
Sublobectomy versus lobectomy for peripheral small-sized pulmonary mucinous adenocarcinoma
Highlight box
Key findings
• For peripheral small-sized pulmonary mucinous adenocarcinoma (PMA), sublobectomy yielded a recurrence-free survival (RFS) and overall survival (OS) comparable to those of lobectomy.
What is known and what is new?
• Multiple previous studies have demonstrated that segmentectomy is noninferior to lobectomy for early-stage lung cancers located in the peripheral one-third of the pulmonary parenchyma, providing comparable disease-free survival, progression-free survival, and OS.
• The prognosis of PMA, a rare type of adenocarcinoma, remains controversial. We conducted this study to compare the oncological outcomes of lobectomy and sublobectomy for peripheral small-sized PMA.
What is the implication, and what should change now?
• Compared to lobectomy, sublobectomy can provide a comparable RFS and OS for peripheral small-sized PMA.
Introduction
Histologically described as adenocarcinoma with goblet cell or columnar cell shape, pulmonary mucinous adenocarcinoma (PMA) is a rare subtype of lung adenocarcinoma, accounting for around 3–10% of cases (1). Due to its low incidence, there is little or often contradicting survival data (2-6). PMA is characterized radiologically into two types: solitary and pneumonic. The pneumonic type has a significantly poorer prognosis than does solitary type (7,8), and pneumonic type PMA represents a stepwise progression from the solitary type (9). Therefore, treatment of early nodular mucinous adenocarcinoma is critical. Sublobectomy (including segmentectomy and wedge resection) can preserve as much lung function as possible while serving as a therapeutic resection and is highly recommended by the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines (version 3, 2024) for peripheral T1a and T1b, N0 non-small cell lung cancer (10). Multiple studies have demonstrated that segmentectomy is noninferior to lobectomy for early-stage lung cancers located in the peripheral one-third of the pulmonary parenchyma, providing comparable disease-free survival (DFS), progression-free survival (PFS), and overall survival (OS) (11,12). However, most of the patients enrolled in these previous studies had nonmucinous adenocarcinomas, and whether these findings also apply to PMA is unclear due to a lack of evidence. Therefore, we conducted this study to investigate the treatment strategies for patients with peripheral small-sized PMA. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2096/rc).
Methods
Study design
The retrospective cohort study focused on PMA conducted with sublobectomy (wedge resection and segmentectomy) and lobectomy. From January 2015 to December 2018, there were 503 patients with PMA who underwent sublobectomy and lobectomy in the Shanghai Pulmonary Hospital. According to the inclusion and exclusion criteria, 279 eligible patients were finally recruited. Demographic, clinical features, surgical outcomes were collected, and oncological outcomes were recorded after follow-up. As the baseline data were not all balanced, 186 patients were selected for propensity score matching (PSM) (Figure 1), and the surgical and oncological outcomes were compared between two cohorts to identify whether sublobectomy was feasibility and safety for peripheral small-sized PMA.

Data sources
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai Pulmonary Hospital (No. K23-290) on September 15, 2023. The data collection process followed Institutional Review Board guidelines, who waived the necessity for written informed consent due to the retrospective nature of the analysis. Patients were included in the study if they met the following inclusion criteria: (I) a pathological diagnosis of primary PMA; (II) completion of lobectomy, segmentectomy, or wedge resection between January 2015 and December 2018; (III) age 20–79 years; (IV) nodules with a diameter ≤2 cm; (V) nodules with a location in the peripheral (outer one-third) lung parenchyma; (VI) Eastern Cooperative Oncology Group performance score ≤1; (VII) surgery performed with selective or systematic lymph node dissection in lobectomy and segmentectomy or with lymph node sampling in wedge resection; and (VIII) a margin distance more than or equal to 2 cm or the maximal tumor diameter in sublobectomy.
Meanwhile, the exclusion criteria were as follows: (I) mucinous adenocarcinoma in situ (AIS); (II) metastatic lung cancer; (III) synchronous or metachronous multiple primary lung cancer; (IV) positive for clinical lymph nodes; (V) distant metastasis; and (VI) missing preoperative chest thin-section computed tomography (CT).
