
Metastatic sites, prognosis, and a new nomogram for predicting overall survival among small cell lung cancer patients with liver metastasis: a retrospective study based on SEER
Highlight box
Key findings
• This study constructed a nomogram to predict the survival of small cell lung cancer (SCLC) patients with liver metastasis (LM) and validated its predictive accuracy.
What is known and what is new?
• LM is common in SCLC patients and has a poor prognosis.
• We constructed a nomogram and risk scoring system capable of accurately predicting the survival rate of SCLC patients with LM. This system effectively differentiates between high-risk and low-risk groups based on the risk score, thereby identifying patients with a high-risk prognosis. Additionally, we explored the prognosis and mortality risk of SCLC patients with LM according to different extrahepatic metastatic sites.
What is the implication, and what should change now?
• This study can provide accurate overall survival (OS) prediction for SCLC patients with LM and identify high-risk patients. Using it in clinical practice will help guide treatment decisions and optimize treatment strategies.
Introduction
Lung cancer is one of the most common cancers and a leading cause of cancer-related deaths worldwide. It is estimated that there are 2 million new cases and 1.76 million deaths from lung cancer each year (1). Small cell lung cancer (SCLC) is the most aggressive histological type, accounting for 15% of all lung cancer cases (2). It is characterized by its high malignancy, short doubling time, rapid growth, easy metastasis, and poor prognosis. There are 60% to 75% of SCLC patients already with metastasis at the time of diagnosis (3). Common sites of metastasis include the contralateral lungs, liver, adrenal glands, brain, bone, and/or bone marrow (4). In CASPIAN study, the subgroup analysis results of distant metastases in SCLC were released, with liver metastasis (LM) being the highest proportion of 52.8% (5). Furthermore, it has been found in other studies that whether it is solitary organ metastasis or multi-organ metastasis, the liver is the most common site of metastasis (4,6,7). SCLC patients with isolated LM or combined metastasis with other organs have the poorest prognosis (6,7). Compared to other metastatic sites, the liver is the organ where metastasis occurs earliest (8).
Immunotherapy combined with chemotherapy has improved the median overall survival (OS) of SCLC patients to 12–15 months (9), and the 5-year OS rate in advanced-stage patients increased to 12% (10,11). For SCLC patients with LM, the drugs available for second-line and subsequent therapies are limited (12). As the most prevalent and earliest site of SCLC metastasis, LM in SCLC patients is an important consideration for various treatment modalities including chemotherapy and immunotherapy and effective control of LM can help achieve overall benefits. Therefore, accurate evaluation of the prognosis of SCLC patients with LM can better identify high-risk groups, help to choose the best treatment methods and improve the survival rate of patients.
In recent years, several studies have identified factors such as age, gender, race, Tumor Node Metastasis (TNM) stage, treatment modalities, and metastatic sites that are associated with the prognosis of SCLC patients (13,14). To the best of our knowledge, there is currently no available nomogram to predict the survival probability of SCLC patients with LM. In this study, we utilized a large SCLC cohort from the Surveillance, Epidemiology, and End Results (SEER) database to investigate prognostic factors in SCLC patients with LM and developed a novel risk stratification system to predict their OS. Furthermore, we validated the nomogram in an independent cohort and conducted a series of tests to evaluate its performance and clinical utility. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1423/rc).
