Diagnostic management of patients with suspected ocular sarcoidosis
Introduction
Sarcoidosis is a systemic granulomatous disease with unknown etiology. Although the disease presentation shows considerable variable depending on environment or ethnic factors, ocular lesions are common among patients with sarcoidosis, with a reported incidence of 11-83% during the disease course (1,2) and 1.5-12.4% at first presentation (3-5). In Japan, an ocular lesion is the most common presentation of sarcoidosis, and its incidence has recently increased (6). To diagnose sarcoidosis and to exclude other conditions, pathological confirmation is essential. Sarcoidosis can be clinically diagnosed in patients with the specific radiographic feature of bihilar lymphadenopathy (BHL); however, sarcoidosis often occurs without apparent abnormality on chest radiographs (7). Biopsy of intraocular lesions is not commonly performed in patients with suspected ocular sarcoidosis (1); it is usually performed from a more easily accessible site, such as the lung, skin, or palpable lymph node. Because the lungs or thoracic lymph nodes are involved in up to 90% of patients (2,8), the lung is the preferred biopsy site. Therefore, ophthalmologists often refer patients with suspected ocular sarcoidosis to pulmonologists for diagnostic examination for sarcoidosis.
Bronchoalveolar lavage (BAL) is a relatively safe procedure, but transbronchial lung biopsy (TBLB) is invasive and can have fatal complications in extremely rare cases (9). Considering that sarcoidosis is a benign condition and that most patients with suspected ocular sarcoidosis do not present with respiratory symptoms, it is important to assess whether TBLB can be avoided as a means of diagnosis, especially in patients with risky comorbidities or where consent for TBLB cannot be obtained. Although international criteria for the diagnosis of ocular sarcoidosis1 using intraocular signs or investigational tests have been developed for use by ophthalmologists, a method of investigation of the lung for definitive diagnosis of sarcoidosis in patients with suspected ocular sarcoidosis, as well as information about the results of systemic examinations for sarcoidosis and its natural history, is lacking.
We prospectively conducted a systematic survey for sarcoidosis in patients with suspected ocular sarcoidosis using examinations available worldwide. This study aims to investigate the diagnostic value of each examination for sarcoidosis and to propose a method to survey patients with suspected ocular sarcoidosis.
Methods
From March 2000 to August 2012, patients suspected to have ocular sarcoidosis by ophthalmologists at Komaki City Hospital, a territorial referral hospital, were prospectively evaluated by systematic survey, including medical history, chest radiography, high-resolution chest CT, serum angiotensin-converting enzyme (ACE), BAL, and TBLB. Patients who did not undergo all above-mentioned examinations were excluded. Institutional Review Board of Komaki City Hospital approved this study and all patients included in the survey provided written informed consent.
Patient characteristics, namely, age, sex, smoking history, respiratory symptoms, and ocular lesion classification (10), were recorded. Chest radiographs and chest CT scans were evaluated by two or more experienced pulmonologists and radiologists. Chest radiographs were graded by the modified Scadding classification (11), and abnormal features on chest CT consistent with sarcoidosis were classified into hilar lymphadenopathy, mediastinal lymphadenopathy, or pulmonary lesion. The lymphadenopathy was defined by its apparent size. Serum ACE was measured by the ELISA method, and a positive value was defined as greater than 21.4 IU/L.
Initial direct observation using bronchofiberscopy (BF) was performed, followed by BAL and then TBLB. BAL was carried out in the right middle lobe or lingula by injection of 50 mL of sterile saline 3 times, and a recovery ratio of less than 30% was excluded. In recovered lavage fluid, lymphocytosis and elevated CD4/CD8 ratio were defined as a lymphocyte count of greater than 15% of cell differential count and CD4/CD8 ratio greater than 3.5, respectively. TBLB was performed more than two times in each of the upper and lower lobes.
The diagnosis of sarcoidosis was pathologically established by the presence of non-caseating granulomas, with negative acid-fast bacterium and fungus cultures.
During the follow-up period, newly developing lesions due to sarcoidosis and changes in initial diagnosis were recorded.