CT imaging acquisition and analysis
A multisection spiral CT scanner (Somatom Definition AS+, Siemens Healthineers, Erlangen, Germany; Brilliance iCT, Philips Healthcare, Amsterdam, the Netherlands) was used to obtain the CT images. A tumor with glass-ground opacity on the lung window was considered to be a ground-glass nodule (GGN).
Surgical approach and postoperative treatment
Trained and professional thoracic surgeons performed all pulmonary resections. The operative procedures included wedge resection, segmentectomy or lobectomy. The size and location of the tumor were considered in the selection of the surgical extent to ensure there was a sufficient margin distance for sublobectomy. For patients undergoing lobectomy or segmentectomy, systematic or selective lymph node dissection was carried out, and lymph node sampling was completed during wedge resection. Selective node dissection or lymph node sampling were performed for the patients with low risk of lymph node metastases (13). The margin distance was required to be more than or equal to 2 cm or the maximal tumor diameter. After the staplers used in sublobectomy were removed, the margin distance was assessed intraoperatively as the shortest distance between the segmental bronchus margin or surgical margin and the tumor edge. Extended resection or conversion to lobectomy was conducted in sublobectomy if the margin distance was less than 2 cm or less than the maximal tumor diameter. Negative margins were validated with a frozen section examination.
Follow-up
Follow-up appointments were scheduled every half year after surgery for the first 2 years and then every year thereafter. Postoperative imaging including chest CT scan, ultrasound or upper abdomen CT scan, brain magnetic resonance imaging (MRI), et al. Records of the complications, recurrence, and their management were kept. Follow-ups that took place outside of hospital were conducted over the phone.
Endpoint definitions
The time interval between the date of the operation and the date of any documented lung cancer relapse was considered to be the recurrence-free survival (RFS). The time between the date of the procedure and the date of death from any cause was considered to be the OS. A histology biopsy was performed to confirm the recurrence. If the biopsy was not available, recurrence was diagnosed by a multidisciplinary oncology committee based on radiological evidence from brain MRI, chest CT, and positron emission tomography-CT. Tumor recurrence in the same thoracic area that involved any of the mediastinal or hilar lymph nodes, ipsilateral lung was considered to be regional recurrence. Metastasis to the pleura, contralateral lung, or other organs was classified as distant recurrence.
Statistical analysis
Stata 18.0 (StataCorp LLC, College Station, TX, USA) was used for data analysis. For nonnormally distributed data, continuous variables are expressed as the median and interquartile range (IQR) and were subjected to the Mann-Whitney test. Categorical variables are expressed as frequencies and percentages and were compared with the Fisher’s exact test or Pearson χ2 test.
The log-rank test was used to compare RFS and OS between the peripheral and nonperipheral groups for the survival study. Univariable regression analysis with the Cox proportional hazards regression model was used to identify the risk factor for RFS and OS. The recurrence patterns of the two groups were compared with the Fine-Gray test.
Results
A total of 279 patients were included, comprising 176 cases in the lobectomy group and 103 cases in the sublobectomy group. The baseline of demographic and clinical features are shown in Table 1. Among the 279 patients, the median age was 61 years, 196 (70.3%) patients were women, 250 (89.6%) patients had no smoking history, 186 (66.7%) patients had tumors in the lower lobe, 178 (63.8%) patients had GGN, and the median tumor size was 1.2 cm. Excluding location, radiological features, and tumor size, the baseline characteristics were balanced. Considering the differences in location, radiological features, and tumor size would have a great impact on oncological outcomes, PSM method with a 1:1 matching ratio was used to balance the variables (Figure 1).