Methods
Selection of study population
In this retrospective study, SEER program was the basis of our analysis. The SEER*stat 8.3.9.2 software was used to extract data from the SEER Research Plus Data 18 Registries (2000–2018) dataset. We selected patient records diagnosed with SCLC between 2010 and 2018. The International Classification of Diseases for Oncology, third edition (ICD-O-3) codes (8002, 8041, 8042, 8043, 8044, 8045) were used to identify SCLC cases (15). The inclusion criteria for this study were as follows: (I) patients with a pathological diagnosis of SCLC as the sole primary tumor; (II) patients with LM; (III) age ≥18 years; (IV) availability of complete clinical and pathological information. The study population was randomly divided into a training cohort and a validation cohort in a 7:3 ratio. The classification process was performed using R version 3.6.3 software. The external validation cohort data was obtained from patients treated at the Jilin Cancer Hospital between 2013 and 2023, with patient screening criteria consistent with those used in the training cohort. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Jilin Province Cancer Hospital (Ethics No. 202406-008-01, date of approval: 24 June 2024). The external validation data for this study were derived from the electronic medical record system of Jilin Cancer Hospital. Only the clinical information of the patients was collected retrospectively, and any characters with the subjects’ identities were deleted from the research results to ensure that personal privacy was not disclosed. Consequently, the Ethics Committee granted a waiver for the requirement of written informed consent. The flowchart of the whole procedure is shown in Figure 1.
Statistical analysis
The Chi-squared test was used for analyzing categorical variables. To facilitate analysis, age was categorized into three groups: <65, 65–75, and >75 years. Univariate and multivariate Cox proportional hazards regression analyses were performed on the training cohort to identify independent prognostic factors for constructing the nomogram. The receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to evaluate the discriminatory performance of the nomogram. Calibration curves were used to assess the consistency between predicted probabilities and actual survival outcomes. Decision curve analysis (DCA) was employed to further evaluate the clinical utility of the predictive model. The bootstrap values were calculated with 1,000 resamples. X-tile software (Yale University, New Haven, CT, USA) was used to identify the optimal cutoff value of the risk score, and all patients were divided into high-risk and low-risk groups. Kaplan-Meier analysis was used to assess the survival time of each subgroup of patients, and the log-rank test was utilized to determine differences in survival time. Median OS was defined as the median time from diagnosis to death as the result of any cause. Statistical analysis was conducted using R software version 3.6.3. In this study, a P value <0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
Ultimately, there were 8,858 patients diagnosed with LM from SCLC from the SEER database, and they were randomly divided into a training cohort (n=6,200) and a validation cohort (n=2,658) in a 7:3 ratio. Table 1 summarizes the demographic and clinical characteristics of SCLC patients with LM. Among all patients, 3,303 (37.3%) were below the age of 65 years, and the majority were male (53.1%) and married (51.7%) individuals. There were 1,944 patients (21.9%) with lung metastasis, 1,550 patients (17.5%) with brain metastasis, and 3,803 patients (42.9%) with bone metastasis. The most common stages among the patients were T4 stage with 3,592 cases (40.6%) and N2 stage with 5,194 cases (58.6%).
