
Exploring and verifying key thin-section computed tomography features for accurately differentiating granulomas and peripheral lung cancers
Highlight box
Key findings
• Satellite lesions, halo sign, calcification, and special shapes are highly indicative of granulomas, while well-defined solid nodules (SNs) with lobulation should raise suspicion for peripheral lung cancers (PLCs), especially those with a vacuole sign. Spiculation, pleural indentation, and vascular convergence signs alone are not sufficient for differentiating them.
What is known and what is new?
• Recent studies have demonstrated that satellite lesions, halo sign, and calcification are commonly observed features of benign SNs, whereas the presence of lobulation sign, spiculation sign, pleural indentation sign, and vascular convergence sign indicates lung cancers.
• We explored and verified thin-section computed tomography (CT) features that can effectively distinguish granulomas and PLCs, and identified three new special shapes predominantly found in granulomas. Furthermore, we established a novel classification system to facilitate accurate differentiation between granulomas and PLCs.
What is the implication, and what should change now?
• Based on the key CT indicators, a classification system including eight types with distinct features was developed, which may serve as an effective guideline for differentiating granulomas and PLCs in clinical practice.
Introduction
With the widespread application of computed tomography (CT) in clinical practice, there has been a notable increase in the detection of pulmonary nodules (1), which are classified into solid nodules (SNs) and sub-SNs (SSNs). At present, extensive research has already revealed the CT characteristics of neoplastic and non-neoplastic SSNs (2,3). In contrast, SNs present a wider range of pathological natures and complex CT features, making the accurate differentiation between benign and malignant ones become one of the most challenging issues. In addition, malignant SNs tend to grow more rapidly and have poorer prognoses compared to SSNs (4,5). Thus, misdiagnosis of SNs may lead to more severe consequences for patients, including unnecessary surgical resection and delays in receiving the most appropriate treatments.
In fact, malignant pulmonary SNs are predominantly indicative of lung cancers, whereas benign ones often vary more in histology. Among the benign SNs, granulomatous lesions are a notable example, which more frequently present similar characteristics to peripheral lung cancers (PLCs). Traditionally, the size of nodules has been a key indicator of potential malignancy, an SN diameter of 8 mm or greater should raise concerns for malignancy, often necessitating further evaluation with positron emission tomography (PET)/CT scans (6). However, the granulomas can be large and some even appear as masses similar to PLCs (7,8). Additionally, granulomas are more likely to exhibit growth over time compared to other benign nodules (9). Furthermore, both granulomas and PLCs can exhibit heterogeneous enhancement on contrast-enhanced CT scans and high fluorodeoxyglucose uptake on PET/CT images during the acute phase (10-12). Lastly, the granulomas may be also frequently associated with lymphadenopathy (13). These similarities pose significant diagnostic challenges between granulomas and PLCs.
To address these challenges, it is vital to deeply explore the CT features specific to granulomas or PLCs. Current research on CT features of benign SNs is extensive (14,15), yet studies specifically addressing granulomas are relatively scarce. Although some studies have suggested that satellite lesions are typical in tuberculous granulomas, and halo signs are common in fungal granulomas (8,16,17), further research is needed to confirm the efficacy of these indicators in differentiation and to identify more distinctive features. Additionally, it was found that spiculation can be present in up to 44% of granulomas (8), further complicating their differentiation from PLCs. Thus, the features which are effective for differentiating granulomas and PLCs remain uncertain and require further clarification through additional research.
Given the limitations of previous studies, which often had small sample sizes and lacked individual granuloma analysis, it is crucial to explore distinct CT features and combine multiple features from a larger sample to enhance the differentiation between granulomas and PLCs. By focusing on thin-section CT (TSCT) features specific to granulomas and PLCs, a more comprehensive understanding may be achieved. This study aimed to establish a classification system that incorporates key CT features to guide the differentiation between granulomas and PLCs effectively. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1505/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional review board of The First Affiliated Hospital of Chongqing Medical University (No. K2023-083), and the requirement for informed consent from the patients was waived due to the retrospective nature of this study.
