Comparison of robot-assisted esophagectomy and thoracoscopic esophagectomy in esophageal squamous cell carcinoma
Introduction
Surgical resection remains a standard treatment for esophageal squamous cell carcinoma (ESCC), based on the principles of complete primary tumor removal and radical lymphadenectomy. However, the invasiveness of surgery involving the chest and abdomen and a relatively high postoperative complication rate are major concerns for esophagectomy. To improve outcomes, minimally invasive esophagectomy (MIE) has been gradually accepted as a reliable surgical procedure for esophageal cancer (1,2). Although the procedure is technically demanding it has been suggested that MIE can be performed with low pulmonary complication rates (1,3) and comparable long-term oncological outcomes (4,5).
Most MIEs have been performed using endoscopic techniques utilizing thoracoscopy and laparoscopy. However, recent developments in robotic technology have made robot-assisted esophagectomy (RE) as another surgical option for MIE (6,7). Robotic technology has several technical advantages over the thoracoscopic technique, such as, the free articulation of robotic arms and superior imaging quality, including three-dimensional vision, which are regarded as optimal technologies for radical oncologic surgery. However, the advantages of RE over thoracoscopic esophagectomy (TE) have not been clearly defined, and perhaps as a result, RE has not been widely applied for the treatment of ESCC. The aim of this study was to compare the short-term and long-term outcomes of RE and TE and to identify any clinical or oncological benefits of RE as compared with TE in ESCC.
Methods
Patients
This study was approved by the institutional review board of our hospital and the patients’ consent was waived (approval number: 1407-137-597). The inclusion criterion was the patients who underwent RE or TE between 2006 Jan and 2014 Jun for the treatment of ESCC. The exclusion criteria applied were; (I) the patients who underwent three field lymphadenectomy; (II) the receipt of laparoscopic transhiatal esophagectomy; and (III) the use of the colon as a substitute graft.
During the study period 435 patients underwent esophagectomy in our institute. MIE was performed in 136 patients (31%) during the same period. After applying the above-mentioned criteria, 105 patients (62 in the RE group and 43 in the TE group) were enrolled in the study. Initially the indication for MIE at our institute was limited to early esophageal cancer, but indications were gradually expanded to advanced esophageal cancer. Currently multi-station lymph node metastasis, invasion to adjuvant organs, and severe pleural adhesion are regarded as contraindications for MIE at our institute. The same indications for MIE were applied to both the TE and RE groups.
Surgical technique of RE
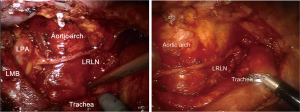
Surgeries were conducted using four-arm technique for thoracic and abdominal procedures and one additional assistant port was made (Figure 1). With a patient positioned in the prone or lateral decubitus position (the prone position was favored for cervical anastomosis and the lateral decubitus for thoracic anastomosis) a camera port was made in the 7th intercostal space just below the scapula tip. Number 1 robotic port was made at the 5th intercostal space at the medial border of scapula, number 2 robotic port was placed at the intersection between the vertical line from number 1 port and the 10th intercostal space. Number 3 robotic port was made in the posterior axillary line in the 3rd intercostal space, and the assistant port was made in the 8th intercostal space at posterior axillary line. Cadiere forceps and robotic scissors were used interchangeably between number 1 and number 3 arms (right arms). Lymph node dissection was performed in whole mediastinal nodal stations. Right and left recurrent laryngeal nerve (RLN) dissections were performed precisely by completely exposing nerves and removing whole lymphatic tissues up to the thoracic inlet and contralateral hilum (Figure 2).

Preoperative evaluation and postoperative follow-up
All patients underwent an intensive preoperative evaluation. Endoscopy, endoscopic ultrasound, chest CT, abdominal CT, PET-CT, cervical ultrasonography, and pulmonary function and blood testing were performed routinely. Bronchoscopy was performed if indicated. For the assessment of RLN injury, vocal cord function was assessed by nasal laryngoscopy on the 3rd postoperative day in all patients. Postoperative surveillance of recurrence was conducted intensively. A PET-CT scan was performed at 1st, 2nd, and 5th years postoperatively and a chest CT scan was performed at 6th months, 18th months, 3rd years, and 4th years postoperatively. Endoscopic examinations were performed annually.