Table 1
| Variables | Subgroups | Validation cohort (n=2,658) | Training cohort (n=6,200) | Total cohort (n=8,858) |
|---|---|---|---|---|
| Sex, n (%) | Female | 1,248 (47.0) | 2,909 (46.9) | 4,157 (46.9) |
| Male | 1,410 (53.0) | 3,291 (53.1) | 4,701 (53.1) | |
| Race, n (%) | White | 2,395 (90.1) | 5,530 (89.2) | 7,925 (89.5) |
| Black | 176 (6.6) | 465 (7.5) | 641 (7.2) | |
| Other | 87 (3.3) | 205 (3.3) | 292 (3.3) | |
| Age, years, n (%) | <65 | 978 (36.8) | 2,325 (37.5) | 3,303 (37.3) |
| 65–75 | 1,003 (37.7) | 2,258 (36.4) | 3,261 (36.8) | |
| >75 | 677 (25.5) | 1,617 (26.1) | 2,294 (25.9) | |
| Marital status, n (%) | No | 1,252 (47.1) | 3,024 (48.8) | 4,276 (48.3) |
| Yes | 1,406 (52.9) | 3,176 (51.2) | 4,582 (51.7) | |
| Radiation, n (%) | No | 1,881 (70.8) | 4,390 (70.8) | 6,271 (70.8) |
| Yes | 777 (29.2) | 1,810 (29.2) | 2,587 (29.2) | |
| Surgery, n (%) | No | 2,649 (99.7) | 6,169 (99.5) | 8,818 (99.5) |
| Yes | 9 (0.3) | 31 (0.5) | 40 (0.5) | |
| Laterality, n (%) | Bilateral | 39 (1.5) | 94 (1.5) | 133 (1.5) |
| Left | 1,123 (42.2) | 2,619 (42.2) | 3,742 (42.2) | |
| Right | 1,496 (56.3) | 3,487 (56.2) | 4,983 (56.3) | |
| Bone metastasis, n (%) | No | 1,506 (56.7) | 3,549 (57.2) | 5,055 (57.1) |
| Yes | 1,152 (43.3) | 2,651 (42.8) | 3,803 (42.9) | |
| Brain metastasis, n (%) | No | 2,200 (82.8) | 5,108 (82.4) | 7,308 (82.5) |
| Yes | 458 (17.2) | 1,092 (17.6) | 1,550 (17.5) | |
| Lung metastasis, n (%) | No | 2,065 (77.7) | 4,849 (78.2) | 6,914 (78.1) |
| Yes | 593 (22.3) | 1,351 (21.8) | 1,944 (21.9) | |
| T stage, n (%) | T0 | 7 (0.3) | 27 (0.4) | 34 (0.4) |
| T1 | 280 (10.5) | 622 (10.0) | 902 (10.2) | |
| T2 | 729 (27.4) | 1,616 (26.1) | 2,345 (26.5) | |
| T3 | 574 (21.6) | 1,411 (22.8) | 1,985 (22.4) | |
| T4 | 1,068 (40.2) | 2,524 (40.7) | 3,592 (40.6) | |
| N stage, n (%) | N0 | 317 (11.9) | 669 (10.8) | 986 (11.1) |
| N1 | 152 (5.7) | 385 (6.2) | 537 (6.1) | |
| N2 | 1,571 (59.1) | 3,623 (58.4) | 5,194 (58.6) | |
| N3 | 618 (23.3) | 1,523 (24.6) | 2,141 (24.2) | |
| Primary site, n (%) | Main bronchus | 309 (11.6) | 755 (12.2) | 1,064 (12.0) |
| Upper lobe | 1,322 (49.7) | 2,987 (48.2) | 4,309 (48.6) | |
| Middle lobe | 107 (4.0) | 225 (3.6) | 332 (3.7) | |
| Lower lobe | 587 (22.1) | 1,396 (22.5) | 1,983 (22.4) | |
| Overlapping | 42 (1.6) | 95 (1.5) | 137 (1.5) | |
| Lung Nos | 291 (10.9) | 742 (12.0) | 1,033 (11.7) |
SCLC, small cell lung cancer; LM, liver metastasis; Nos, not otherwise specified.
Prognostic factors for SCLC patients with LM
The prognostic factors for SCLC patients with LM were selected using univariate and multivariate Cox regression models. The results of Cox regression analysis on the training cohort are shown in Table 2. In the univariate Cox regression analysis, age, gender, surgery, chemotherapy, radiotherapy, bone metastasis, brain metastasis, lung metastasis, N stage, and primary tumor site were significantly associated with the OS of patients. Finally, the results of the multivariate Cox regression analysis indicated that age, gender, primary tumor site, surgery, radiotherapy, chemotherapy, brain metastasis, and lung metastasis were independent prognostic factors (Table 2). Patients who received surgery [hazard ratio (HR) =0.46; 95% confidence interval (CI): 0.31–0.67; P<0.001], chemotherapy (HR =0.23; 95% CI: 0.21–0.24; P<0.001), and radiotherapy (HR =0.72; 95% CI: 0.68–0.77; P<0.001) had a lower risk of death. Additionally, male gender, advanced age, brain metastasis, lung metastasis, and different primary tumor sites were associated with higher risks of death.