Study design and patient selection
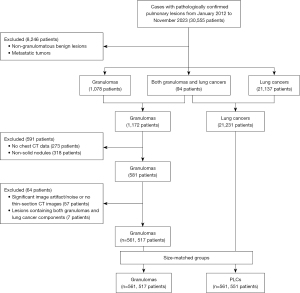
The Pathology Information System (PIS) was searched for patients confirmed to have pulmonary lesions through radical surgical resection from January 2012 to November 2023 (30,555 patients) at The First Affiliated Hospital of Chongqing Medical University, identifying 1,172 patients with granulomas and 21,231 with lung cancers after excluding 8,246 patients with non-granulomatous benign lesions or metastatic tumors. For these 1,172 granulomatous patients, a fellowship-trained thoracic radiologist (H.B.X.) manually reviewed on the picture archiving and communication system workstation (Carestream Vue; Carestream, Rochester, NY, USA), resulting in exclusion of 273 patients who had not undergone a chest CT scan within 3 weeks prior to their resection at our hospital. The previously mentioned radiologist (H.B.X.) and an additional senior thoracic radiologist (Z.G.C.) then jointly reviewed the preoperative chest CT examinations, resulting in exclusion of 318 patients whose lesions did not qualify as peripheral SNs (diameter ≤30 mm) on CT (5), including 207 patches, 91 masses, and 20 SSNs. These exclusions resulted in 581 patients with solid granulomatous nodules on chest CT performed before resection. Additional patients were excluded due to significant image artifact/noise or no thin-section images (≤1.5 mm) (57 patients), and lesions containing both granulomas and lung cancer components (7 patients). The study included a final cohort of 517 patients with 561 granulomatous nodules. Due to the diameter of the nodule may influence its characteristics, the diameter of both granulomas and PCLs should ideally remain consistent (4). Additionally, CT scans technology may be updated with the increase of years, an equal number of granulomas and PLCs should be enrolled in the same year. Thus, patients with PLCs as a control group were continuously selected by individually matching with the similar nodule size, same year of resection, and same inclusion and exclusion criteria at a ratio of 1:1, resulting in a total of 551 patients with 561 nodules (Figure 1).
CT examinations
The chest CT scans were performed using SOMATOM Perspective (Siemens Healthineers, Erlangen, Germany), Discovery CT750 HD (GE Healthcare, Milwaukee, WI, USA), SOMATOM Definition Flash (Siemens Healthineers), SOMATOM Force (Siemens Healthineers), and Aquilion ONE pureViSION (Canon Medical System, Otawara-shi, Japan). Patients were positioned supine with arms elevated above the head and instructed to maintain breath-holding following deep inspiration to optimize imaging quality. The imaging coverage extended from the thoracic inlet to the costophrenic angle. Scanning parameters included: tube voltage range of 110–130 kVp, tube current time settings between 50 and 140 mAs (employing automatic current modulation), 5 mm slice thickness, 0.5 s rotation time, 1–1.1 pitch, and 0.6/0.625 mm collimation. Reconstruction parameters comprised 0.625/1 mm slice thickness with identical intervals and a 512×512 matrix. All participants initially underwent non-contrast CT examinations. Contrast-enhanced scans were performed in 342 cases (30.5%), including 197 granuloma patients and 145 PLC cases, using a total of 80–100 mL of nonionic iodinated contrast material (iopamidol, 3.0 mL/s; 320 mgI/mL; Shanghai Bracco Sine Pharmaceutical Co., Ltd., Shanghai, China). Image acquisition employed two distinct window settings: mediastinal [width: 400 Hounsfield units (HU); level: 30 HU] and lung (width: 1,500 HU; level: −600 HU).
Image analysis
H.B.X. and Z.G.C. evaluated the SNs on axial, multi-planar reconstruction (MPR), and maximum intensity projection (MIP) images. In case of disagreement, a consensus was reached after a joint discussion and/or consultation with a third senior radiologist (F.J.L.).
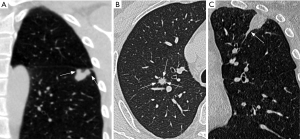
The CT features of SNs were evaluated in the following aspects: distribution (upper lobes, or other lobes), size (the mean of the longest diameter and the perpendicular diameter on axial CT images), shape (round, oval, irregular, or special shape), CT value on non-enhanced and enhanced images, ΔCT value, boundary (well-defined or ill-defined), margin (smooth or coarse), lobulation sign, spiculation sign, vacuole sign, calcification, satellite lesions, halo sign, pleural indentation sign, and vascular convergence sign. In this study, three special shapes of SNs were identified, (I) multi-nodular fusion shape was defined as a nodule manifesting as a fusion of two or more smaller nodules, distinct from the lobulated nodule; (II) gear-like shape was characterized a nodule with multiple small nodular protrusion at the margin, resembling a gear; and (III) nodule with a tail was defined as lesion growing along the proximal bronchus and resembling a tail of the nodule (Figure 2). The ΔCT value was determined by the difference between the peak CT value on enhanced CT images and the CT value on non-enhanced CT images. Lobulation sign referred to an abrupt protrusion in the contour of the lesion (18). Spiculation sign denoted linear strands extending from the nodule surface into the lung parenchyma without reaching the pleural surface (18). The vacuole sign indicated the potential presence of air or gas within the nodule, suggesting a specific growth type or abnormality (19). Satellite lesions were defined as small nodules around the dominant nodules (16). The halo sign was characterized by ground-glass opacity surrounding a nodule or mass in the lung parenchyma (17). The pleural indentation sign denoted the indentation or drawing in of the pleura, the thin tissue covering the lungs and lining the interior chest wall (20). The vascular convergence sign described the phenomenon where blood vessels converge towards a specific point or region within the lungs (21).
