Percutaneous imaging-guided cryoablation for lung cancer
Introduction
Lung cancer is the most common major cancer in the world and the leading cause of cancer-related mortality, accounting for 1.59 million deaths every year (1). Treatment intention in lung cancer patients varies with the stage of disease. Curative treatment such as surgical resection with lobectomy (2), with or without adjuvant/neoadjuvant chemotherapy, is usually the best choice for early-stage cancer. On the other hand, advanced-stage disease is usually treated with palliative chemotherapy and radiation, which provides 1-year survival of only 15–35% (3).
Lung cancer is an insidious, smoking-related disease, and most patients are not suitable candidates for surgery, either due to the presence of advanced-stage disease or because of compromised cardiopulmonary function (4). Chemotherapy and radiotherapy are the traditional choices for these patients. Various ablative techniques have also been applied for this category of patients in the past. Percutaneous cryoablation under imaging guidance has been proved to be a safe and effective method for ablating and debulking tumors, and can provide radical cure or palliation, as the case may be, for patients with different stages of disease (5,6). In this paper we review the technique of imaging-guided cryoablation and review the studies being conducted at various centers, focusing on the recent advances.
Technique and mechanism
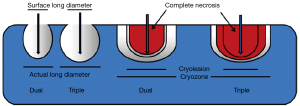
Percutaneous cryoablation of tumors in lung is usually carried out under CT guidance, with sedation plus local anesthesia. The commonly applied argon-based cryoablation system is able to generate temperatures as low as −140 °C (7). As the temperature falls into the freezing range, ice crystals begin to form in the extracellular fluid, which leads to cell dehydration. With further cooling, intracellular ice forms, disrupting cell membranes and organelles and threatening cell viability. Vascular stasis and thrombosis caused by cold injury also play a role in the mechanism of cell damage during cryoablation (8). The degree of cold-induced cytotoxicity depends on thermal parameters such as the cooling rate, end temperature, hold time, and thawing rate. Factors that aggravate cellular injury include rapid freezing, lower temperature, longer time at the minimum temperature, slow thawing, and repeated freeze-thaw cycles (8,9). The number of freeze–thaw cycles applied differs in clinical practice and also in the studies that have been conducted to date, with double or triple cycles being most commonly adopted. It has been demonstrated that when cryozones (actual destruction zones on pathology) and cryolesions (zones of destruction observed by the naked eye) are similar, triple-freeze protocols induce more necrosis (10) (Figure 1). Thus, a triple-freeze protocol may be best for pulmonary cryoablation as it can help decrease the risk of tumor recurrence.
Imaging guidance and evaluation
One advantage of cryoablation, as compared to other thermal ablative methods, is that good visualization is possible under computed tomography (CT) guidance (5). However, accurate imaging evaluation after cryoablation is challenging during postoperative follow-up, when both a residual mass and an ablation zone are present. According to various reports, ablation zones enlarge significantly on day 0 with significant decrease in size being obvious at 1 month after ablation. Rapid size reduction is recorded during 1–3 months and slows down after 6 months. According to Ito et al. (11), after cryoablation, the ablation zone shows five different patterns on CT imaging: i.e., consolidation/atelectasis, nodular pattern, stripe pattern, pleural thickening, and disappearance of lesion of these, consolidation or the nodular pattern are the commonest; atypical shape transformation indicates local progression (11). The majority of local progression cases arose from an atypical shape transformation and later turn into the stripe pattern after 6-month’s follow-up, thus cases should receive special attention and close follow-up when ablation zone reverses from stripe pattern to nodular pattern.
Cryoablation in early-stage lung cancer
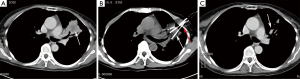
Cryoablation offers a relatively safe option for patients with medically inoperable stage I lung cancer, in whom treatment intention should be to achieve radical cure (Figure 2). Several studies have focused on efficacy and safety issues in these patients and reported local control and survival rates.
Moore et al. (12) retrospectively analyzed survival and recurrence in 45 patients with medically inoperable T1N0M0 NSCLC who were treated with cryoablation. The 5-year overall survival (OS) rate, cancer-specific survival rate, and progression-free survival rate were 67.8%±15.3%, 56.6%±16.5%, and 87.9%±9%, respectively. The local and regional recurrence rates combined was 36.2%. Major complications included hemoptysis and need for prolonged pleural effusion drainage. No death occurred in the first 30 days after ablation.
In 2010, Zemlyak and colleagues (13) compared survival between stage I NSCLC patients treated with cryoablation, radiofrequency ablation (RFA), and sublobar resection (SLR). In their study, 64 high-risk patients (defined as those deemed medically unfit for standard resection according to American College of Surgeons Oncology Group/NIH inoperability criteria) received one of the treatments mentioned above. The 3-year OS rates were 77%, 87.5%, and 87.1% (P>0.05), respectively, in the three groups. The 3-year cancer-specific survival rates in the three groups were 90.2%, 90.6%, and 87.5%, respectively.