Table 2
| Characteristics | Univariate Cox regression | Multivariate Cox regression | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Sex (vs. female) | |||||||
| Male | 1.16 | 1.10–1.22 | <0.001 | 1.18 | 1.12–1.24 | <0.001 | |
| Race (vs. White) | 0.20 | ||||||
| Black | 0.93 | 0.85–1.03 | 0.18 | ||||
| Other | 0.9 | 0.78–1.05 | 0.18 | ||||
| Age, years (vs. <65) | <0.001 | ||||||
| 65–75 | 1.21 | 1.14–1.29 | <0.001 | 1.1 | 1.03–1.16 | 0.005 | |
| >75 | 1.59 | 1.49–1.70 | <0.001 | 1.19 | 1.11–1.27 | <0.001 | |
| Radiation (vs. no) | |||||||
| Yes | 0.61 | 0.57–0.64 | <0.001 | 0.72 | 0.68–0.77 | <0.001 | |
| Chemotherapy (vs. no) | |||||||
| Yes | 0.21 | 0.20–0.22 | <0.001 | 0.23 | 0.21–0.24 | <0.001 | |
| Surgery (vs. no) | |||||||
| Yes | 0.55 | 0.37–0.80 | 0.002 | 0.46 | 0.31–0.67 | <0.001 | |
| Bone metastasis (vs. no) | |||||||
| Yes | 0.95 | 0.89–0.99 | 0.04 | ||||
| Brain metastasis (vs. no) | |||||||
| Yes | 1.09 | 1.02–1.16 | 0.02 | 1.27 | 1.18–1.37 | <0.001 | |
| Lung metastasis (vs. no) | |||||||
| Yes | 1.14 | 1.07–1.21 | <0.001 | 1.07 | 1.00–1.14 | 0.04 | |
| T stage (vs. T0) | 0.05 | ||||||
| T1 | 1.14 | 0.76–1.72 | 0.53 | ||||
| T2 | 1.31 | 0.87–1.96 | 0.19 | ||||
| T3 | 1.29 | 0.86–1.94 | 0.21 | ||||
| T4 | 1.29 | 0.86–1.92 | 0.22 | ||||
| N stage (vs. N0) | 0.002 | ||||||
| N1 | 0.87 | 0.77–0.99 | 0.04 | ||||
| N2 | 0.97 | 0.89–1.05 | 0.42 | ||||
| N3 | 0.87 | 0.79–0.96 | 0.005 | ||||
| Primary site (vs. main bronchus) | <0.001 | ||||||
| Upper lobe | 1.01 | 0.93–1.09 | 0.89 | 1.01 | 0.93–1.09 | 0.87 | |
| Middle lobe | 0.95 | 0.81–1.11 | 0.48 | 0.98 | 0.84–1.15 | 0.83 | |
| Lower lobe | 1.05 | 0.95–1.15 | 0.34 | 0.98 | 0.89–1.07 | 0.62 | |
| Overlapping | 1.29 | 1.04–1.61 | 0.02 | 1.27 | 1.02–1.58 | 0.03 | |
| Lung Nos | 1.18 | 1.06–1.31 | 0.002 | 1.14 | 1.03–1.27 | 0.01 | |
Nos, not otherwise specified; HR, hazard ratio; CI, confidence interval; SCLC, small cell lung cancer; LM, liver metastasis.
Development and validation of the prognostic nomogram
Based on the prognostic factors selected in the training cohort, we developed a prognostic nomogram to predict the OS of SCLC patients with LM (Figure 2). In the nomogram, each patient was represented by a line on the variable axis, indicating the score for each variable. The scores for each variable were summed to calculate the individual’s total risk score, and a line was then drawn on the survival probability axis to visually estimate the patient’s 6-, 12-, and 18-month OS.