Statistical analysis
SPSS 25.0 (IBM Corporation, Armonk, NY, USA) was used for the statistical tests. Continuous data were presented as mean ± standard deviation, and categorical data as counts and percentages. The intraclass correlation coefficient (ICC) and Cohen’s kappa coefficient were employed to assess interobserver agreement for continuous and categorical variables, respectively. Interobserver agreement according to ICC was classified as poor (<0.500), moderate (0.500–0.740), good (0.750–0.890), or excellent (≥0.900) (22). Interobserver agreement according to kappa coefficients was categorized as poor (<0.000), slight (0.000–0.200), fair (0.210–0.400), moderate (0.410–0.600), substantial (0.610–0.800), or almost perfect (0.810–1.000) (22). The Mann-Whitney U test was used for comparing continuous variables in clinical and CT features between granulomas and PLCs, and the Pearson Chi-squared test was used to compare categorical variables between them. Variables with statistically significant differences in univariate analysis were further included in multivariate logistic regression analysis to determine independent factors. A classification system was established mainly based on the independent CT features. Each type contained a single morphological feature or a combination of multiple features, and the positive predictive value (PPV) was evaluated to assess its accuracy for predicting granulomas or PLCs in each type. Receiver operating characteristic (ROC) analysis was conducted to determine optimal threshold values, and to evaluate the diagnostic performance of the regression model and each classification type. Area under the curve (AUC) was categorized as fail (0.50–0.59), poor (0.60–0.69), fair (0.70–0.79), good (0.80–0.89), or excellent (0.90–1.00) (23). Differences with P values of less than 0.05 were considered statistically significant.
Results
Interobserver agreement
Table 1 summarizes the interobserver agreement for the CT features. For the continuous variables, agreement for both non-enhanced CT value and ΔCT value was good, nodule size was excellent. For the categorical indicators, agreements were all almost perfect.
Table 1
| Parameters | Metric | 95% CI | P value |
|---|---|---|---|
| Size (mm) | 0.967 | 0.963–0.971 | <0.001 |
| Non-enhanced CT value (HU) | 0.747 | 0.704–0.783 | <0.001 |
| ΔCT value (HU) | 0.887 | 0.708–0.943 | <0.001 |
| Boundary | 0.862 | 0.811–0.913 | <0.001 |
| Margin | 0.953 | 0.942–0.964 | <0.001 |
| Special shape | 0.960 | 0.921–0.999 | <0.001 |
| Lobulation sign | 0.952 | 0.934–0.970 | <0.001 |
| Spiculation sign | 0.919 | 0.891–0.947 | <0.001 |
| Vacuole sign | 0.973 | 0.953–0.993 | <0.001 |
| Calcification | 1.000 | 1.000–1.000 | <0.001 |
| Pleural indentation sign | 0.982 | 0.970–0.994 | <0.001 |
| Vascular convergence sign | 0.810 | 0.747–0.873 | <0.001 |
| Satellite lesions | 0.970 | 0.950–0.990 | <0.001 |
| Halo sign | 0.907 | 0.827–0.987 | <0.001 |
Metric represents ICC for continuous variables and kappa coefficient for categorical variables. CI, confidence interval; CT, computed tomography; HU, Hounsfield unit; ICC, intraclass correlation coefficient.
Patients’ clinical characteristics and TSCT features of lesions
Among 561 granulomas, 441 cases (78.6%) were tuberculosis (TB), 70 cases (12.5%) were fungal infections, 2 cases (0.4%) showed concurrent TB and fungal infection, and the remaining 48 cases (8.6%) had undetermined etiology due to lack of further examination. In comparison, among the 561 PLCs, cases included 453 (80.7%) adenocarcinomas, 70 (12.5%) squamous cell carcinomas, 11 (2.0%) small cell lung cancers, and 27 (4.8%) other types of lung cancers including large cell, carcinoid, and adenosquamous carcinomas. On preoperative CT images, 340 (60.6%) of 561 granulomas were initially suspected as lung cancers, while 103 (18.4%) of 561 PLCs were initially suspected as benign lesions.
