
Basic fibroblast growth factor-loaded hydrogel biomaterial patches for promoting the healing of esophageal anastomosis: an experimental animal study
Highlight box
Key findings
• The application of hyaluronic acid methacrylate (HAMA) hydrogel biomaterial patches loaded with basic fibroblast growth factor (bFGF) in esophageal surgery improved the mechanical strength of the esophageal anastomosis, facilitated fibroblast proliferation and collagen secretion, and promoted the growth of the tissues around the esophageal anastomosis.
What is known and what is new?
• Technical optimization involving anastomotic reinforcement has demonstrated potential in mitigating fistula risks, particularly in the implementation of biomechanically compatible reinforcement strategies during surgical reconstruction.
• Building upon the application of biological mesh reinforcement at the anastomotic site, we implemented a HAMA hydrogel-based sustained-release system for localized delivery of bFGF to enhance perianastomotic tissue regeneration.
What is the implication, and what should change now?
• The HAMA hydrogel biomaterial patches loaded with bFGF in this study could promote the proliferation of fibroblasts and the secretion of collagen fibers, which may enhance the tissue healing in esophageal anastomosis. Overall, this approach may serve as a novel strategy to prevent esophageal anastomotic fistula.
Introduction
Esophageal cancer ranks fifth in the incidence among malignant tumors in China, which has a particularly high incidence rate, with thoracic surgery as the primary treatment strategy (1,2). Currently, the diagnosis and treatment of esophageal malignant tumors are based on surgical resection, which may be combined with chemotherapy, immunotherapy, or radiotherapy or in other multidisciplinary, integrated treatment plans, effectively prolonging the survival time and improving the quality of life of patients. Although the treatment concepts and therapeutic means for esophageal cancer have advanced considerably in recent years, the occurrence of esophageal anastomotic fistula remains an issue for thoracic surgeons in the treatment cycle of esophageal malignancies, especially in the perioperative period of resectable esophageal cancer. Anastomotic fistula is the most common serious complication of esophageal cancer surgery, with an incidence rate of about 8–15% and an even higher mortality rate of around 50% (3-6). Moreover, the occurrence of esophageal anastomotic fistula is often accompanied by infections of the surrounding tissues, with thoracic anastomotic fistula being more likely to cause serious infections of the thoracic cavity and mediastinum, which can lead to infectious shock and even death in severe cases. The clinical occurrence of esophageal anastomotic fistula is typically treated conservatively and it involves a substantially prolonged hospital stay and higher treatment costs, with a less than satisfactory therapeutic effect. An alternative to conservative treatment is surgical treatment of esophageal anastomotic fistula; however, it is not only difficult to complete during the operative procedure but would also result in a greater risk of anastomotic fistula recurrence after surgery, leading to unsatisfactory therapeutic effects.
However, with the rapid development of material science, especially with a variety of biomaterials with excellent biocompatibility and degradability, reducing the incidence of esophagus anastomotic fistula may now be feasible. Numerous studies have explored means to prevent esophageal anastomotic fistula. For instance, it has been reported that the use of a tipped greater omentum wrap to reinforce the anastomosis during esophageal cancer surgery can reduce anastomotic tension to prevent anastomotic fistula (7,8). Moreover, intraoperative extraction of the greater omentum from autologous tissues has been found not to trigger rejection nor foreign body reaction; however, extraction of the tipped greater omentum may cause some damage to healthy structures. Shao et al. (9) and Hong et al. (10) each reported the use of biomaterial patches to repair and reinforce the esophageal anastomosis in McKeown and Ivor-Lewis surgeries for esophageal cancer, but the application of biomaterial patches did not significantly reduce esophageal anastomotic fistula. In 2015, Afaneh et al. (11) used the scaffold membrane made of pig bladder acellular epithelial basement membrane to wrap and suture the esophagojejunal anastomosis after total gastrectomy and achieved good results. The incidence of anastomotic fistula was reduced to 2.7%, but the mechanical strength was unsatisfactory, and its vascular regeneration ability was limited. In 2016, Dua et al. (12) placed a metal scaffold covered by extracellular matrix on the esophageal defect and sprayed the patient’s autologous with platelet-rich plasma adhesion gel, which was used to stimulate tissue regeneration and restore the continuity of the esophageal lumen. This was also the first reported human case of the application of tissue-engineering principles to regenerate part of the esophagus.
