Patient-prosthesis mismatch: surgical aortic valve replacement versus transcatheter aortic valve replacement in high risk patients with aortic stenosis
A recent study by Zorn and colleagues evaluated the incidence and significance of patient prosthesis mismatch (PPM) in high risk patients with severe aortic stenosis who were randomized to transcatheter aortic valve replacement (TAVR) or surgical aortic valve replacement (SAVR) (1). The objective of our manuscript is to provide perspective on this contemporary study. PPM can occur when a prosthetic aortic valve has an effective orifice area (EOA) less than that of a native valve (2). Although the clinical significance of PPM has been frequently debated, surgeons assiduously attempt to avoid moderate or severe PPM following SAVR by implantation of an appropriate size valve in relation to the patient’s size (3). Because bioprosthetic and mechanical aortic valve leaflets are mounted into prosthetic space-occupying frames, EOA is slightly decreased which may contribute to PPM, especially in small aortic roots and larger patients.
The management of aortic stenosis is rapidly evolving with the development of TAVR. The design of TAVR valves is radically different than traditional SAVR valves. TAVR valves are mounted in a nitinol stent and lack a sewing ring, which is hypothesized to increase EOA and decrease risk for PPM. In 2014, Pibarot and colleagues evaluated the influence of PPM on outcomes in the Placement of Aortic Transcatheter Valve (PARTNER) Trial in high risk surgical patients who received the balloon expandable Edwards Sapien (Edwards Lifesciences, Irving, CA, USA) valve (4). They found that the incidence of severe PPM was greater in SAVR versus TAVR patients (28.1% vs. 19.7%; P<0.001), with increased 2-year mortality in SAVR patients with severe PPM (HR =1.78; P=0.04). Furthermore they found that severe PPM was more likely to develop in patients with aortic annulus <20 mm and concluded that TAVR may be preferable to SAVR in patients with small aortic annulus to avoid PPM. Although still developing, two TAVR valves have been most widely utilized: the balloon expandable Edwards Sapien valve and the self-expanding Medtronic CoreValve (Medtronic, Minneapolis, Minnesota). These two valves have differing hemodynamic profiles, with the CoreValve possessing a supra-annular and funnel shape design that may further increase EOA and lower gradient (5).
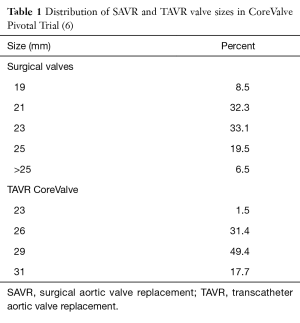
Zorn and colleagues recently evaluated the incidence of PPM in SAVR (n=353) and TAVR (n=389) patients in the CoreValve US High Risk Pivotal Trial (1,6). PPM was stratified as moderate when EOA index was between 0.65 and 0.85 cm2/m2 and severe when EOA index was ≤0.65 cm2/m2. Similar to the Sapien Valve, the CoreValve was associated with decreased incidence of severe PPM compared to SAVR (6.2% vs. 25.7%; P<0.0001). The incidence of moderate or greater aortic insufficiency (AI) was 5.1% in TAVR and 0.8% in SAVR patients. The distribution of SAVR and TAVR valve sizes utilized in this study is delineated in Table 1. In this patient cohort, 40.8% of patients received SAVR valves ≤21 mm, similar to the 38.2% rate in a recent study of the Society of Thoracic Surgeons (STS) database of 141,905 SAVR patients (7). By comparison, implanted TAVR valves were larger in size with 29 mm being the most common implanted size. Of note, additional surgical procedures for SAVR patients included aortic root replacement (2.2%), aortic root enlargement (0.6%), septal myomectomy (0.3%), and mitral valve replacement (0.3%) (6).
In this study, few predictive risk factors for development of PPM were identified. Higher body mass index was noted in patients with severe PPM for both TAVR (31.9 vs. 27.8 kg/m2; P=0.002) and SAVR (30.4 vs. 28.0 kg/m2; P=0.013). TAVR patients with severe PPM had smaller preoperative EOA index (0.32 vs. 0.40; P=0.0005) and smaller aortic annulus (2.07 vs. 2.23 cm; P=0.0006). SAVR patients with severe PPM also had smaller preoperative EOA index (0.36 vs. 0.41; P=0.01), but no difference in aortic annulus size (2.14 vs. 2.19; P=0.068). The study did not evaluate the relationship of implanted prosthesis size and development of PPM.