Definition of assessment parameters
Grade of dysphagia was scored from 0 to 4 using the scoring system proposed by Mellow and Pinkas (8). Performance status was graded from 0 to 5 according to the European clinical oncology group performance status scoring system (9). Dissected lymph node locations were classified using three mediastinal groups. Upper mediastinal lymph nodes were defined as 2R (right upper paratracheal nodes), 4R (right lower paratracheal nodes), 2L (left upper paratracheal nodes), 4L (left lower paratracheal nodes), 3P (posterior mediastinal nodes), 5 (aortopulmonary nodes) and lymph nodes along right RLN. Middle mediastinal lymph nodes were defined as 7 (subcarinal nodes), 8M (middle paraesophageal lymph nodes), 10L (left tracheobronchial nodes), and 10R (right tracheobronchial nodes), and lower mediastinal lymph nodes as 8L (lower paraesophageal lymph nodes), 9 (pulmonary ligament nodes), and 15 (diaphragmatic nodes). Postoperative morbidity was prospectively recorded during bi-monthly morbidity conferences and severities of complications were graded using the Clavien-Dindo classification (10).
Statistical methods
The student’s t-test or Wilcoxon’s rank-sum test were used to compare continuous group variables, depending on normality of distribution. The Chi-square test or Fishers’ exact test were used to compare categorical variables. Survival was estimated using the Kaplan-Meier method and the significances of differences were determined using the log-rank test. All statistical tests were two-sided and SPSS software (version 21, IBM, Armonk, NY, USA) was used throughout. Statistical significance was accepted for P values <0.05.
Results
Preoperative characteristics
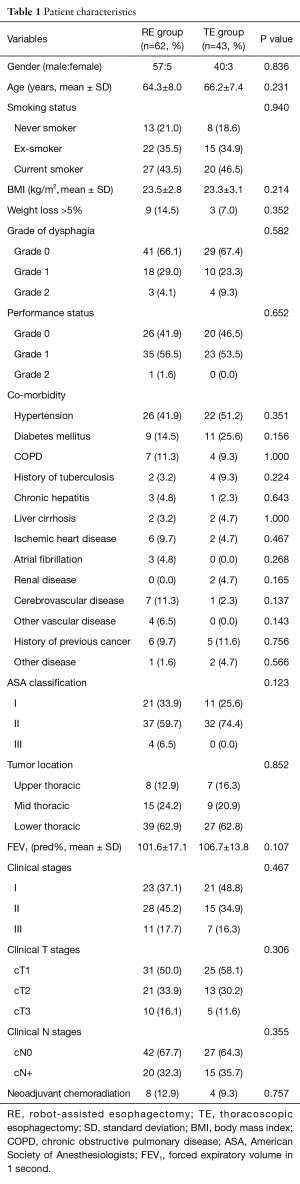
Demographic and preoperative features were comparable in the RE and TE groups (Table 1). Asymptomatic patients in whom ESCC was incidentally detected during endoscopic screening constitute 66% in the RE group and 67% in the TE group. The co-morbidity rate was significantly high in both groups and 66% of the RE group and 74% of the TE group had an American Society of Anesthesiologists (ASA) class of more than II.
Full table
Operation
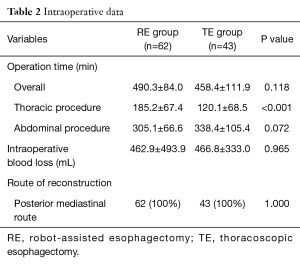
For thoracic procedures, robotic and thoracoscopic surgery were performed in all 105 patients. For abdominal procedures, robot-assisted surgery was performed in 36 patients (58%) in the RE group and laparoscopic procedures were performed in 21 patients (49%) in the TE group and the proportion of laparotomy was comparable in the two groups. Cervical anastomosis was performed in 56 patients (90%) in the RE group and in 35 patients (81%) in the TE group (P=0.186). The posterior mediastinal route was employed in all patients. Conversion to thoracotomy was necessary in one patient in each group. Total operation time was not different between the two groups (490.3±84.0 minutes in the RE group vs. 458.4±111.9 minutes in the TE group; P=0.118). However, one-lung ventilation time, which represent the time required for the main thoracic procedure was significantly greater in the RE group (185.2±67.4 minutes vs. 120. ±68.5 minutes; P<0.001). Intraoperative blood loss amounts were not different between the two groups (462.9±493.9 mL in the RE group vs. 466.8±333.0 mL in the TE group; P=0.965) (Table 2). Complete resection rates were also comparable (98% in the RE group vs. 98% in the TE group; P=1.000).