To validate the prognostic nomogram, ROC curves were plotted for both the training and validation cohorts, and the corresponding AUC values were calculated. As shown in Figure 3, in the training cohort, the AUC values of the nomogram for 6-, 12-, and 18-month OS were 0.82, 0.75, and 0.75, respectively. In the validation cohort, the AUC values were 0.81, 0.74, and 0.71, respectively. Furthermore, we compared the AUC values of the nomogram with those of all the independent prognostic factors. The results showed that the nomogram had higher AUC values for 6-, 12-, and 18-month OS than any individual factor in both the training and validation cohorts. As shown in Figure 4, calibration curves were generated to assess the consistency between the predicted survival rates by the nomogram and the actual observed values. These points were close to the 45-degree diagonal line, indicating a good agreement between the predicted and actual survival rates. Finally, DCA curves were plotted (Figure 5), and the results showed that the prognostic nomogram had a wider and more practical range of threshold probabilities, significantly increasing the net benefit. This suggested that the nomogram had high clinical utility in predicting OS for SCLC patients with LM.

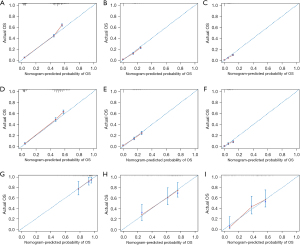

External validation of the nomogram’s predictive accuracy for OS
External validation was conducted to assess the predictive accuracy of the established nomogram for SCLC patients with LM. There were 128 patients treated at Jilin Cancer Hospital from 2013 to 2023 included in this validation set, following the same inclusion and exclusion criteria as the training cohort. The C-index was found to be 0.69 (95% CI: 0.64–0.75), with corresponding AUCs of 0.73, 0.77, and 0.78 for 6-, 12-, and 18-month, respectively (Figure 3G). The calibration curves (Figure 4G-4I) for 6-, 12-, and 18-month survival illustrate a strong concordance between projected values and observed survival probabilities. These findings from the external validation process suggested that the nomogram constructed in this investigation possessed a notable degree of precision and reliability, rendering it appropriate for forecasting 6-, 12-, and 18-month OS rates in SCLC patients with LM.
Establishment of risk stratification system
By using X-tile to calculate the optimal cutoff value of the risk score, all patients including the training cohort and the validation cohort were divided into low-risk group and high-risk group. The Kaplan-Meier survival curve clearly showed that the prognosis of the high-risk group patients was worse than that of the low-risk group patients (Figure 6).

Survival rates of SCLC patients with LM in different metastatic sites
All patients were further divided into 8 groups based on whether LM was accompanied by other metastases. There were statistically significant differences in OS among these 8 groups (Figure 7). Figure 8 shows that when comparing the HR under different metastasis patterns, it was found that patients with multiple metastatic sites had a higher risk of death. In patients presenting with two metastatic sites, it was observed that individuals with liver and brain metastases exhibited an 11% elevated mortality risk compared to those with solely LM, with a median survival duration of 4 months (P=0.02). Similarly, patients with liver and lung metastases demonstrated a 19% increased risk of death and a median survival time of 2 months (P<0.001). Additionally, among patients with three metastatic sites, those with liver, brain, and lung metastases experienced a 23% higher risk of mortality in comparison to individuals with only LM, with a median survival time of 3 months (P<0.001). Furthermore, among patients with four metastatic sites, those with liver, brain, bone, and lung metastases had a 19% higher risk of death compared to those with only LM, with a median survival time of 3 months (P=0.01).


Other potential prognostic factors for OS
Due to the limited information of the SEER database, we explored the potential prognostic factors affecting the OS of SCLC-LM patients based on our external validation data of 128 cases. We analyzed several relevant prognostic factors including: Eastern Cooperative Oncology Group Performance Status (ECOG PS), pleural metastasis, adrenal metastasis, smoking history, and neutrophil-to-lymphocyte ratio (NLR). Different subgroup analyzes were conducted based on the number of metastases in different metastasis sites, types of systemic treatment, and whether they received prophylactic cranial irradiation (PCI). The results are shown in Table 3. None of the above potential influencing factors was found to be significantly associated with the OS of SCLC-LM patients.