Similar to prior SAVR studies, severe PPM in the CoreValve Pivotal Trial was associated with increased all-cause mortality (20.6% vs. 12.0%; P=0.0145) (8). Interestingly, there was no relationship in New York Heart Association (NYHA) Classification improvement at 1 year and presence of PPM. In TAVR patients with PPM, 82.5% of patients were NYHA III/IV at baseline, but only 5.3% were NYHA III/IV at 1 year postoperatively. Similarly, in SAVR patients 81.8% of patients were NYHA III/IV at baseline and 5.5% were NYHA III/IV at 1 year.
SAVR was associated with increased left ventricular (LV) remodeling and mass regression compared with TAVR. TAVR patients noted LV mass regression of 6.8% with severe PPM and 5.4% without severe PPM. SAVR patients noted a regression of 15.1% with severe PPM and 15.7% without severe PPM.
The major finding of this recent study by Zorn and colleagues is that the incidence of PPM may be greater with SAVR than TAVR in high risk patients. Similar findings were noted in the PARTNER trial. We believe that this finding is not surprising and largely driven by valve design. Traditional SAVR valves are placed in a stiff space occupying prosthesis, which can be cumbersome to place in small aortas and small annulus. Newer designed SAVR valves, partly based on the TAVR platform, such as the “sutureless” rapid-deployment aortic valves may reduce PPM (9). The adoption of these newer SAVR valves will likely reduce or eliminate any disparity of PPM compared to TAVR valves.
In this study, 40.1% of the patients had a 21 mm or smaller valve placed and 8.5% had a 19 mm valve placed. Although a root enlargement is an effective procedure, it is infrequently performed in “real-world” practice. Our preference is to implant a valve with a predicted EOA index of ≥0.8 and when necessary perform a root enlargement utilizing a Nicks procedure. However, our decision to perform a root enlargement also takes into account the patient’s overall surgical risk and functional status. As the CoreValve Pivotal study was performed in a high risk cohort, there may have been a surgical bias to “settle” for a smaller valve to avoid increased operative risk in this cohort. Based on this study and others, we would advocate for a more vigorous approach to root enlargement in SAVR especially in cases where a 19 mm valve would be placed.
Interestingly, there was no relationship to PPM and NYHA functional status. Although all-cause mortality was higher in PPM patients, it is unclear if PPM was causal as there was no difference in cardiovascular mortality. Future studies could evaluate the role of PPM on patient functional and activity status to better determine clinical significance in this patient population.
Another interesting finding of this study is that LV mass regression was nearly three times greater following SAVR compared to TAVR. The etiology of this is unclear, and the authors point out that cardiac MRI would better quantitate LV mass than echocardiography. This increased favorable LV remodeling may have important consequences in long term LV function, freedom from heart failure, and potentially survival.
In summary, Zorn and colleagues recently evaluated the incidence of PPM after TAVR and SAVR in high risk patients with severe aortic stenosis. TAVR is associated with decreased incidence of severe PPM compared to traditional SAVR valves. Severe PPM increases risk for death at 1 year postoperatively in high risk patients. This study should increase consideration and development of newer SAVR valves to reduce risk for PPM. In addition more vigorous approaches to root enlargement in small annulus should be performed with SAVR to prevent PPM. Future studies should evaluate the effect of LV mass regression following TAVR and SAVR on survival.
Acknowledgements
None.
Footnote
Provenance: This is an invited Perspective commissioned by the Section Editor Hui Xue (The First Affiliated Hospital of Tsinghua University, Beijing, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zorn GL 3rd, Little SH, Tadros P, et al. Prosthesis-patient mismatch in high-risk patients with severe aortic stenosis: A randomized trial of a self-expanding prosthesis. J Thorac Cardiovasc Surg 2016;151:1014-22, 1023.e1-3. [Crossref] [PubMed]
- Rahimtoola SH. The problem of valve prosthesis-patient mismatch. Circulation 1978;58:20-4. [Crossref] [PubMed]
- David TE. Is prosthesis-patient mismatch a clinically relevant entity? Circulation 2005;111:3186-7. [Crossref] [PubMed]
- Pibarot P, Weissman NJ, Stewart WJ, et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort--a analysis. J Am Coll Cardiol 2014;64:1323-34. [Crossref] [PubMed]
- Nombela-Franco L, Ruel M, Radhakrishnan S, et al. Comparison of hemodynamic performance of self-expandable CoreValve versus balloon-expandable Edwards SAPIEN aortic valves inserted by catheter for aortic stenosis. Am J Cardiol 2013;111:1026-33. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Thourani VH, Suri RM, Gunter RL, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg 2015;99:55-61. [Crossref] [PubMed]
- Head SJ, Mokhles MM, Osnabrugge RL, et al. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J 2012;33:1518-29. [Crossref] [PubMed]
- Kocher AA, Laufer G, Haverich A, et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110-5; discussion 115-6. [Crossref] [PubMed]