Full table
Pathology and lymph node yields
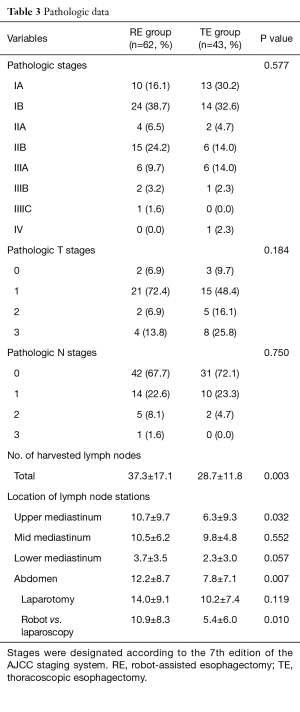
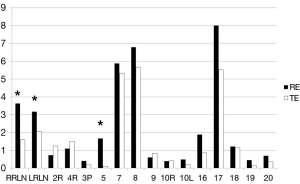
The distributions of pathologic stages in the two groups were comparable (Table 3). The mean number of dissected lymph nodes was significantly greater in the RE group (37.3±17.1 in the RE group vs. 28.7±11.8 in the TE group; P=0.003). To examine this difference in further detail, we classified lymph node stations into four categories, that is, upper mediastinum, middle mediastinum, lower mediastinum, and abdomen. A significant intergroup difference was evident in upper mediastinum (10.7±9.7 in the RE group vs. 6.3±9.3 in the TE group; P=0.032). For abdominal lymph node dissection, the mean number of lymph nodes dissected by the robot was greater than that by laparoscopy (10.9±8.3 in the RE group vs. 5.4±6.0 in the TE group; P=0.010). Numbers of dissected lymph nodes per station are plotted in Figure 3. The difference was evident in lymph nodes of both RLNs and number 5.
Full table
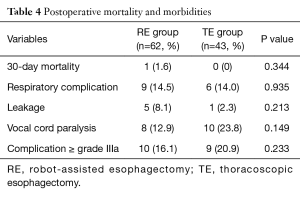
Early outcomes (Table 4)
One 30-day mortality occurred in the RE group (1.6%), but no 30-day mortality occurred in the TE group. The incidences of respiratory complications and anastomosis leakages were not different. The incidence of vocal cord paralysis was lower in the RE group, however the difference was not statistically significant (13% vs. 24%; P=0.149). The incidence of major complication more than grade IIIa according to Clavien-Dindo classification was not different between the two groups (16% vs. 21%; P=0.233).
Full table
Long-term outcomes
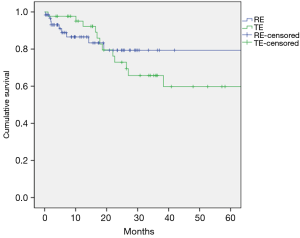
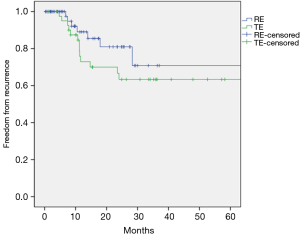
The median follow-up duration was 22 months (17 months in RE group and 26 months in TE group). The 5-year overall survivals were not different between the two groups (69% in the RE group vs. 59% in the TE group; P=0.737; Figure 4). The 5-year freedom from locoregional recurrence was 88% in the RE group and 74% in the TE group. However the difference was not statistically significant (P=0.100, Figure 5). The 5-year freedom from distant recurrence was not different between the two groups (72% in the RE group and 71% in the TE group; P=0.594).
Discussion
In the present study, the short-term and long-term surgical outcomes achieved by RE and TE in ESCC were compared. The two study groups were comparable in terms of preoperative clinical characteristics and clinical stages. In this study, the RE group was found to have an advantage over the TE group in terms of numbers of lymph nodes dissected, especially in the upper mediastinum. But on the other hand, the RE group also required significantly longer one-lung ventilation time. Other clinical outcomes including mortality and morbidities were comparable. In terms of long-term oncologic outcomes, overall survival and freedom from recurrence were not different between two groups. Although lower locoregional recurrence was identified in the RE group, the difference was not statistically significant.