The results for patients with and without sarcoidosis were compared using the chi-square test or Fisher’s exact test for categorical variables and Student’s t-test or Mann-Whitney’s U test for continuous variables. Analyses were performed using StatView® version 5.0 (SAS Institute Inc., Cary, NC, USA) and a P value of <0.05 was considered statistically significant. Diagnostic values of each examination were expressed as sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Based on these results, a flow chart for examining patients with suspected ocular sarcoidosis was proposed.
Results
Of 51 patients undergoing examination for sarcoidosis, 42 patients were included, of whom 27 (64.3%) were diagnosed with biopsy-proven sarcoidosis by TBLB. Nine patients do not undergo all examinations due to patients’ refusal.
Patient characteristics are shown in Table 1, divided into with- and without-sarcoidosis groups. Female gender was dominant (71.4%); age was 56.2±14.8 years (mean ± SD), and no respiratory symptoms were recorded in any patient. All patients suffered from uveitis, with anterior uveitis being the most common and secondary glaucoma seen in 9.5% of patients. No significant difference was detected among patient characteristics and ocular findings. Extrapulmonary lesions (all of which were skin lesions) were recorded at diagnosis in 6 patients (22.2%) in the sarcoidosis group.
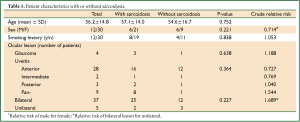
Full table
The results of each examination are presented in Table 2. No findings were seen on chest radiography in 71.4% of patients. All patients with sarcoidosis had some abnormalities on chest CT, while 59.2% of those patients had a normal chest X-ray. Chest CT revealed some features consistent with sarcoidosis, the most common finding being small plural mediastinal lymphadenopathy without fusion. In 19.0% of patients, pulmonary lesions were detected, all of which were multiple small nodules predominantly in the upper lobes, and most were undetectable on chest radiography. In the sarcoidosis group, the percentage of patients with negative findings on radiography and positive findings on CT was 74.2%.
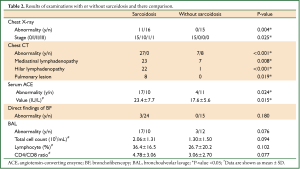
Full table
In patients with negative findings on radiography or CT, TBLB results were positive in 50.0% and 55.9% of patients, respectively.
Direct findings of BF, all of which were network vascularization, were revealed in 7.1% of all patients. BAL findings were available in all 42 cases, and no significant difference was detected. A complication of a small pneumothorax caused by TBLB was recorded in 1 patient.
With regard to both its value and the number of patients in whom results were positive, serum ACE was significantly greater in the sarcoidosis group.
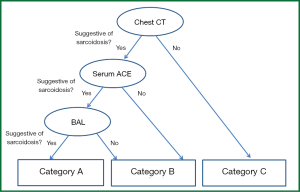
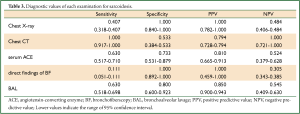
The diagnostic value of each examination is shown in Table 3. Chest CT shows low specificity and very high sensitivity, whereas chest radiography and direct findings of BF show the opposite: high specificity and low sensitivity. To make practical use of these values, a flow chart for examination of patients with suspected ocular sarcoidosis was proposed (Figure 1). Direct findings of BF were excluded because of the potential for lack of objectivity on the part of those interpreting the results. Category C reflects very low clinical probability of sarcoidosis; category A reflects a high clinical probability. Therefore, TBLB can be avoided in categories A and C. Application of this flow chart to our patients showed that the prevalence of sarcoidosis was 100.0%, 66.7%, and 0.0% in categories A, B and C, respectively (Table 4), and the requirement for TBLB was reduced to 50.0%.
Full table
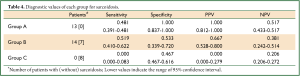
Full table
Follow-up was performed at a median of 51 months (range, 3-145 months). In 7 patients from the sarcoidosis group (25.9%), newly developing lesions were reported (heart, 2; skin, 2; skeletal muscle, 1; and peripheral nerve, 1; skeletal muscle and heart, 1). No patient in the non-sarcoidosis group was diagnosed with sarcoidosis after the first survey.