With the emergence of various new biomaterial-prepared biomaterial patches or scaffolds and the continuous improvement of tissue-engineering construction, the full application of biomaterials in esophageal surgery is anticipated and should be verified. Although many obstacles remain in resolving the issue of esophageal anastomotic fistula, promoting the early healing of esophageal anastomoses through the use of appropriate materials and methods appears to be an attainable goal. Therefore, the aim of this study was to examine the use of a hydrogel biomaterial patch loaded with basic fibroblast growth factor (bFGF) to encapsulate and reinforce the esophageal anastomosis and promote the healing of perianastomotic tissues. Moreover, we sought to determine whether this simple and feasible method could be used to reduce the incidence of anastomotic fistula during esophageal cancer surgery. We present this article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-623/rc).
Methods
Materials
Experimental animals
Forty large, healthy adult New Zealand white rabbits were purchased from the Animal Experiment Center of Naval Military Medical University (Shanghai, China), aged 5–7 months old and weighed 1.20–1.80 kg. The experimental animals were randomly divided into the experimental group (n=20) and the control group (n=20) via a random number table. The experimental animal feeding and experimental procedures were carried out in accordance with the National Regulations on the Management of Experimental Animals for the care and use of animals and were approved by the Ethics Committee of Shanghai Changzheng Hospital [No. CZEC(2024)-38]. A protocol was prepared before the study without registration.
Main reagents and instruments
Reagents and instruments used in this study are as follows: porcine bladder basement membrane combined with porcine small intestine submucosal decellularized biomaterial patches (Zhuoruan Medical Technology, Suzhou, China), hyaluronic acid methacrylate (HAMA) hydrogel (Suzhou Yongqinquan Intelligent Equipment Co., Ltd., Suzhou, China), LAP (lithium phenyl-2,4,6-trimethylbenzoylphosphinate) photoinitiator (Suzhou Yongqinquan Intelligent Equipment Co., Ltd.), heat-stabilized bFGF recombinant protein (Thermo Fisher Scientific, Waltham, MA, USA), hematoxylin-eosin (HE) reagent (Wuhan Servicebio Technology Co., Ltd., Wuhan, China), Masson staining reagent (Wuhan Servicebio Technology Co., Ltd.), DAPI (4',6-diamidino-2'-phenylindole) staining reagent (Wuhan Servicebio Technology Co., Ltd.), CY3-labeled goat anti-mouse IgG (immunoglobulin G) (Wuhan Servicebio Technology Co., Ltd.), anti-S100A4 antibody (Wuhan Servicebio Technology Co., Ltd.), Su-Mian-Xin II injection (Jilin Shengda Co., Ltd., Jilin, China), 5-0 suture with needle (Ningbo Lingqiao Co., Ltd., Ningbo, China), a ultraviolet light emitting diode (UV-LED) light source lamp (Beijing Fotanxie, Beijing, China), a pathology sectioning machine (Leica Biosystems, Nussloch, Germany), a microscope and imaging system (Nikon, Tokyo, Japan), and an orthogonal fluorescence microscope (Nikon).
Experimental section
Preparation of biomaterial patches loaded with bFGF
A total of 0.5 g of HAMA-150K freeze-dried powder at 2% weight/volume (w/v) concentration was dissolved in 0.25% w/v LAP photoinitiator solution to form a 25-mL solution; subsequently, 100 µg of heat-stabilized bFGF recombinant protein was added and mixed evenly (13). The porcine bladder basement membrane combined with porcine small intestine submucosal decellularized biomaterial patches was placed in the HAMA solution and dissolved in bFGF for 30 s in the dark until infiltration, after which it was removed and irradiated with a 405-nm wavelength light source for 20 s to form a gel (Figure 1).