Table 2 shows the patients’ clinical characteristics and TSCT features of lesions. Patients with granulomas were significantly younger than those with PLCs (P<0.05). Compared with PLCs, granulomas exhibited lower density and enhancement on non-enhanced and enhanced CT images (each P<0.05). ROC analysis revealed the optimal cutoff value of patients’ age, CT value on non-enhanced images, and ΔCT value for distinguishing granulomas and PLCs was 58 years, 10 HU, and 30 HU, respectively. Regarding their CT features, granulomas were more frequently demonstrated ill-defined boundary, smooth margin, special shape, satellite lesions, and halo sign (each P<0.05), while lobulation sign, spiculation sign, vacuole sign, pleural indentation sign, and vascular convergence sign were more common in PLCs (each P<0.05).
Table 2
| Parameters | Granulomas (n=561, 517 patients) | PLCs (n=561, 551 patients) | P value |
|---|---|---|---|
| Age (years) | 51.71±12.17 | 61.97±10.01 | <0.001 |
| Gender | 0.20 | ||
| Male | 308 (59.6) | 307 (55.7) | |
| Female | 209 (40.4) | 244 (44.3) | |
| Size (mm) | 13.44±5.27 | 13.42±5.32 | 0.98 |
| 0–10 | 201 (35.8) | 201 (35.8) | |
| 11–20 | 292 (52.0) | 292 (52.0) | |
| 21–30 | 68 (12.1) | 68 (12.1) | |
| Non-enhanced CT value (HU) | 16.47±41.19 | 26.64±24.05 | 0.001 |
| ΔCT value (HU)† | 30.44±22.60 | 41.44±17.29 | <0.001 |
| Location | 0.18 | ||
| Upper lobes | 324 (57.8) | 301 (53.7) | |
| Middle and lower lobes | 237 (42.2) | 260 (46.3) | |
| Boundary | <0.001 | ||
| Well-defined | 476 (84.8) | 535 (95.4) | |
| Ill-defined | 85 (15.2) | 26 (4.6) | |
| Margin† | n=476 | n=535 | 0.001 |
| Coarse | 345 (72.5) | 434 (81.1) | |
| Smooth | 131 (27.5) | 101 (18.9) | |
| Special shape | 48 (8.6) | 3 (0.5) | <0.001 |
| Multi-nodular fusion shape | 22 (3.9) | 1 (0.2) | |
| Gear-like shape | 17 (3.0) | 2 (0.4) | |
| Nodule with a tail | 9 (1.6) | 0 (0.0) | |
| Lobulation sign | 178 (31.7) | 436 (77.7) | <0.001 |
| Spiculation sign | 99 (17.6) | 150 (26.7) | <0.001 |
| Vacuole sign | 58 (10.3) | 95 (16.9) | 0.001 |
| Calcification | 70 (12.5) | 15 (2.7) | <0.001 |
| Pleural indentation sign | 208 (37.1) | 288 (51.3) | <0.001 |
| Vascular convergence sign | 37 (6.6) | 57 (10.2) | 0.03 |
| Satellite lesions | 187 (33.3) | 13 (2.3) | <0.001 |
| Halo sign | 23 (4.1) | 2 (0.4) | 0.03 |
Data are expressed as number (percentage) or mean ± standard deviation. Gender is calculated using the number of patients, and others are calculated using the number of nodules. N represents the number of nodules. †, only the patients with this data were compared. CT, computed tomography; HU, Hounsfield unit; PLC, peripheral lung cancer; SN, solid nodule.
Logistic regression analysis for granulomas and PLCs
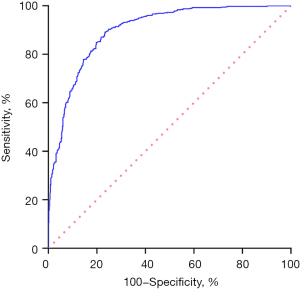
Table 3 depicts the clinical characteristics and TSCT features independently distinguishing granulomas from PLCs via multivariate logistic regression. Age <58 years, CT value <10 HU on non-enhanced images, ill-defined boundary, special shape, calcification, satellite nodules, and halo sign were identified as independent predictive indicators of granulomas (each P<0.05), while lobulation sign and vacuole sign were identified as independent predictive indicators of PLCs (each P<0.05). Spiculation sign, pleural indentation sign, and vascular convergence sign were not independent indicators for predicting PLCs or granulomas (each P>0.05). The sensitivity, specificity, accuracy, and AUC of the model for predicting granulomas were 80.0%, 84.0%, 82.0%, and 0.893 [95% confidence interval (CI): 0.875–0.912; P<0.001], respectively (Figure 3).