Table 1
| Variables | Before matching (n=279) | After matching (n=186) | |||||
|---|---|---|---|---|---|---|---|
| Lobectomy group (n=176) | Sublobectomy group (n=103) | P value | Lobectomy group (n=83) | Sublobectomy group (n=103) | P value | ||
| Age (years) | 61 (54–66) | 62 (55–68) | 0.35 | 60 (54–66) | 62 (55–68) | 0.38 | |
| Sex | 0.32 | 0.56 | |||||
| Male | 56 (31.8) | 27 (26.2) | 25 (30.1) | 27 (26.2) | |||
| Female | 120 (68.2) | 76 (73.8) | 58 (69.9) | 76 (73.8) | |||
| Smoking | 0.27 | 0.47 | |||||
| No | 155 (88.1) | 95 (92.2) | 74 (89.2) | 95 (92.2) | |||
| Yes | 21 (11.9) | 8 (7.8) | 9 (10.8) | 8 (7.8) | |||
| Location | 0.01* | 0.054 | |||||
| Right upper lobe | 19 (10.8) | 19 (18.4) | 8 (9.6) | 19 (18.4) | |||
| Right middle lobe | 13 (7.4) | 5 (4.9) | 7 (8.4) | 5 (4.9) | |||
| Right lower lobe | 85 (48.3) | 30 (29.1) | 39 (47.0) | 30 (29.1) | |||
| Left upper lobe | 19 (10.8) | 18 (17.5) | 9 (10.8) | 18 (17.5) | |||
| Left lower lobe | 40 (22.7) | 31 (30.1) | 20 (24.1) | 31 (30.1) | |||
| Radiological feature | 0.02* | 0.64 | |||||
| GGN | 103 (58.5) | 75 (72.8) | 57 (68.7) | 75 (72.8) | |||
| Solid | 73 (41.5) | 28 (27.2) | 26 (31.3) | 28 (27.2) | |||
| Tumor size (cm) | 1.40 (1.10–1.60) | 1.00 (0.80–1.30) | <0.001* | 1.09 (0.95–1.42) | 1.00 (0.80–1.30) | 0.12 | |
Data are presented as median (IQR) or n (%). *, P value ≤0.05. GGN, glass-ground nodule; IQR, interquartile range.
All the margin distances were more than or equal to 2 cm or the maximal tumor diameter in sublobectomy, and all the margins were negative. In the cohort of 103 patients who underwent sublobectomy, 40 (38.8%) were treated with wedge resections. The surgical and oncological outcomes of the two groups are listed in Table 2. Compared to the sublobectomy group, the lobectomy group had a longer operation time (lobectomy: median 120 min, IQR, 85–150 min; sublobectomy: median 75 min, IQR, 50–100 min), more blood loss (lobectomy: median 50 mL, IQR, 40–100 mL; sublobectomy: median 50 mL, IQR, 20–60 mL), more drainage volume (lobectomy: median 560 mL, IQR, 400–847 mL; sublobectomy: median 320 mL, IQR, 200–500 mL), and longer drainage time (lobectomy: median 3 days, IQR, 2–4 days; sublobectomy: median 2 days, IQR, 2–3 days). The pathological diagnosis showed that no pleural invasion or lymph node metastases were found in any patients. Spread through air spaces (STAS) was detected in 5 (1.7%) patients, and all patients with STAS underwent lobectomy. Perioperative complications, including bronchopleural fistula, hydrothorax requiring redrainage, chylothorax, respiratory failure, and pulmonary embolism, were comparable between the groups.