Lymphadenectomy in esophageal cancer is technically challenging, and this is especially true in ESCC. The most common location of mediastinal nodal metastasis in ESCC is in the lymph nodes along RLNs (11,12). Many authors have emphasized that the extent of mediastinal lymphadenectomy in ESCC should include lymph nodes along bilateral RLNs (13,14). Furthermore, bilateral RLN dissection requires a meticulous technique to achieve the complete removal of lymphatic chains and the safe preservation of nerves. However, it remains debatable whether thoracoscopic surgery can achieve comparable quality of lymphadenectomy to that of thoracotomy. Because of the steep learning curve, lower dissected lymph node yields in thoracoscopic surgery have been reported as compared to open surgery (15,16). The other issue regarding RLN dissection is the incidence of vocal cord paralysis which has been reported to range from 6% to 40% after thoracoscopic RLN dissection (17,18). Whether thoracoscopic RLN lymph node dissection can achieve comparable incidence of vocal cord paralysis is still questionable. Therefore better surgical technique to improve the quality of lymphadenectomy in MIE is necessary.
Thoracoscopic instruments have their limitations with respect to dissection in a small and narrow space, and thus, thoracoscopic dissection along a deeply located RLN in the thoracic inlet area is technically challenging. Instead robotic surgery has several technological advantages over thoracoscopic surgery in this respect, as it provides a three-dimensional view, ten times magnification, and freely articulated movement of the robotic arms, which enables more meticulous dissection, and thus, reduces the risk of damaging nearby nerves. However, the actual benefits of robotic surgery remain controversial. Kim et al. concluded that robot-assisted surgery could improve the quality of lymphadenectomy. They compared conventional upper mediastinal lymphadenectomy in 18 patients and extended upper mediastinal lymphadenectomy in 22 patients, and found that extensive dissection around the RLN increased numbers of retrieved lymph nodes from 35 to 48 (19). However, other studies failed to demonstrate increased yields of dissected lymph nodes during robotic surgery (17,20). In the present study, a greater number of lymph nodes was dissected in the RE group, and this advantage was more obvious in the upper mediastinum around both the RLN and aortopulmonary window lymph nodes where thoracoscopic dissection is technically difficult and dangerous. Although no statistical significance was found, the incidence of vocal cord paralysis was also lower in the RE group, which concurs with the findings of Suda et al. (17). In our opinion, these findings represent advantages of robot-assisted surgery over thoracoscopic surgery.
However, early postoperative outcomes were comparable between the RE and TE groups. Overall complication rates and severities of complications were not different in the two groups. Both groups had relatively low pulmonary complication and anastomosis leakage rates, which agree with previous studies (17,20). These comparable early results were expected because the invasiveness of both surgical methods is not greatly different. Instead, we believe that the advantages of RE over TE are better oncologic surgery and improved long-term survival. We have already indicated RE is superior in terms of quality of lymphadenectomy, but we were not able to demonstrate improved survival in the RE group. We did find that 5-year overall survival and freedom from locoregional recurrence rates were higher in the RE group by 10% and 14%, respectively. However these differences were not large enough to show significance, presumably because of the relatively small number of patients recruited. Furthermore, as several studies have reported correlations between dissected lymph node numbers and long-term survival (21,22), we recommend that a larger scale comparative study should be conducted on RE and TE.
Several disadvantages of robotic surgery were identified in this study. The first disadvantage was its longer operation time, especially one-lung ventilation time, which has the potential to increase postoperative respiratory complications (23). Although no significant increase in the risk of respiratory complications was identified in the RE group, this increase in operation time could adversely affect postoperative outcomes. The second disadvantage is the cost of robotic surgery. In our country robotic surgery is not reimbursed by national medical insurance system, and thus, additional charges should be paid by patients. This is the undoubtedly the most important obstacle to the widespread use of robotic surgery in our country. Accordingly, more evidence on the superiority of robotic surgery as compared with conventional MIE is required to justify the cost of robotic surgery.