Table 3
| Parameters | Odds ratio (95% CI) | P value |
|---|---|---|
| Age (years) | <0.001 | |
| ≥58 | 1 | |
| <58 | 4.645 (3.315, 6.508) | |
| Non-enhanced CT value (HU) | <0.001 | |
| ≥10 | 1 | |
| <10 | 2.545 (1.756, 3.687) | |
| Boundary | 0.007 | |
| Well-defined | 1 | |
| Ill-defined | 2.260 (1.246, 4.100) | |
| Special shape | <0.001 | |
| No | 1 | |
| Yes | 23.089 (6.372, 83.669) | |
| Lobulation sign | <0.001 | |
| No | 1 | |
| Yes | 0.222 (0.158, 0.313) | |
| Spiculation sign | 0.45 | |
| No | 1 | |
| Yes | 1.175 (0.773,1.788) | |
| Vacuole sign | 0.009 | |
| No | 1 | |
| Yes | 0.516 (0.314, 0.848) | |
| Calcification | <0.001 | |
| No | 1 | |
| Yes | 7.145 (3.516, 14.518) | |
| Pleural indentation sign | 0.41 | |
| No | 1 | |
| Yes | 0.771 (0.504, 1.106) | |
| Vascular convergence sign | 0.71 | |
| No | 1 | |
| Yes | 0.890 (0.485, 1.634) | |
| Satellite lesions | <0.001 | |
| No | 1 | |
| Yes | 22.330 (11.765, 42.383) | |
| Halo sign | 0.02 | |
| No | 1 | |
| Yes | 6.945 (1.337, 36.078) |
CI, confidence interval; CT, computed tomography; HU, Hounsfield unit.
Establishing a classification system for differentiation based on key TSCT features
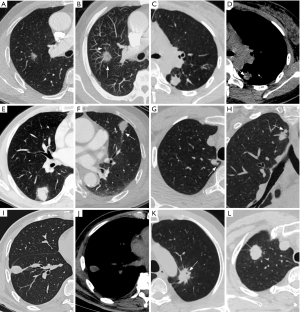
A novel classification system is outlined in Table 4. Lesions were classified into eight types mainly based on independent CT features, and each type contained a single morphological feature or a combination of multiple features. Given that the margin characteristics of lesions are valuable for nodule classification, and the margin of nodules with ill-defined boundary cannot be evaluated, ill-defined nodules were classified as a distinct type. Types I to V were significantly more common in granulomas compared to PLCs, with a PPV of 0.93, 0.84, 0.93, 0.67, and 0.78, respectively (each P<0.05). In contrast, types VI to VIII were more frequently observed in PLCs, with a PPV of 0.64, 0.92, and 0.80, respectively (each P<0.05) (Figure 4). Regarding the diagnostic performance of each type within the classification system, it was observed that all of them exhibited relatively lower sensitivity but higher specificity. Types I, II, III, V, VII, and VIII showed higher efficiency in differentiating (each P<0.05), whereas types IV (P=0.22) and VI (P=0.07) did not show significant differential capability according to ROC analysis.
Table 4
| Types | Description | Granulomas (n=561) | PLCs (n=561) | P value | PPV† | ROC analysis | |||
|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | Sensitivity (%) | Specificity (%) | P value | ||||||
| I | Nodule with satellite lesions | 159 (28.3) | 12 (2.1) | <0.001 | 0.93 | 0.631 (0.598–0.664) | 28.3 | 97.9 | <0.001 |
| II | Nodule with halo sign, calcification, or both | 92 (16.4)‡ | 17 (3.0) | <0.001 | 0.84 | 0.567 (0.533–0.600) | 16.4 | 97.0 | <0.001 |
| III | Nodule with a special shape | 43 (7.7) | 3 (0.5) | <0.001 | 0.93 | 0.536 (0.502–0.569) | 7.7 | 99.5 | 0.04 |
| IV | Nodule with ill-defined boundary | 48 (8.6) | 24 (4.3) | 0.002 | 0.67 | 0.521 (0.488–0.555) | 8.6 | 95.7 | 0.22 |
| V | Nodule with well-defined boundary and smooth margin, but no lobulation | 90 (16.0) | 26 (4.6) | <0.001 | 0.78 | 0.557 (0.523–0.591) | 16.0 | 95.4 | 0.001 |
| VI | Nodule with well-defined boundary and coarse margin, but no lobulation | 44 (7.8) | 79 (14.1) | 0.001 | 0.64 | 0.531 (0.497–0.565) | 14.1 | 92.2 | 0.07 |
| VII | Nodule with well-defined boundary, lobulation, and a vacuole sign | 7 (1.2) | 76 (13.5) | <0.001 | 0.92 | 0.561 (0.528–0.595) | 13.5 | 98.8 | <0.001 |
| VIII | Nodule with well-defined boundary, and lobulation, but no vacuole sign | 83 (14.8) | 324 (57.8) | <0.001 | 0.80 | 0.715 (0.684–0.745) | 57.8 | 85.2 | <0.001 |
Data in parentheses are expressed as number (percentage). n represents the number of nodules. †, in type I to V, PPV is used to assess the accuracy of predicting granulomas, while in type VI to VIII, PPV is employed to evaluate the accuracy of predicting PLCs. Similarly, for type I to V, the ROC curve is used to evaluate the diagnostic value of granulomas, while for type VI to VII, the ROC curve is used to evaluate that of PLCs. ‡, five granulomas having both satellite lesions and halo sign are repeatedly counted in type I and type II. In addition, all other data in this classification system were mutually exclusive between different types. AUC, area under the curve; CI, confidence interval; CT, computed tomography; PLC, peripheral lung cancer; PPV, positive prediction value; ROC, receiver operating characteristic.