Table 2
| Variables | Before matching (n=279) | After matching (n=186) | |||||
|---|---|---|---|---|---|---|---|
| Lobectomy group (n=176) | Sublobectomy group (n=103) | P value | Lobectomy group (n=83) | Sublobectomy group (n=103) | P value | ||
| Operation time (min) | 120 (85–150) | 75 (50–100) | <0.001* | 125 (90–160) | 75 (50–100) | <0.001* | |
| Blood loss (mL) | 50 (50–100) | 50 (20–50) | <0.001* | 50 (50–100) | 50 (20–50) | <0.001* | |
| Drainage volume (mL) | 560 (400–847) | 320 (200–500) | <0.001* | 570 (350–950) | 320 (200–450) | <0.001* | |
| Drainage (days) | 3 (2–4) | 2 (2–3) | <0.001* | 3 (2–4) | 2 (2–3) | <0.001* | |
| Pleural invasion | 0 (0.0) | 0 (0.0) | – | 0 (0.0) | 0 (0.0) | – | |
| Positive node | 0 (0.0) | 0 (0.0) | – | 0 (0.0) | 0 (0.0) | – | |
| STAS | 5 (2.8) | 0 (0.0) | 0.16 | 1 (1.2) | 0 (0.0) | 0.45 | |
| Postoperative complications | |||||||
| Bronchopleural fistula | 1 (0.6) | 0 (0.0) | 0.44 | 0 (0.0) | 0 (0.0) | – | |
| Hydrothorax requiring redrainage | 1 (0.6) | 2 (1.9) | 0.44 | 0 (0.0) | 0 (0.0) | – | |
| Chylothorax | 1 (0.6) | 1 (1.0) | 0.70 | 0 (0.0) | 1 (1.0) | 0.55 | |
| Respiratory failure | 1 (0.6) | 0 (0.0) | 0.44 | 1 (1.2) | 0 (0.0) | 0.26 | |
| Pulmonary embolism | 1 (0.6) | 0 (0.0) | 0.44 | 1 (1.2) | 0 (0.0) | 0.26 | |
| Recurrence | 0.67 | 0.29 | |||||
| Regional | 1 (0.6) | 0 (0.0) | 1 (1.2) | 0 (0.0) | |||
| Distant | 5 (2.8) | 2 (1.9) | 4 (4.8) | 2 (1.9) | |||
Data are presented as median (IQR) or n (%). *, P value ≤0.05. STAS, spread through air spaces; IQR, interquartile range.
During the median 5-year follow-up, recurrence occurred in 8 patients, and 6 patients died. Before and after PSM, log-rank tests showed that the lobectomy and sublobectomy groups were not significantly different in terms of 5-year RFS (before PSM: 97.0% vs. 98.9%; after PSM: 94.6% vs. 98.9%) or 5-year OS (before PSM: 96.9% vs. 98.8%; after PSM: 94.8% vs. 98.8%) (Figure 2). The recurrence patterns were similar between the two groups (Table 2). The Cox proportional-hazards regression model showed that surgery (lobectomy vs. sublobectomy) was not a risk factor for RFS or OS (Table 3).
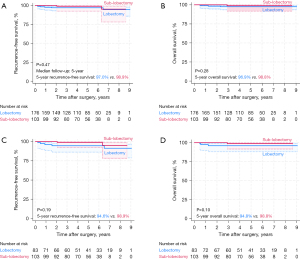
Table 3
| Variables | Recurrence-free survival | Overall survival | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 1.02 (0.95–1.05) | 0.64 | 0.99 (0.92–1.07) | 0.82 | |
| Sex (male vs. female) | 0.38 (0.10–1.52) | 0.17 | 0.21 (0.04–1.13) | 0.07 | |
| Smoking | 1.40 (1.71–11.41) | 0.76 | 1.75 (0.21–15.00) | 0.61 | |
| Location (lower lobe vs. others) | 0.28 (0.04–2.30) | 0.28 | 0.03 (0.00–28.18) | 0.30 | |
| Radiological feature (GGN vs. solid) | 1.63 (0.41–6.51) | 0.49 | 1.73 (0.35–8.55) | – | |
| Tumor size (≤1 vs. >1 and ≤2 cm) | 0.93 (0.22–3.92) | 0.92 | 2.99 (0.35–25.59) | 0.32 | |
| STAS | –† | 0.82 | –† | 0.82 | |
| Surgery (lobectomy vs. sublobectomy) | 0.56 (0.13–2.79) | 0.46 | 0.33 (0.04–2.80) | 0.31 | |
†, the STAS (+) group all underwent lobectomy, with none experiencing recurrence or death. HR, hazard ratio; CI, confidence interval; GGN, glass-ground nodule; STAS, spread through air spaces.