Esophageal anastomosis in experimental animals
New Zealand white rabbits were fasted on the day of the experiment and anesthetized via the intramuscular injection of 0.2 mL/kg of Su-Mian-Xin II. The rabbits were fixed in the supine position, and an incision was made in the middle of the neck. The fascial and muscular layers were cut and separated sequentially, and the cervical esophagus was separated from the back of the trachea. A 5-mm of section of esophagus was cut off, the esophagus in the experimental animal was removed, and the distal and proximal ends of the esophagus were manually anastomosed with 5-0 silk thread. In the control group, a drainage tube was placed next to the anastomosis, and the neck incision was closed layer by layer. In the experimental group, bFGF-loaded biomaterial patches were wrapped around the anastomosis after esophageal anastomosis, the anastomosis was reinforced by 5-0 silk thread in interrupted sutures, the drainage tube was then left in place, and the incision was closed layer by layer (Figure 2). The experimental animals were fasted for 1 day after the operation, and the cervical drain was removed 3 days after the operation.
Incidence of anastomotic leakage
Four weeks after the surgery, the surviving experimental animals in the experimental group and the control group were killed via an overdose of anesthesia, and their body weights were recorded. A neck incision was made on the experimental animal, the healing of the neck anastomosis was observed, and the incidence of anastomotic leakage was calculated.
Esophageal anastomosis tension experiment
Eight animals without anastomotic fistula were randomly selected from each of the experimental and control groups. A 5-cm section of esophagus was cut off, with the esophageal anastomosis serving as the midpoint. One end of the esophagus was fixed to the test bench, and the other end was connected to a tensile dynamometer. The esophagus was pulled horizontally, the tensile force was increased slowly until the anastomosis was completely torn, and the peak tensile force was recorded.
Histology and immunohistochemistry
The cervical esophagus of the remaining experimental animals was obtained to observe the anastomosis healing. These esophageal anastomosis tissues specimens were subjected to paraffin-embedding in preparation for immunofluorescence staining, anti-S100A4 antibody was added according to a previously described process (14), and the nuclei were stained with the fluorescent DAPI dye to facilitate the observation and evaluation of the proliferation of fibroblasts at the anastomosis. The remaining paraffin sections were subjected to HE staining and Masson staining to observe the morphology and structure of the tissues at the anastomosis site and to compare the collagen formation at the anastomosis site.
Statistical analysis
All data were statistically analyzed with SPSS 23.0 software (IBM Corp., Armonk, NY, USA). The measured data were expressed as mean ± standard deviation and were analyzed with the t-test; meanwhile, the count data were analyzed with the Fisher’s exact probability method. A P value <0.05 was considered to indicate a statistically significant difference between two sets of data.
Results
Incidence of anastomotic fistula
Three experimental animals died of systemic depletion due to refusal to eat within 5 days after surgery: two in the experimental group and one in the control group. The deaths of these three experimental animals were not related to anastomotic leakage, so they were not included in the statistical analysis. At the 4-week postoperative endpoint, one animal in the experimental group had an anastomotic fistula (Figure 3) (combined with severe infection leading to death), representing an anastomotic fistula incidence rate of 1/18; seven experimental animals in the control group had an anastomotic fistula (of which three were combined with severe infection leading to death) (Figure 4), representing an anastomotic fistula incidence rate of 7/19; and the difference in the incidence rates of anastomotic fistula between the two groups was statistically significant (P=0.04).
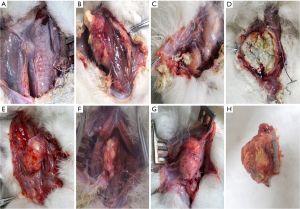
Esophageal anastomosis tension experiment
At 4 weeks after the endpoint of postoperative observation, eight animals in each of the experimental group and the control group were randomly selected for the anastomotic tension experiment. The experimental group weighed 1.50±0.11 kg (n=8), and the control group weighed 1.46±0.12 kg (n=8), and the difference in body weight between the two groups of experimental animals was not statistically significant (P>0.05). The peak tensile force of complete anastomotic tear in the experimental group was 6.49±0.17 N (n=8), and the peak tensile force of complete anastomotic tear in the control group was 6.33±0.12 N (n=8), representing a statistically significant difference (P<0.05).