Table 3
| Characteristics | Univariate Cox regression | |
|---|---|---|
| HR (95% CI) | P value | |
| ECOG PS (vs. 0) | ||
| 1 | 0.81 (0.39–1.68) | 0.57 |
| 2 | 1.76 (0.60–5.13) | 0.30 |
| Pleural metastasis (vs. no) | ||
| Yes | 1.68 (0.86–3.27) | 0.13 |
| Adrenal metastasis (vs. no) | ||
| Yes | 1.06 (0.61–1.84) | 0.85 |
| Smoking (vs. no) | ||
| Yes | 1.28 (0.84–1.95) | 0.25 |
| Chemotherapy type (vs. chemotherapy alone) | ||
| Chemotherapy plus immunotherapy | 0.79 (0.52–1.19) | 0.26 |
| PCI (vs. no) | ||
| Yes | 2.01 (0.69–5.84) | 0.20 |
| Number of liver metastases (vs. single) | ||
| Multiple | 1.29 (0.76–2.22) | 0.37 |
| Number of lung metastases (vs. single) | ||
| Multiple | 1.68 (0.87–3.25) | 0.12 |
| Number of bone metastases (vs. single) | ||
| Multiple | 2.51 (0.64–9.87) | 0.19 |
| NLR | 1.03 (0.99–1.07) | 0.10 |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; PCI, prophylactic cranial irradiation; NLR, neutrophil-to-lymphocyte ratio; HR, hazard ratio; CI, confidence interval.
Discussion
This study constructed a prognostic nomogram incorporating variables such as age, gender, surgery, chemotherapy, radiotherapy, bone metastasis, brain metastasis, lung metastasis, N stage, and primary tumor site to predict the OS probability of individuals diagnosed with SCLC-LM patients. The validity of the nomogram model was confirmed through analysis of an independent cohort, demonstrating significant discriminatory power and accuracy in both the training and validation cohorts. Furthermore, patients were categorized into 8 subgroups based on their distinct metastasis locations, and the OS rates were compared among patients with varying metastatic conditions.
Currently, there is a lack of research that comprehensively documents the characteristics and prognosis of SCLC patients with LM. To the best of our knowledge, our study is the first to establish a prognostic prediction nomogram for SCLC-LM patients. This nomogram offers the benefit of visually representing the score for each variable and the personalized probability of survival, thereby enhancing its utility in clinical settings.
Our research results indicate that among SCLC-LM patients, females had a better prognosis than males, which may be related to the stimulatory effect of androgens on lung cancer growth (16). In previous studies, advanced age has been reported as an unfavorable prognostic factor for SCLC patients (17-19). Our study showed that elderly SCLC patients diagnosed with LM at an early stage had a higher risk of death. This increased risk may be associated with age-related degenerative changes in various organ functions and an increased incidence of other diseases (20). Additionally, older patients may be more susceptible to the toxic reactions caused by systemic treatments, while younger patients have better overall health and greater tolerance to the side effects of chemotherapy and radiotherapy (21,22). Surgery should only be considered in those with stage I to IIA disease (T1–2N0M0) (23), analyses of the SEER database also suggest that surgery may be appropriate for some patients with localized disease (24,25). In our study, due to the advanced stage at which most patients were diagnosed, only a small percentage (0.5%) underwent surgical intervention. Those patients who were eligible for surgery demonstrated a more favorable prognosis. For late-stage SCLC patients, chemotherapy alone or combined with radiotherapy is considered standard treatment (2). Our research confirmed that patients who underwent chemotherapy and radiation therapy had a significantly increased survival period.