Discussion
For peripheral small-sized (≤2 cm) lung cancers sublobectomy can provide a comparable prognosis to that of lobectomy (11,14). However, whether sublobectomy is effective for PMA remains uncertain. Prior studies have demonstrated conflicting outcomes for PMA. For instance a recent study by Ueda et al. demonstrated that intrapulmonary recurrence occurs 2.3 times more often with adenocarcinoma with mucin (15). These results suggest that lobectomy may be preferential for patients with PMA.
In our study, which included 279 patients with peripheral small-sized PMA, we found that sublobar resection was comparable to lobectomy in terms of RFS and OS at 5 years of follow-up. Cui et al. analyzed 303 patients with stage I–III PMA and concurrent non-PMA in the Surveillance, Epidemiology, and End Results database and found sublobar resection to be a risk factor for prognosis (16). However, their study included only 74 patients with small-sized PMA, and no subgroup analysis was performed for early-stage PMA. Kim et al. studied 91 patients with PMA less than 3 cm in size through 42-month follow-up and found that sub-lobar resection resulted in comparable DFS and OS compared with lobectomy (17). Lin et al. (18) and Wang et al. (19) also reported that sublobectomy for early-stage PMA was comparable to lobectomy and yielded equivalent oncological outcomes. The small sample size and the early stage in our study lead to a lower recurrence rate, larger size and longer follow-up were needed to validate the results.
Lymph node dissection is an important issue in sublobar resection. For sublobar resection, especially wedge resection, is difficult to achieve lymph node dissection. The target population staged as cT1a-1bN0M0, and the NCCN guidelines strongly recommend sublobectomy with mediastinal lymph node dissection or systematic lymph node sampling for them (10). Matsui and peers reported low lymph node metastasis (4%) occurred in PMA staged IA–IIIB (6). There was no lymph node metastasis in our target patients, and only one patient who underwent lobectomy had lymph node metastasis during follow-up, thus wedge resection and lymph node sampling did not affect the pattern of recurrence in our patient. Larger number of samples were essential to analyze the lymph node metastasis of PMA.
PMA has different clinical features to pulmonary non-mucinous adenocarcinoma (PNMA). Clinically, PMA tends to arise in older women with a never or light smoking history (20), is mainly distributed in the periphery of the lower lobe (21,22), is less likely to result in lymph node metastases, and is discovered at an earlier pathological stage (15,19). Recurrence of PMA tends to occur in the lungs (23,24). Our study was consistent with previous studies: in our study, the median age was 61 years, 196 (70.3%) patients were women, 250 (89.6%) patients had no smoking history, and 186 (66.7%) patients had tumors in the lower lobe. Among the 7 cases of recurrence in our study, 4 occurred in the lung, and the others occurred in the regional lymph node (2 cases) or bone (1 case).
PMA progresses in stepwise fashion. Radiologically, PMA is classified into two types: solitary and pneumonic (25). PMAs may have the same genetic origin. In the early stage, the tumor region is surrounded by an intra-alveolar air space filled with mucin, and the remaining intra-alveolar air space in the solitary type with ground-glass opacity is gradually filled with mucin as the tumor grows (8). As the tumor grows and mucus production increases, the tumor cells can easily disseminate quickly through the airways, drifting away with the mucus (21). This intrapulmonary dissemination can result in the development of the pneumonic type (25). The prognosis of the pneumonic type is worse than that of the solitary type (8,26). At the solitary stage, the tumor is relatively limited, and it is the window period for cure; therefore, identifying early-stage solitary PMA is critical to successful treatment.