Histological study
HE staining of the esophageal tissue at the anastomosis site revealed that the experimental group had thickening of the whole layer at the esophageal anastomosis, with clearer tissue levels, in which a large number of fibroblasts were seen to be proliferating, accompanied by a small amount of inflammatory cell infiltration. The tissue around the esophagus grew well and in close proximity to the biomaterial patches, and a large number of fibroblasts could be observed adhering closely to the surface of the biomaterial patches. In the control group, the tissue at the anastomosis of the esophagus was obviously thinned as compared with the surrounding area, and the tissue layers were not clear; moreover, fibroblast proliferation with a small amount of inflammatory cell infiltration could be observed, but connective tissue proliferation was obviously weakened as compared with the experimental group. Masson staining results showed a large amount of collagen fiber proliferation at the anastomosis of the esophagus in the experimental group, and collagen fiber proliferation at the anastomosis in the control group was obviously reduced as compared with that in the experimental group (Figure 5).

Immunohistochemistry
Immunofluorescence staining of fibroblasts observed in 10× field of view showed the experimental group fibroblast count of 2,979.8±1,238.2 (n=10), and the control group fibroblast count of 1,059±451.52 (n=11), representing a statistically significant difference (P<0.05). Immunofluorescence staining with fibroblast-specific antibodies (FSP1 and S100A4) showed a significant increase in fibroblast proliferation in the tissues around the anastomosis in the experimental group as compared with the control group (Figure 6).
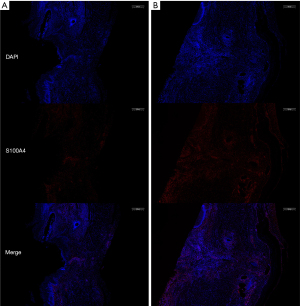
Discussion
In the clinical treatment of esophageal cancer, various surgical complications seriously restrict the surgical effect and affect the quality of life of patients. Among these, the negative impact of esophageal anastomotic fistula is particularly prominent, as it inflicts additional pain on the patients and adds considerable burden to the medical system. Based on the development of existing devices and clinical treatment concepts, innovations in surgical approaches for the treatment of esophageal cancer have plateaued to a degree, and it is unlikely that significant improvements to the procedures will emerge in the short-term. Inspired by other work in this field (11,15-17), we examined the ability of biomaterial patches combined with HAMA hydrogel and sustained release of bFGF to promote the rapid growth of tissues around the esophageal anastomosis in the early postoperative period.
Studies indicate that bFGF plays an important role in promoting angiogenesis, cell proliferation, and matrix remodeling, and as a key regulator of human trauma repair, it has been widely used in clinic (13,16,18,19). In 2023, Ji et al. (15) reported that HAMA hydrogel had a release cycle of 7–10 days in response to the loaded drug, and the release cycle included a burst release in the early stage and a stable sustained release in the late stage; this contributes to the rapid onset of action in the initial stage and the maintenance of drug action in the middle and late stages. The sustained and controlled release of bFGF around the anastomosis can be achieved by the use of HAMA hydrogel for sustained release of the drug without causing any damage to the human body.
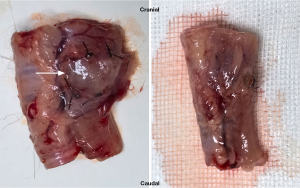
In this study, we applied a porcine bladder basement membrane combined with porcine small intestine submucosal decellularized biomaterial patches and HAMA hydrogel containing sustained-released bFGF to reinforce esophageal anastomosis. The biomaterial patches improve the mechanical strength of the anastomosis and provide cellular scaffolding, while bFGF is slowly released through HAMA hydrogel to promote the proliferation and migration of surrounding tissue cells, especially fibroblasts; this facilitates angiogenesis and promotes anastomotic healing and tissue regeneration (17,20,21) (Figure 7).