Several limitations of the present study warrant consideration. The first concern is the relatively small number of patients enrolled. Importantly, several outcome variables were improved in the RE group but these improvements were not significant despite large differences. The vocal cord paralysis rate was decreased by 11% and 5-year freedom from locoregional recurrence was increased by 14%. However, the difference was not statistically significant. The second limitation stems from the retrospective nature of this study, for example, although preoperative parameters were comparable in the two groups, unidentified, uncontrolled selection bias might have existed.
Conclusions
In the present study, we compared RE and TE and found RE enabled more radical lymphadenectomy, especially in the upper mediastinum. On the other hand, RE was associated with longer operation times. Considering that balance between radicality and safety is an important goal of minimally invasive surgery, however unfortunately, we were not able to determine whether robotic surgery is a better proposition for esophagectomy. However, the better quality lymphadenectomy offered by robotic surgery has the potential to improve oncologic outcomes in the long-term follow-up. Well-designed large scale studies are required to compare RE and TE to define the role of robotic surgery in ESCC.
Acknowledgements
Funding: This work was supported by Seoul National University College of Medicine (grant number 800-20130290).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was reviewed by the Institutional Review Board and was approved as a minimal risk retrospective study (approval number: 1407-137-597), which did not require individual consent based on the institutional guidelines for waiving consent.
References
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. [Crossref] [PubMed]
- Smithers BM, Gotley DC, Martin I, et al. Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg 2007;245:232-40. [Crossref] [PubMed]
- Watanabe M, Baba Y, Nagai Y, et al. Minimally invasive esophagectomy for esophageal cancer: an updated review. Surg Today 2013;43:237-44. [Crossref] [PubMed]
- Watson TJ. Robotic esophagectomy: is it an advance and what is the future? Ann Thorac Surg 2008;85:S757-9. [Crossref] [PubMed]
- Boone J, Schipper ME, Moojen WA, et al. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg 2009;96:878-86. [Crossref] [PubMed]
- Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Arch Intern Med 1985;145:1443-6. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72; discussion 372-3. [Crossref] [PubMed]
- Tan Z, Ma G, Zhao J, et al. Impact of thoracic recurrent laryngeal node dissection: 508 patients with tri-incisional esophagectomy. J Gastrointest Surg 2014;18:187-93. [Crossref] [PubMed]
- Fujita H, Kakegawa T, Yamana H, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg 1995;222:654-62. [Crossref] [PubMed]
- Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 1991;48:411-20. [Crossref] [PubMed]
- Decker G, Coosemans W, De Leyn P, et al. Minimally invasive esophagectomy for cancer. Eur J Cardiothorac Surg 2009;35:13-20; discussion 20-1. [Crossref] [PubMed]
- Hamouda AH, Forshaw MJ, Tsigritis K, et al. Perioperative outcomes after transition from conventional to minimally invasive Ivor-Lewis esophagectomy in a specialized center. Surg Endosc 2010;24:865-9. [Crossref] [PubMed]
- Suda K, Ishida Y, Kawamura Y, et al. Robot-assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: technical report and short-term outcomes. World J Surg 2012;36:1608-16. [Crossref] [PubMed]
- Noshiro H, Iwasaki H, Kobayashi K, et al. Lymphadenectomy along the left recurrent laryngeal nerve by a minimally invasive esophagectomy in the prone position for thoracic esophageal cancer. Surg Endosc 2010;24:2965-73. [Crossref] [PubMed]
- Kim DJ, Park SY, Lee S, et al. Feasibility of a robot-assisted thoracoscopic lymphadenectomy along the recurrent laryngeal nerves in radical esophagectomy for esophageal squamous carcinoma. Surg Endosc 2014;28:1866-73. [Crossref] [PubMed]
- Weksler B, Sharma P, Moudgill N, et al. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus 2012;25:403-9. [Crossref] [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [Crossref] [PubMed]
- Darling G. The role of lymphadenectomy in esophageal cancer. J Surg Oncol 2009;99:189-93. [Crossref] [PubMed]
- Tekinbas C, Ulusoy H, Yulug E, et al. One-lung ventilation: for how long? J Thorac Cardiovasc Surg 2007;134:405-10. [Crossref] [PubMed]