The prognosis for PMA with STAS is poor (27). Tumor cells floating in pools of abundant extracellular mucin are more likely to spread via mucin proteins and then disseminate to the adjacent alveoli, resulting in the high occurrence of STAS in patients with PMA (28). In the study of Lee et al., patients with STAS and larger-sized PMA (median size 3.8 cm) had a higher number of lymph node metastases, while the incidence of locoregional recurrence was significantly higher than that in patients without STAS (1.2% vs. 22.6%) (24). However, STAS was only detected in 5 (1.7%) patients in the study, and we speculate that abundant mucus and extensive STAS does not appear in early-stage PMA. Nicotra’s study also indicated that lower STAS occurred in early-stage adenocarcinoma, and STAS did not affect prognosis in early-stage adenocarcinoma (29). Moreover, STAS in our study was not a risk factor for RFS or OS, and patients with STAS did not experience recurrence. In our study, 5 cases of PMA with STAS underwent lobectomy, which resulted in complete resection of lesions with STAS and good prognosis. Han et al. (30) demonstrated that STAS can be identified at distances of 2,500 µm or more from the tumor margin. Despite all margin distances being more than or equal to 2 cm or the maximum tumor diameter in sublobectomy, the risk of undiagnosed STAS in the residual pulmonary parenchyma remained. Our study involves a few limitations which should be acknowledged. First, we employed a retrospective, single-institution design, and selection bias could not be avoided. Further multicenter randomized controlled trials are required to corroborate our findings. Second, postoperative pulmonary function tests were not typically conducted (unless symptoms such as dyspnea and chest tightness appeared). Thus, it is hard to evaluate the effect of sublobectomy on pulmonary function preservation.
Conclusions
Our analysis indicates that sublobectomy for peripheral small-sized PMA could provide a comparable RFS and OS to those of lobectomy. Clinically, it is not essential to extend the resection to lobectomy when these nodules are pathologically confirmed to be PMA.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2096/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2096/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2096/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2096/coif). H.C.F. serves as an unpaid editorial board member of Journal of Thoracic Disease from April 2024 to June 2026. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Ethics Committee of Shanghai Pulmonary Hospital (No. K23-290) on September 15, 2023. The data collection process followed Institutional Review Board guidelines, and the board waived the necessity for written informed consent due to the retrospective nature of the analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362-87. [Crossref] [PubMed]
- Liu QH, Zhang SJ. Clinical status and research progress of primary pulmonary mucinous adenocarcinoma. Clinical Medicine of China 2023;39:261-65.
- Chen Z, Li M, Ma K, et al. Analysis of the clinicopathological characteristics, genetic phenotypes, and prognostic of pure mucinous adenocarcinoma. Cancer Med 2020;9:517-29. [Crossref] [PubMed]
- Moon SW, Choi SY, Moon MH. Effect of invasive mucinous adenocarcinoma on lung cancer-specific survival after surgical resection: a population-based study. J Thorac Dis 2018;10:3595-608. [Crossref] [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Matsui T, Sakakura N, Koyama S, et al. Comparison of Surgical Outcomes Between Invasive Mucinous and Non-Mucinous Lung Adenocarcinoma. Ann Thorac Surg 2021;112:1118-26. [Crossref] [PubMed]
- Watanabe H, Saito H, Yokose T, et al. Relation between thin-section computed tomography and clinical findings of mucinous adenocarcinoma. Ann Thorac Surg 2015;99:975-81. [Crossref] [PubMed]
- Goto E, Takamochi K, Kishikawa S, et al. Stepwise progression of invasive mucinous adenocarcinoma based on radiological and biological characteristics. Lung Cancer 2023;184:107348. [Crossref] [PubMed]