Discussion
Granulomas are more easily to be misdiagnosed as lung cancers due to their similarities, while the differences between them remain not fully to be elucidated. In this study, the TSCT features of granulomas and PLCs were comprehensively evaluated and compared. It was observed that granulomas displayed some unique features alongside indicators commonly seen in PLCs. Based on their independent TSCT indicators, a classification system including eight types with distinct features was developed and most of them exhibited promising efficiency in distinguishing them. This newly established classification system may serve as an effective guideline for differentiating granulomas and PLCs in clinical practice.
In the present study, it was confirmed that the satellite lesion, halo sign, and calcification were more associated with granulomas. Histologically, satellite lesions in granulomas typically consist of small granulomatous lesions around the central lesion, more common in tuberculomas (16). In contrast, only a small proportion of PLCs in this study exhibited satellite lesions, likely due to tumor dissemination (4). Furthermore, granulomas may also exhibit peripheral inflammatory infiltration or alveolar hemorrhage in the active stage, leading to the presence of halo sign, while calcification tends to occur in the chronic stage (10,16,17). Although the halo sign could also been seen in PLCs, they were relative rare, likely due to neoplasm invasiveness (15,17). The frequency of the halo sign in granulomas in this study was lower than that in benign nodules in previous studies (15,17), probably because only pathologically confirmed lesions suspected to be malignant were studied while the halo sign was often considered as a benign feature. Calcifications in PLCs may originate from other diseases but be encapsulated by tumor or were of their own origin (24), while the present results indicated they were rarely detected in PLCs manifested as nodules.
Besides these indicators, this study also identified three special shapes, which were almost exclusively found in granulomas and had not been described in prior studies. The multi-nodular fusion and gear-like shape SNs may result from the formation and merging of multiple granulomas, with a mechanism akin to that of satellite lesions. The nodule with a tail shape may stem from the growth of peripheral granulomas along the proximal bronchus, indicating a distinct benign growth pattern. While lung cancer can produce similar manifestations in the distal bronchus due to bronchial destruction or obstruction, such changes in the proximal bronchus are uncommon (25,26). Nevertheless, squamous cell carcinoma and small cell lung cancer, which are closely associated with the bronchus, should be considered in the differential diagnosis of granulomas.
Lobulation is often associated with lung cancer and can indicate the continuous growth of malignant tumors (15,19). In this study, lobulation was more commonly seen in PLCs but was also present in a significant proportion of granulomas, similar to the previous findings (8). This could be attributed to the tendency of granulomas to exhibit ongoing growth compared to inflammatory pseudotumors and other benign nodules (9,27). Thus, granulomas with lobulation may be prone to misdiagnosis (8). This study found that combining multiple features proved effective in distinguishing lobulated granulomas from PLCs. It revealed that well-defined SNs with lobulation but lacking satellite lesions, halo sign, or calcification (type VIII) were typically PLCs. In addition, vacuole sign is related to the specific growth pattern of PLCs (25). By incorporating the vacuole sign, the positive prediction value and specificity of this feature (type VII) in diagnosing PLC improved. In contrast, because the non-calcified SNs with smooth margin were eventually confirmed to be benign regardless of growth during follow-up (28), the smooth SNs without lobulation (type V) were more likely to be granulomas in this study.