Discussion
Sarcoidosis is a systemic granulomatous disease with unknown etiology frequently affecting the lung, eye, skin, and liver. The lungs and thoracic lymph nodes are the most common sites, with a reported prevalence of 90% (2,8). Among sarcoidosis patients, the most common (54.8%) extrapulmonary manifestation is eye involvement, which has recently increased in Japan (6). Previous studies have shown that 1.5-12.4% of sarcoidosis patients have eye lesions at first presentation (3-5). Therefore, systemic survey, including pathological examination, is required for patients with suspected ocular sarcoidosis to rule out sarcoidosis as well as other diseases causing uveitis, such as tuberculosis, Vogt-Koyanagi-Harada disease, and Behçet disease. Because of the risk involved in ocular biopsy (1), this procedure is rarely performed for patients with suspected ocular sarcoidosis; where there is no involvement of skin or surface lymph nodes, TBLB has often been performed because of its effectiveness and relative safety.
TBLB has been reported to have a high detection rate for non-caseating granuloma, even in patients without pulmonary lesion: 42.7% and 84.2% of stage 0 and I on chest radiography, respectively (7); 61.7% of stage 0 (12); and 43% of stage 0 and I (13), similar to the findings of this study. However, TBLB is an invasive procedure with potentially fatal complications; 0.1% mortality and 6.8% major complications have been reported (9). TBLB performed for detecting sarcoidosis has been also reported to result in pneumothorax in 10% of patients without pulmonary lesions (13). Therefore, pulmonologists should restrict indications for TBLB, if possible. Although some studies have retrospectively presented results of surveys in suspected ocular sarcoidosis (11,14-17), none, unfortunately, has recommended a method by which a survey can proceed to diagnose sarcoidosis.
We performed a prospective survey of 42 patients with suspected ocular sarcoidosis. Patient characteristics and type of ocular lesions did not differ significantly for presence of sarcoidosis. For diagnosing sarcoidosis, the sensitivity of chest CT and the specificity of chest radiography and direct findings of BF were very high. Serum ACE and BAL did not have high diagnostic value. Combining these examinations, we proposed a flow chart for diagnosing sarcoidosis; using this chart, we could reduce the requirement for TBLB to half in our population. In general, pathological examinations should be performed in all cases; however, the flow chart is especially effective in patients with risky comorbidities or from whom consent for TBLB cannot be obtained. During the follow-up period, one-fourth of the sarcoidosis group developed other lesions from sarcoidosis; therefore, regular systemic survey is required for patients with ocular sarcoidosis. To our knowledge, no study has prospectively evaluated a pulmonary survey for sarcoidosis and recommended a method by which sarcoidosis can be diagnosed in patients with suspected ocular sarcoidosis.
In our study, no differences were detected in patient characteristics and ocular lesions. It is apparently simply because of sample size that all ocular lesions were found to be uveitis in this population and no other ocular lesion type, such as optic neuritis or lacrimal gland swelling, was found, although uveitis is the most common type of ocular sarcoidosis (2,10). The most common cause of intraocular inflammatory disease is sarcoidosis, with a Japanese national survey of 3,060 patients reporting a 13.3% incidence of sarcoidosis (18). Consistent with findings of other studies, the anterior or bilateral type was dominant (3,14).
We found chest radiography to have low sensitivity and high specificity and chest CT to have high sensitivity and low specificity, similar to previous studies. Although some studies mention risk of radiation exposure due to CT in sarcoidosis surveys (19) and the statement on sarcoidosis by ATS/ERS/WASOG advises against chest CT for every patient (2), chest CT appears to be useful for all patients with suspected ocular sarcoidosis without BHL because of its very high NPV. In this study, the incidence of patients with features consistent with sarcoidosis only on chest CT was 74.2%; the reported incidence ranges from 64.7% to 90.9% (15-17,20). As mentioned above, chest CT is deemed necessary for survey unless BHL is detected on radiography.