- Park BJ, Woo W, Cha YJ, et al. Proposal of a revised International Association for the Study of Lung Cancer grading system in pulmonary non-mucinous adenocarcinoma: The importance of the lepidic proportion. Lung Cancer 2023;175:1-8. [Crossref] [PubMed]
- Riely GJ, Wood DE, Ettinger DS, et al. Non-Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2024;22:249-74. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Damman B, et al. Lobectomy, segmentectomy, or wedge resection for peripheral clinical T1aN0 non-small cell lung cancer: A post hoc analysis of CALGB 140503 (Alliance). J Thorac Cardiovasc Surg 2024;167:338-347.e1. [Crossref] [PubMed]
- Zhang Y, Deng C, Zheng Q, et al. Selective Mediastinal Lymph Node Dissection Strategy for Clinical T1N0 Invasive Lung Cancer: A Prospective, Multicenter, Clinical Trial. J Thorac Oncol 2023;18:931-9. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Ueda K, Kawaguchi Y, Itoh Y, et al. Surgical outcome in patients with lung adenocarcinoma with mucin. Gen Thorac Cardiovasc Surg 2024; Epub ahead of print. [Crossref]
- Cui D, Xie S, Liu Q. Postoperative survival of pulmonary invasive mucinous adenocarcinoma versus non-mucinous invasive adenocarcinoma. BMC Pulm Med 2023;23:9. [Crossref] [PubMed]
- Kim DH, Bae SY, Na KJ, et al. Radiological and clinical features of screening-detected pulmonary invasive mucinous adenocarcinoma. Interact Cardiovasc Thorac Surg 2022;34:229-35. [Crossref] [PubMed]
- Lin W, Su H, Xie H, et al. Limited resection is comparable to lobectomy for tumor size ≤2 cm pulmonary invasive mucinous adenocarcinoma. World J Surg Oncol 2024;22:109. [Crossref] [PubMed]
- Wang L, Zhang G, Zheng C, et al. Long-term outcomes of lobectomy vs . sublobectomy for stage I lung invasive mucinous adenocarcinoma. Chin Med J (Engl) 2024;137:1879-81. [Crossref] [PubMed]
- Yang SR, Chang JC, Leduc C, et al. Invasive Mucinous Adenocarcinomas With Spatially Separate Lung Lesions: Analysis of Clonal Relationship by Comparative Molecular Profiling. J Thorac Oncol 2021;16:1188-99. [Crossref] [PubMed]
- Pan X, Fang R, Zhang B, et al. Pathological and imaging features of pulmonary invasive mucinous adenocarcinoma-a retrospective cohort study. Transl Lung Cancer Res 2024;13:1376-82. [Crossref] [PubMed]
- Qi L, Jia J, Zhang G, et al. Radiological Features of Primary Pulmonary Invasive Mucinous Adenocarcinoma Based on 312 Consecutive Patients. Clin Respir J 2024;18:e13820. [Crossref] [PubMed]
- Shim HS, Kenudson M, Zheng Z, et al. Unique Genetic and Survival Characteristics of Invasive Mucinous Adenocarcinoma of the Lung. J Thorac Oncol 2015;10:1156-62. [Crossref] [PubMed]
- Lee J, Cho S, Chung JH, et al. Prognosis of spread through air spaces in invasive mucinous lung adenocarcinoma after curative surgery. Eur J Surg Oncol 2024;50:108053. [Crossref] [PubMed]
- Nie K, Nie W, Zhang YX, et al. Comparing clinicopathological features and prognosis of primary pulmonary invasive mucinous adenocarcinoma based on computed tomography findings. Cancer Imaging 2019;19:47. [Crossref] [PubMed]
- Li W, Yang Y, Yang M, et al. Clinicopathologic Features and Survival Outcomes of Primary Lung Mucinous Adenocarcinoma Based on Different Radiologic Subtypes. Ann Surg Oncol 2024;31:167-77. [Crossref] [PubMed]
- Chang WC, Zhang YZ, Nicholson AG. Pulmonary invasive mucinous adenocarcinoma. Histopathology 2024;84:18-31. [Crossref] [PubMed]
- Lee MA, Kang J, Lee HY, et al. Spread through air spaces (STAS) in invasive mucinous adenocarcinoma of the lung: Incidence, prognostic impact, and prediction based on clinicoradiologic factors. Thorac Cancer 2020;11:3145-54. [Crossref] [PubMed]
- Nicotra S, Melan L, Pezzuto F, et al. Significance of Spread Through Air Spaces and Vascular Invasion in Early-stage Adenocarcinoma Survival: A Comprehensive Clinicopathologic Study of 427 Patients for Precision Management. Am J Surg Pathol 2024;48:605-14. [Crossref] [PubMed]
- Han YB, Kim H, Mino-Kenudson M, et al. Tumor spread through air spaces (STAS): prognostic significance of grading in non-small cell lung cancer. Mod Pathol 2021;34:549-61. Erratum in: Mod Pathol 2021;34:1038. [Crossref] [PubMed]
(English Language Editor: J. Gray)