Types IV and VI showed significant differences between granulomas and PLCs, but their diagnostic value was lower compared to other types. An ill-defined boundary in granulomas typically indicates the spread of inflammation, while in PLCs, it may result from tumor infiltration into the surrounding lung tissue (15,29). Additionally, the spiculation, pleural indentation, and vascular convergence sign, which have been more associated with PLCs than benign SNs (4,21,27,30), were not independent indicators for predicting granulomas or PLCs. Pathologically, the growth and shrinkage of PLCs result in irregular margins, which are often coarse, even lobulated or spiculated (15,25). However, granulomas in chronic phase are more likely to undergo fibrosis and contraction than general inflammatory nodules, leading to coarse margins, spiculation, pleural indentation, and vascular convergence signs (10,16). Differentiating between SNs with these features can be challenging in the absence of other distinguishing characteristics. Fortunately, coarse granulomas in the chronic phase often exhibit a lower degree of enhancement, which can be detected through contrast-enhanced CT scans (16,31). Therefore, contrast-enhanced CT scans may be essential for further differentiation in such cases.
In this study, patients with granulomas were significantly younger than those with PLCs, and the age was an independent indicator for distinguishing them. This difference could be attributed to the fact that granulomas, being inflammatory lesions with diverse etiologies, can occur in individuals of any age, whereas older individuals are at a higher risk of developing lung cancer due to age-related immune system decline and increased exposure to carcinogens (4,5,32,33). Furthermore, the density of granulomas on non-enhanced CT images was significantly lower than that of PLCs, likely due to the higher prevalence of necrosis in granulomas (34). Therefore, patients’ age and lesion density on non-enhanced CT images can be considered key indicators for discrimination, particularly when the CT features of SNs are atypical.
In clinical practice, when it is difficult to determine an SN as a granuloma or PLC, certain features should be prioritized for assessment. Firstly, the presence of satellite lesions, halo sign, calcification, and special shapes should be evaluated, as their existence suggests a potential granuloma. Subsequently, attention should be focused on determining whether the boundary of the nodule is well-defined. For a well-defined SN, the presence of lobulation should be assessed. A lack of lobulation in an SN with smooth margin indicates a higher likelihood of a granuloma. Conversely, an SN with lobulation, especially if it exhibits a vacuole sign, is more indicative of a PLC. If an SN does not exhibit typical distinguishing features, additional clues for differential diagnosis can be obtained by considering the patient’s age, density of the lesion on non-enhanced CT images, and degree of enhancement.
This study has several limitations that should be considered. Firstly, the specific etiology of some granulomas remains unclear, primarily due to the retrospective nature of the study. Secondly, the analysis and comparison were limited to granulomas and PLCs, so the findings are specifically focused on distinguishing between these two types of lesions. Thirdly, while the classification system provided guidance for distinguishing most lesions, there were still some cases that could not be effectively differentiated, highlighting the need for further research in this area. Fourthly, the study was retrospective and conducted at a single center, suggesting that the findings may benefit from validation in larger, multicenter studies. Finally, not all patients underwent contrast-enhanced CT examinations, which limited the ability to further differentiate between certain types of lesions.
Conclusions
Granulomas and PLCs indeed share some similar features, leading to potential misdiagnosis. To accurately distinguish between them, it is essential to explore their distinct features and consider a combination of multiple indicators. Features such as satellite lesions, halo sign, calcification, and special shapes are highly indicative of granulomas. On the other hand, well-defined SNs with lobulation should raise suspicion for PLCs, especially those with a vacuole sign. It is important to note that spiculation, pleural indentation, and vascular convergence signs alone are not sufficient for distinguishing between PLCs and granulomas.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1505/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1505/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1505/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1505/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional review board of The First Affiliated Hospital of Chongqing Medical University (No. K2023-083), and the requirement for informed consent from the patients was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The Lancet Respiratory Medicine. Lung cancer screening in Europe: hurdles to overcome. Lancet Respir Med 2018;6:885. [Crossref] [PubMed]
- Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology 2009;253:606-22. [Crossref] [PubMed]
- Gao F, Li M, Ge X, et al. Multi-detector spiral CT study of the relationships between pulmonary ground-glass nodules and blood vessels. Eur Radiol 2013;23:3271-7. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Mazzone PJ, Lam L. Evaluating the Patient With a Pulmonary Nodule: A Review. JAMA 2022;327:264-73. [Crossref] [PubMed]
- The American College of Radiology. Lung-RADS® v2022. 29 September 2023. Available online: https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/Lung-RADS-2022.pdf
- Karpathiou G, Batistatou A, Boglou P, et al. Necrotizing sarcoid granulomatosis: A distinctive form of pulmonary granulomatous disease. Clin Respir J 2018;12:1313-9. [Crossref] [PubMed]
- Thiessen R, Seely JM, Matzinger FR, et al. Necrotizing granuloma of the lung: imaging characteristics and imaging-guided diagnosis. AJR Am J Roentgenol 2007;189:1397-401. [Crossref] [PubMed]
- Zhang R, Tian P, Qiu Z, et al. The growth feature and its diagnostic value for benign and malignant pulmonary nodules met in routine clinical practice. J Thorac Dis 2020;12:2019-30. [Crossref] [PubMed]
- Ohshimo S, Guzman J, Costabel U, et al. Differential diagnosis of granulomatous lung disease: clues and pitfalls: Number 4 in the Series "Pathology for the clinician" Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev 2017;26:170012.