SCLC is an aggressive malignant tumor that commonly metastasizes to multiple organs, often progressing from one organ to another in most patients (26). One study has found that SCLC patients are prone to multiple-site metastases, especially LM combined with metastases in other parts of the body (27). Cai et al. conducted a retrospective cohort study and found that nearly 42% of extensive-stage SCLC patients had metastases in at least two distant sites (7). In our study, we found that 42.9% of patients had concomitant bone metastases, 17.5% had concomitant brain metastases, and 21.9% had concomitant lung metastases. Among them, SCLC patients with concomitant brain or lung metastases in addition to LM had a worse prognosis. Therefore, incorporating metastatic indicators into the prognostic system and quantifying their impact on LM can improve the accuracy of the prognostic nomogram. Adding them to the nomogram may be a better choice.
Compared to other conventional staging systems, the nomogram constructed in this study showed higher accuracy in predicting the survival of patients with SCLC-LM at 6, 12, and 18 months based on ROC analysis. However, excellent discrimination or calibration performance does not necessarily translate into excellent clinical utility in practice (28). Therefore, DCA was used to evaluate the clinical utility of the new nomogram. The predictive model developed in this study demonstrated significantly better clinical utility compared to conventional staging systems.
There are still certain limitations in this study. First, it takes a lot of time and money to evaluate a study as large as this study as a prospective study. Thus, this research employed a retrospective study design, which inherently introduces selection bias. Second, the constraints inherent in the SEER database precluded the acquisition of data pertaining to general health status, genetic mutations, and treatment plans, thereby impeding more comprehensive prognostic analyses. Furthermore, this study did not incorporate several potential prognostic factors for SCLC, including ECOG PS, pleural and adrenal metastases, receipt of PCI, the types of systemic treatment, the number of metastatic lesions at each site, smoking history, and NLR. Although we examined these variables using our own external validation data, the limited sample size precluded the identification of any significant associations with prognosis. Future prospective studies with more samples are needed to verify the results of this study.
Conclusions
In conclusion, a prognostic nomogram has been developed for SCLC patients with LM, incorporating 10 variables such as age, gender, surgery, chemotherapy, radiotherapy, bone metastasis, brain metastasis, lung metastasis, N stage, and primary tumor site. This tool aids in the precise evaluation of OS and the identification of high-risk individuals. Identifying the locations of synchronous extra LM and their corresponding prognoses can aid in guiding treatment decisions and optimizing therapeutic strategies.
Acknowledgments
The authors thank all the patients who participated in this study, and especially appreciate the public access provided by the SEER database.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1423/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1423/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1423/prf
Funding: The present study was financially supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1423/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Jilin Province Cancer Hospital (Ethics No. 202406-008-01, date of approval: 24 June 2024). The external validation data for this study were derived from the electronic medical record system of Jilin Cancer Hospital. Only the clinical information of the patients was collected retrospectively, and any characters with the subjects’ identities were deleted from the research results to ensure that personal privacy was not disclosed. Consequently, the Ethics Committee granted a waiver for the requirement of written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw 2013;11:78-98. [Crossref] [PubMed]
- Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi99-105. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78-84. [Crossref] [PubMed]
- Reinmuth N, Garassino MC, Trukhin D, et al. P48. 03 First-line durvalumab plus platinum-etoposide in ES-SCLC: exploratory analyses based on extent of disease in CASPIAN. J Thorac Oncol 2021;16:S500.
- Nakazawa K, Kurishima K, Tamura T, et al. Specific organ metastases and survival in small cell lung cancer. Oncol Lett 2012;4:617-20. [Crossref] [PubMed]
- Cai H, Wang H, Li Z, et al. The prognostic analysis of different metastatic patterns in extensive-stage small-cell lung cancer patients: a large population-based study. Future Oncol 2018;14:1397-407. [Crossref] [PubMed]
- Megyesfalvi Z, Tallosy B, Pipek O, et al. The landscape of small cell lung cancer metastases: Organ specificity and timing. Thorac Cancer 2021;12:914-23. [Crossref] [PubMed]
- Cheng Y, Han L, Wu L, et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022;328:1223-32. [Crossref] [PubMed]
- Liu SV, Dziadziuszko R, Sugawara S, et al. Five-Year Survival in Patients with ES-SCLC Treated with Atezolizumab in IMpower133: Imbrella a Extension Study Results. J Thorac Oncol 2023;18:S44-S5.