Yamauchi et al. (14) performed CT-guided percutaneous cryoablation in 22 patients with medically inoperable stage I NSCLC; in all, 34 tumors were treated. After median follow-up of 23 months (range, 12–68 months), local tumor progression was detected in only 1 of the 34 (3%) tumors (this occurred 8 months after cryoablation). The mean local tumor progression-free interval was 69±2 months. Of the 22 patients, 3 died; 1 patient died at 68 months due to lung cancer progression. The 2- and 3-year OS rates were 88% and 88%, respectively, and the disease-free survival was 78% and 67%, respectively.
Studies conducted in recent years show that CT-guided percutaneous cryoablation of early-stage lung cancer has a low local failure rate; moreover, the OS is comparable to that achieved with sublobar resection and RFA. In view of these findings, and the minimal invasiveness of the procedure, percutaneous cryoablation is a reasonable choice for unresectable early-stage lung cancer patients.
Palliative cryoablation of advanced-stage lung cancer
Cryoablation in advanced-stage lung cancer can achieve tumor debulking and provide symptom relief. Tumors are often large and/or adherent to adjacent organs and tissues and therefore percutaneous cryoablation cannot always guarantee complete ablation. However, the efficacy of cryoablation has been confirmed by recent studies.
Ismail reported a single center experience with cryoablation of advanced-stage (IIIB/IV) NSCLC patients (15). Of the 72 patients treated over a 5-year span, 71 patients received CT-guided cryoablation and 1 received video-assisted thoracoscopic surgery (VATS). Tumor size and location varied. In 9 patients, cryoablation covered over 90% of the cancer mass, while in 63 patients, it covered 60–90% of the tumor mass.
Niu and colleagues retrospectively analyzed the efficacy of imaging-guided cryoablation in stage IV lung cancer patients (6). Of the 54 patients enrolled in their study, 31 received comprehensive cryoablation of both primary and metastatic tumors, and 23 received other palliative treatments. After 6.5 years’ follow-up, the OS in the cryoablation group was significantly better than that in the other group (median OS: 14 vs. 7 months; P<0.01). In subgroup analysis, multiple cryoablation sessions (cryoablation was administered 2 times in 12 patients, 3 times in 5 patients, and 4 times in 1 patient) was shown to play an important role in improving OS (median OS: 7 vs. 14 months; P<0.05).
In another study conducted at the same center, 840 NSCLC patients with various stages of disease were enrolled; the OS in this group ranged from 5 to 61 months (16). The 1-, 2-, 3-, 4-, and 5-year OS rates were 68%, 52%, 34%, 26%, and 21%, respectively. The 1- and 2-year OS of stage IIIB/IV lung cancer patients treated at this center since 2008 is 58% and 48%, respectively.
Li et al. investigated the long-term efficacy and risk factors of percutaneous cryoablation in 253 advanced-stage lung cancer patients (17). Median survival was for 11.98 months, and the 1- and 2-year OS rates were 41.1% and 27.6%. Multivariate analysis showed that tumor staging, tumor size and location, and combination chemotherapies were significantly associated with the prognosis. Another study conducted at the FUDA Cancer Hospital revealed that female gender, stage of disease, previous treatment, and followed chemotherapies were predictors of better survival (18).
A meta-analysis of 44 studies (19) assessed survival and quality of life in intermediate- and advanced-stage NSCLC patients receiving cryoablation. The results showed that cryoablation can improve the quality of life in this group of patients, and that chemotherapy or radiotherapy do not have any clinical advantages over cryoablation alone.
Emerging application: cryoablation in ground-glass nodules (GGNs)
The correct treatment of pure GGNs in the lungs is still controversial. Some surgeons prefer to closely follow-up cases and operate only when there is significant change in size or the appearance of pure GGN (20), while others advocate immediate surgery (21). However surgery is not suitable for patients with limited pulmonary reserve. In such patients, treatment with minimally invasive percutaneous imaging-guided cryoablation may offer a safer solution. Kim and colleagues (22) reported a case where a 5-mm-sized pure GGN was treated successfully with cryoablation, without recurrence after 6 months of follow-up. No large-scale study has been conducted till date, but given the continuing rapid advances in high-resolution imaging technology and the consequent increase in the detection of GGNs, cryoablation is likely to find greater application in the treatment of these lesions.