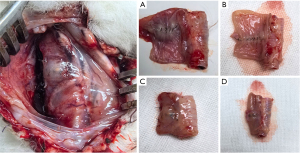
In our study, the incidence of anastomotic fistula in the experimental group was significantly lower than that in the control group, suggesting that this method can effectively promote the healing of esophageal anastomosis and reduce the incidence of anastomotic fistula. In the early postoperative period, the animals in the experimental group did not have a strong willingness to eat, and two experimental animals died of systemic depletion because they did not eat at all, whereas such cases were less common in the control group. The autopsy of the two dead animals in the experimental group revealed that a large amount of food residue had accumulated in the biomaterial patches wrapped around the anastomotic stoma biomaterial patches. It was surmised that the small size of the biomaterial patches resulted in limited diastole at the anastomosis, preventing the passage of food and resulting in the inability of the experimental animals to eat (Figure 8). As death was unrelated to anastomotic fistula, dead animals need to be excluded in order to accurately determine the effect of patches application on the incidence of esophageal anastomotic fistula. Small patches and poor material compliance may have combined to cause the death of the animals. However, the use of biomaterial patch of a large size may result in a gap between the anastomotic tissue and the biomaterial patches, affecting the growth of tissue cells adhering to the biomaterial patches. Therefore, the selection of biomaterial patches of appropriate size remains an unaddressed challenge.
HE staining and Masson staining of the esophageal anastomosis indicated that the tissue morphology at the anastomosis in the experimental group was good and the connective tissue proliferation was obvious; additionally, the collagen secretion at the anastomosis in the experimental group was superior to that in the control group. In the immunofluorescence histochemical study, the proliferation of fibroblasts at the anastomosis in the experimental group was significantly increased, suggesting that the biomaterial patches with slow-release of bFGF promoted tissue proliferation and collagen matrix expression around the anastomosis and supported the healing of the esophageal anastomosis. In the tensile force experiment of esophageal anastomosis, the peak tensile force of esophageal anastomosis tear in the experimental group was significantly higher than that in the control group, indicating that the use of the biomaterial patches can provide superior mechanical strength to the anastomosis.
The use of biomaterial patches in this study significantly promoted the tissue healing of esophageal anastomosis and greatly reduced the incidence of esophageal anastomotic fistula in the experimental group. However, the incidence of esophageal anastomotic fistula is still higher than the 2.7% reported in the study by Afaneh et al. (11). The results of the esophageal anastomosis tension test demonstrated that the biomaterial patches provided adequate mechanical strength to the anastomosis, but similar to Afaneh et al. (11), we did not observe significant vascular regenerative capacity of the biomaterial patches. Whether the replacement or addition of other growth factors can achieve this goal requires further investigation. In our study, we observed that the use of the biomaterial patches may lead to limited local dilatation of the esophagus to the extent that the animal has difficulty in eating in the perioperative period. This was primarily due to the poor elasticity of the porcine bladder basement membrane combined with porcine small intestine submucosal decellularized biomaterial patches, although it did not provide sufficient mechanical strength to the anastomosis. If the biomaterial patches applied at the anastomosis are too small, this can lead to local stenosis of the esophagus, and there is a long-term risk of anastomosis stenosis; therefore, further animal studies over a longer period are needed to clarify this issue.
Conclusions
In conclusion, the HAMA hydrogel biomaterial patches loaded with bFGF in this study can promote the proliferation of fibroblasts and the secretion of collagen fibers, which can have a positive effect on the tissue healing of esophageal anastomosis and provide novel strategies for the prevention of esophageal anastomotic fistula. Due to the limitations of the sample size and observation time in this study, our findings need to be verified by further work to determine their clinical applicability. It is hoped that these biomaterials can aid in further reducing the incidence of esophageal anastomotic fistula.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-623/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-623/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-623/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-623/coif). All authors report that this work was supported by the Shanghai Science and Technology Commission (No. shdc2022crd0229). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The experimental animal feeding and experimental procedures were carried out in accordance with the National Regulations on the Management of Experimental Animals for the care and use of animals and were approved by the Ethics Committee of Shanghai Changzheng Hospital [No. CZEC(2024)-38].