- Payapilly A, Guilbert R, Descamps T, et al. TIAM1-RAC1 promote small-cell lung cancer cell survival through antagonizing Nur77-induced BCL2 conformational change. Cell Rep 2021;37:109979. [Crossref] [PubMed]
- Cheng Y, Wang Q, Li K, et al. Anlotinib for patients with small cell lung cancer and baseline liver metastases: A post hoc analysis of the ALTER 1202 trial. Cancer Med 2022;11:1081-7. [Crossref] [PubMed]
- Wang S, Yang L, Ci B, et al. Development and Validation of a Nomogram Prognostic Model for SCLC Patients. J Thorac Oncol 2018;13:1338-48. [Crossref] [PubMed]
- Gao H, Dang Y, Qi T, et al. Mining prognostic factors of extensive-stage small-cell lung cancer patients using nomogram model. Medicine (Baltimore) 2020;99:e21798. [Crossref] [PubMed]
- Behera M, Ragin C, Kim S, et al. Trends, predictors, and impact of systemic chemotherapy in small cell lung cancer patients between 1985 and 2005. Cancer 2016;122:50-60. [Crossref] [PubMed]
- Salmerón D, Chirlaque MD, Isabel Izarzugaza M, et al. Lung cancer prognosis in Spain: the role of histology, age and sex. Respir Med 2012;106:1301-8. [Crossref] [PubMed]
- Liu J, Zhou H, Zhang Y, et al. Cause-specific death assessment of patients with stage I small-cell lung cancer: a competing risk analysis. Future Oncol 2019;15:2479-88. [Crossref] [PubMed]
- Wang Y, Pang Z, Chen X, et al. Development and validation of a prognostic model of resectable small-cell lung cancer: a large population-based cohort study and external validation. J Transl Med 2020;18:237. [Crossref] [PubMed]
- Lara JD, Brunson A, Riess JW, et al. Clinical predictors of survival in young patients with small cell lung cancer: Results from the California Cancer Registry. Lung Cancer 2017;112:165-8. [Crossref] [PubMed]
- Aarts MJ, Aerts JG, van den Borne BE, et al. Comorbidity in Patients With Small-Cell Lung Cancer: Trends and Prognostic Impact. Clin Lung Cancer 2015;16:282-91. [Crossref] [PubMed]
- Chen RC, Royce TJ, Extermann M, et al. Impact of age and comorbidity on treatment and outcomes in elderly cancer patients. Semin Radiat Oncol 2012;22:265-71. [Crossref] [PubMed]
- Gregor A, Drings P, Rinaldi M, et al. Acute toxicity of alternating schedule of chemotherapy and irradiation in limited small-cell lung cancer in a pilot study (08877) of the EORTC Lung Cancer Cooperative Group. Ann Oncol 1995;6:403-5. [Crossref] [PubMed]
- Kalemkerian GP, Schneider BJ. Advances in Small Cell Lung Cancer. Hematol Oncol Clin North Am 2017;31:143-56. [Crossref] [PubMed]
- Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 2010;5:215-9. [Crossref] [PubMed]
- Schreiber D, Rineer J, Weedon J, et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer 2010;116:1350-7. [Crossref] [PubMed]
- Oikawa A, Takahashi H, Ishikawa H, et al. Application of conditional probability analysis to distant metastases from lung cancer. Oncol Lett 2012;3:629-34. [Crossref] [PubMed]
- Ren Y, Dai C, Zheng H, et al. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget 2016;7:53245-53. [Crossref] [PubMed]
- Badzio A, Kurowski K, Karnicka-Mlodkowska H, et al. A retrospective comparative study of surgery followed by chemotherapy vs. non-surgical management in limited-disease small cell lung cancer. Eur J Cardiothorac Surg 2004;26:183-8. [Crossref] [PubMed]