Complications
According to various studies, percutaneous cryoablation has a low procedure-related mortality rate, and complications are usually mild and self-limiting. The most common complication is pneumothorax, which occurs in 12–62% of patients, with 0–12% of these patients having collapse of ≥30% of lung and requiring chest tube insertion (23). Use of more numbers of cryoprobes and larger diameter of cryoprobes is related to higher risk of pneumothorax during cryoablation. With the development of finer cryoprobes (from 11 G, diameter 3.0 mm to 17 G, diameter 1.47 mm), the pneumothorax rate has been decreasing in recent years. Other complications are hemoptysis, pleural effusion, cough, skin injury, arm paresis, and tumor implantation (7). Serious complications included cardiac arrest and hemopneumothorax, therefore preventative steps should be taken. Thus, CT guided percutaneous lung cryotherapy yielded lower procedural-related morbidity.
Conclusions
The last several years has seen increasing clinical application of percutaneous cryoablation in lung cancer patients. Several studies have also been conducted. Percutaneous imaging-guided cryoablation of lung cancer is a minimally invasive procedure that is suited for curative treatment of medically inoperable early-stage lung cancer and for tumor debulking in advanced-stage lung carcinoma. The local control is high, and the complications are usually controllable; treatment-related is death rarely seen. However, there have been no large randomized controlled studies and therefore its efficacy, especially in advanced-stage lung cancer, is still not established. Researchers also need to examine the application of cryoablation in GGNs and its use in combination with standard chemotherapy.
Acknowledgements
Funding: This work was supported by International Scientific Fund of Fuda Cancer Hospital, Guangzhou (Y2016-ZD-002).
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 415-6. [Crossref] [PubMed]
- Inoue M, Nakatsuka S, Yashiro H, et al. Percutaneous cryoablation of lung tumors: feasibility and safety. J Vasc Interv Radiol 2012;23:295-302. [Crossref] [PubMed]
- Niu L, Chen J, Yao F, et al. Percutaneous cryoablation for stage IV lung cancer: a retrospective analysis. Cryobiology 2013;67:151-5. [Crossref] [PubMed]
- Niu L, Xu K, Mu F. Cryosurgery for lung cancer. J Thorac Dis 2012;4:408-19. [PubMed]
- Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998;37:171-86. [Crossref] [PubMed]
- Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology 2002;60:40-9. [Crossref] [PubMed]
- Niu L, Li J, Chen J, et al. Comparison of dual- and triple-freeze protocols for pulmonary cryoablation in a Tibet pig model. Cryobiology 2012;64:245-9. [Crossref] [PubMed]
- Ito N, Nakatsuka S, Inoue M, et al. Computed tomographic appearance of lung tumors treated with percutaneous cryoablation. J Vasc Interv Radiol 2012;23:1043-52. [Crossref] [PubMed]
- Moore W, Talati R, Bhattacharji P, et al. Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol 2015;26:312-9. [Crossref] [PubMed]
- Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg 2010;211:68-72. [Crossref] [PubMed]
- Yamauchi Y, Izumi Y, Hashimoto K, et al. Percutaneous cryoablation for the treatment of medically inoperable stage I non-small cell lung cancer. PLoS One 2012;7:e33223. [Crossref] [PubMed]
- Ismail D. 128P: Cryosurgery for advanced stages of non small cell lung cancer (5 years experience). J Thorac Oncol 2016;11:S111. [Crossref] [PubMed]
- Niu L, Xu K, He W, et al. Percutaneous Cryoablation for patients with advanced non-small cell lung cancer. Technol Cancer Res T 2007;6:451-2.
- Li Y, Feng H, Nie Z, et al. The long-term effects and risk factors analysis in 253 cases advanced non-small cell lung cancer treated with percutaneous cryosurgery. Chinese Clinical Oncology 2010;15:346-9.
- Niu LZ, Xu KQ, Mu F, et al. Impact of percutaneous cryotherapy on survival of advanced lung cancer with or without chemotherapy Low Temp Med 2010;36:41-6.
- Du XF, Han BS, Li TZ. Effect of argon-helium cryoablation on intermediate and advanced non-small cell lung cancer: a meta-analysis. J Chin PLA Postgrad Med Sch 2010;31:714-7.
- Nakajima R, Yokose T, Kakinuma R, et al. Localized pure ground-glass opacity on high-resolution CT: histologic characteristics. J Comput Assist Tomogr 2002;26:323-9. [Crossref] [PubMed]
- Park JH, Lee KS, Kim JH, et al. Malignant pure pulmonary ground-glass opacity nodules: prognostic implications. Korean J Radiol 2009;10:12-20. [Crossref] [PubMed]
- Kim KY, Jin GY, Han YM, et al. Cryoablation of a small pulmonary nodule with pure ground-glass opacity: a case report. Korean J Radiol 2015;16:657-61. [Crossref] [PubMed]
- Inoue M, Nakatsuka S, Jinzaki M. Cryoablation of early-stage primary lung cancer. Biomed Res Int 2014;2014:521691.