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lai SP, Su HM, Liu YW, et al. Spatial and temporal distribution characteristics research of esophageal cancer in China. Zhonghua Zhong Liu Za Zhi 2024;46:657-62. [PubMed]
- Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi 2019;41:19-28. [PubMed]
- Dent B, Griffin SM, Jones R, et al. Management and outcomes of anastomotic leaks after oesophagectomy. Br J Surg 2016;103:1033-8. [Crossref] [PubMed]
- Kassis ES, Kosinski AS, Ross P Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. [Crossref] [PubMed]
- Honda M, Kuriyama A, Noma H, et al. Hand-sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta-analysis. Ann Surg 2013;257:238-48. [Crossref] [PubMed]
- Ryan CE, Paniccia A, Meguid RA, et al. Transthoracic Anastomotic Leak After Esophagectomy: Current Trends. Ann Surg Oncol 2017;24:281-90. [Crossref] [PubMed]
- Hou X, Wan Q, Zheng H, et al. Clinical application of omentum ring envelopment in prevention of esophagogastric anastomotic leakage. Cancer Research on Prevention and Treatment 2012;39:118-9.
- Zhao X, Feng Q, Li H. Pedicled omentum flap ring envelop to prevent esophagogastric anastomotic leakage. The Practical Journal of Cancer 2002;17:430-1.
- Shao Z, Zhang J, Chen Y, et al. Application of biomaterial patch in esophageal cancer surgery. Journal of Modern Oncology 2013;21:1225-7.
- Hong L, Lu Y, Wang K, et al. Clinical application of biomaterial patch in esophageal cancer surgery. Journal of Clinical Medicine in Practice 2021;1-3.
- Afaneh C, Abelson J, Schattner M, et al. Esophageal reinforcement with an extracellular scaffold during total gastrectomy for gastric cancer. Ann Surg Oncol 2015;22:1252-7. [Crossref] [PubMed]
- Dua KS, Hogan WJ, Aadam AA, et al. In-vivo oesophageal regeneration in a human being by use of a non-biological scaffold and extracellular matrix. Lancet 2016;388:55-61. [Crossref] [PubMed]
- Yu A, Matsuda Y, Takeda A, et al. Effect of EGF and bFGF on fibroblast proliferation and angiogenic cytokine production from cultured dermal substitutes. J Biomater Sci Polym Ed 2012;23:1315-24. [Crossref] [PubMed]
- Lendahl U, Muhl L, Betsholtz C. Identification, discrimination and heterogeneity of fibroblasts. Nat Commun 2022;13:3409. [Crossref] [PubMed]
- Ji J, Cheng J, Chen C, et al. Pirfenidone-loaded hyaluronic acid methacryloyl hydrogel for preventing epidural adhesions after laminectomy. Drug Deliv Transl Res 2023;13:770-81. [Crossref] [PubMed]
- Jin W, Shen S, Xu X, et al. All-in-one hydrogel patches with sprayed bFGF-loaded GelMA microspheres for infected wound healing studies. Int J Pharm 2024;658:124205. [Crossref] [PubMed]
- Zhang X, Kang X, Jin L, et al. Stimulation of wound healing using bioinspired hydrogels with basic fibroblast growth factor (bFGF). Int J Nanomedicine 2018;13:3897-906. [Crossref] [PubMed]
- Fenn SL, Oldinski RA. Visible light crosslinking of methacrylated hyaluronan hydrogels for injectable tissue repair. J Biomed Mater Res B Appl Biomater 2016;104:1229-36. [Crossref] [PubMed]
- Peng Z, Xue H, Liu X, et al. Tough, adhesive biomimetic hyaluronic acid methacryloyl hydrogels for effective wound healing. Front Bioeng Biotechnol 2023;11:1222088. [Crossref] [PubMed]
- Akita S, Akino K, Hirano A. Basic Fibroblast Growth Factor in Scarless Wound Healing. Adv Wound Care (New Rochelle) 2013;2:44-9. [Crossref] [PubMed]
- Farooq M, Khan AW, Kim MS, et al. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021;10:3242. [Crossref] [PubMed]