Despite not having a high diagnostic value, serum ACE, as has previously been described (2), was helpful in our survey.
The values of lymphocytes and CD4/CD8 ratio in BAL fluid did not differ significantly between groups. Lymphocytosis in BAL among patients with uveitis causing non-sarcoidosis groups has previously been reported as 26.4% (21), and in other extrapulmonary granulomatosis, lymphocytosis in BAL has been reported as subclinical alveolar lymphocytosis (22), indicating the low specificity of BAL. The high sensitivity of BAL is apparently reflected in the fact that lymphocytosis in BAL is related to highly positive TBLB in sarcoidosis (12,13). The CD4/CD8 ratio is also described to have high specificity (2). Consequently, we regarded both lymphocytosis and elevated CD4/CD8 ratio as positive findings of BAL.
During the follow-up period, despite no change in initial diagnosis, new extrapulmonary lesions from sarcoidosis developed in one-fourth of patients in the sarcoidosis group. This reconfirms that a regular systemic survey should be performed in patients with ocular sarcoidosis as well as in those with systemic sarcoidosis. In this study, the heart was the most affected organ, and this is one of the features of Japanese sarcoidosis (6).
A flow chart was proposed using widely available examinations in clinical settings; using this chart, we could decrease the requirement of TBLB to half in our population. However, invasive biopsy, such as TBLB, should be performed promptly for patients with clinical diagnosis of sarcoidosis if the clinical course becomes atypical compared to that of sarcoidosis. In addition, regular survey may be needed in patients without sarcoidosis because the presenting symptoms of sarcoidosis may gradually become obvious, and because TBLB may yield false negative results.
Recently, Kawaguchi et al. (16) reported the diagnostic values of examinations used in surveys of ocular sarcoidosis. Two or more positive results in 5 noninvasive examinations indicate clinical sarcoidosis with a sensitivity and specificity of 83.9% and 97.7%, respectively. Moreover, five typical ocular features of sarcoidosis had different diagnostic values. This survey for suspected ocular sarcoidosis can be carried out only by an ophthalmologist but should be added to examinations for the lung, such as TBLB and BAL, because the lung is the most affected organ. In addition, follow-up and regular survey of patients with ocular sarcoidosis by a pulmonologist is clinically important because of the presence of a systemic disease most frequently involving the lung; however, no systematic recommendation for management of patients with suspected ocular sarcoidosis has been available to pulmonologists.
This study has some limitations. First, the study population is small despite the prospective nature of the study. Our flow chart must be validated. Second, the possibility of false negative findings with TBLB cannot be excluded. However, in this study, the long follow-up period revealed no additional patients with sarcoidosis, and 4 lung biopsies have previously been described as sufficient in a survey for pulmonary sarcoidosis (23). Endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration has been shown to be a safe and valuable procedure for investigating thoracic lymphadenopathy (24); unfortunately, EBUS is available only in a limited number of institutions. Third, most of our study population did not receive the recently advocated ophthalmological survey (1), so the flow chart’s systematic ocular survey seemed to be ideal. Fourth, we did not evaluate other examinations, such as 67Ga-scintigraphy and tuberculin skin test (TST). 67Ga-scintigraphy is nonspecific and very expensive. A negative TST result provides no clinical information in countries without widespread vaccination of BCG; inversely, positive results may suggest the possibility of tuberculosis accompanied by granulomatous uveitis or thoracic lymphadenopathy (8). Serum and urinary calcium measurement also has low diagnostic value; the reported incidences of hypercalcemia and hypercalciuria in sarcoidosis are 10% and 30%, respectively (8).
In summary, we prospectively evaluated a survey for sarcoidosis in patients with suspected ocular sarcoidosis and proposed a flow chart of clinical examinations to reduce invasive procedures. The development of a more comprehensive flow chart, including indication for EBUS as a new procedure, and an established survey of ocular findings are warranted.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Herbort CP, Rao NA, Mochizuki M, et al. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm 2009;17:160-9.
- Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J 1999;14:735-7.
- Rizzato G, Angi M, Fraioli P, et al. Uveitis as a presenting feature of chronic sarcoidosis. Eur Respir J 1996;9:1201-5.
- Foster S. Ocular manifestations of sarcoidosis preceding systemic manifestations. In: Grassi C, Rizzato G, Pozzi E. eds. Sarcoidosis and other granulomatous disorders. Amsterdam: Elsevier, 1988:177-81.
- Rothova A, Alberts C, Glasius E, et al. Risk factors for ocular sarcoidosis. Doc Ophthalmol 1989;72:287-96.
- Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J 2008;31:372-9.
- Ohmichi M. Histologic diagnosis of sarcoidosis. Nihon Rinsho 2002;60:1759-65.
- O’Regan A, Berman JS. Sarcoidosis. Ann Intern Med 2012;156:ITC5-1, ITC5-2, ITC5-3, ITC5-4, ITC5-5, ITC5-6, ITC5-7, ITC5-8, ITC5-9, ITC5-10, ITC5-11, ITC5-12, ITC5-13, ITC5-14, ITC5-15; quiz ITC5-16.
- British Thoracic Society Bronchoscopy Guidelines Committee, a Subcommittee of Standards of Care Committee of British Thoracic Society. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001;56:i1-21.
- Baughman RP, Lower EE, Kaufman AH. Ocular sarcoidosis. Semin Respir Crit Care Med 2010;31:452-62.
- Judson MA, Baughman RP, Teirstein AS, et al. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:75-86.
- Ohara K, Okubo A, Kamata K, et al. Transbronchial lung biopsy in the diagnosis of suspected ocular sarcoidosis. Arch Ophthalmol 1993;111:642-4.
- de Boer S, Milne DG, Zeng I, et al. Does CT scanning predict the likelihood of a positive transbronchial biopsy in sarcoidosis? Thorax 2009;64:436-9.
- Birnbaum AD, Oh FS, Chakrabarti A, et al. Clinical features and diagnostic evaluation of biopsy-proven ocular sarcoidosis. Arch Ophthalmol 2011;129:409-13.
- Takahashi T, Azuma A, Abe S, et al. Significance of lymphocytosis in bronchoalveolar lavage in suspected ocular sarcoidosis. Eur Respir J 2001;18:515-21.
- Kawaguchi T, Hanada A, Horie S, et al. Evaluation of characteristic ocular signs and systemic investigations in ocular sarcoidosis patients. Jpn J Ophthalmol 2007;51:121-6.
- Chung YM, Lin YC, Liu YT, et al. Uveitis with biopsy-proven sarcoidosis in Chinese--a study of 60 patients in a uveitis clinic over a period of 20 years. J Chin Med Assoc 2007;70:492-6.
- Goto H, Mochizuki M, Yamaki K, et al. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol 2007;51:41-4.
- Birnbaum AD, Fagan BM, Tessler HH, et al. Risks of computerized tomography in the evaluation of chronic uveitis. Am J Ophthalmol 2005;139:951-2; author reply 952.
- Kaiser PK, Lowder CY, Sullivan P, et al. Chest computerized tomography in the evaluation of uveitis in elderly women. Am J Ophthalmol 2002;133:499-505.
- Jouveshomme S, Fardeau C, Finet JF, et al. Alveolar lymphocytosis in patients with chronic uveitis: relationship to sarcoidosis. Lung 2001;179:305-17.
- Wallaert B, Dugas M, Dansin E, et al. Subclinical alveolitis in immunological systemic disorders. Transition between health and disease? Eur Respir J 1990;3:1206-16.
- Gilman MJ, Wang KP. Transbronchial lung biopsy in sarcoidosis. An approach to determine the optimal number of biopsies. Am Rev Respir Dis 1980;122:721-4.
- Oki M, Saka H, Kitagawa C, et al. Prospective study of endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes versus transbronchial lung biopsy of lung tissue for diagnosis of sarcoidosis. J Thorac Cardiovasc Surg 2012;143:1324-9.