, Han D, et al. Robotic Approach to Combined Anatomic Pulmonary Subsegmentectomy: Technical Aspects and Early Results. Ann Thorac Surg 2019;107:1480-6. [Crossref] [PubMed]
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 1604-5. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Mogi A, et al. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg 2018;66:81-90. [Crossref] [PubMed]
- Veronesi G, Novellis P, Voulaz E, et al. Robot-assisted surgery for lung cancer: State of the art and perspectives. Lung Cancer 2016;101:28-34. [Crossref] [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2018;268:254-9. [Crossref] [PubMed]
- Houck WV, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery upper lobe trisegmentectomy for early-stage left apical lung cancer. Ann Thorac Surg 2004;78:1858-60. [Crossref] [PubMed]
- Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012;94:929-34. [Crossref] [PubMed]
- Walts AE, Marchevsky AM. Potential errors in staging primary pulmonary adenocarcinomas by sublobar resection. Ann Diagn Pathol 2013;17:471-5. [Crossref] [PubMed]
- Argento G, Maurizi G. Anatomical partial lobectomy for lung cancer: less or more? J Thorac Dis 2024;16:4069-71. [Crossref] [PubMed]
- Frederiksen JG, Christensen TD, Petersen RH. Lung cancer surgery in Denmark. J Thorac Dis 2022;14:3638-47. [Crossref] [PubMed]
- Cao M, Zhao X, Fu Y. A uniportal video-assisted thoracoscopic segmentectomy(S3) for left upper lobe. Curr Chall Thorac Surg 2022;4:10.
(English Language Editor: J. Gray)