- Tateishi U, Kusumoto M, Akiyama Y, et al. Role of contrast-enhanced dynamic CT in the diagnosis of active tuberculoma. Chest 2002;122:1280-4. [Crossref] [PubMed]
- Ambrosini V, Nicolini S, Caroli P, et al. PET/CT imaging in different types of lung cancer: an overview. Eur J Radiol 2012;81:988-1001. [Crossref] [PubMed]
- Bode SFN, Rohr J, Müller Quernheim J, et al. Pulmonary granulomatosis of genetic origin. Eur Respir Rev 2021;30:200152. [Crossref] [PubMed]
- Berliner L. PET-Negative Solid Pulmonary Nodules: Implications for Management Guidelines. Acad Radiol 2021;28:634-5. [Crossref] [PubMed]
- Xiao YD, Lv FJ, Li WJ, et al. Solitary Pulmonary Inflammatory Nodule: CT Features and Pathological Findings. J Inflamm Res 2021;14:2741-51. [Crossref] [PubMed]
- Lee JY, Lee KS, Jung KJ, et al. Pulmonary tuberculosis: CT and pathologic correlation. J Comput Assist Tomogr 2000;24:691-8. [Crossref] [PubMed]
- Ray A, Mittal A, Vyas S. CT Halo sign: A systematic review. Eur J Radiol 2020;124:108843. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Yang XM, Huo MH, Xie YZ, et al. Vacuole sign and small node sign in early peripheral lung cancer. Pathologic basis and diagnostic value. Chin Med J (Engl) 1988;101:818-22.
- Seki N, Fujita Y, Shibakuki R, et al. Easier understanding of pleural indentation on computed tomography. Intern Med 2007;46:2029-30. [Crossref] [PubMed]
- Yang X, Yan H, Liu H, et al. Vascular manifestations of small solitary pulmonary masses. Angiographic-pathologic correlations and clinical significance. Invest Radiol 1996;31:275-9. [Crossref] [PubMed]
- Benchoufi M, Matzner-Lober E, Molinari N, et al. Interobserver agreement issues in radiology. Diagn Interv Imaging 2020;101:639-41. [Crossref] [PubMed]
- Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol 2022;75:25-36. [Crossref] [PubMed]
- Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 2: pretest probability and algorithm. Chest 2013;143:840-6. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Yue JY, Chen J, Zhou FM, et al. CT-pathologic correlation in lung adenocarcinoma and squamous cell carcinoma. Medicine (Baltimore) 2018;97:e13362. [Crossref] [PubMed]
- Xu DM, van der Zaag-Loonen HJ, Oudkerk M, et al. Smooth or attached solid indeterminate nodules detected at baseline CT screening in the NELSON study: cancer risk during 1 year of follow-up. Radiology 2009;250:264-72. [Crossref] [PubMed]
- Kazerooni EA, Bhalla M, Shepard JA, et al. Adenosquamous carcinoma of the lung: radiologic appearance. AJR Am J Roentgenol 1994;163:301-6. [Crossref] [PubMed]
- Aoki T, Nakata H, Watanabe H, et al. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. AJR Am J Roentgenol 2000;174:763-8. [Crossref] [PubMed]
- Snoeckx A, Reyntiens P, Desbuquoit D, et al. Evaluation of the solitary pulmonary nodule: size matters, but do not ignore the power of morphology. Insights Imaging 2018;9:73-86. [Crossref] [PubMed]
- Muhm JR, Roberts CC. AJR teaching file: Solitary pulmonary nodule with enhancing rim sign. AJR Am J Roentgenol 2007;188:S5-6. [Crossref] [PubMed]
- Cui X, Han D, Heuvelmans MA, et al. Clinical characteristics and work-up of small to intermediate-sized pulmonary nodules in a Chinese dedicated cancer hospital. Cancer Biol Med 2020;17:199-207. [Crossref] [PubMed]
- Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health 2019;85:8. [Crossref] [PubMed]
- Rosen Y. Pathology of Granulomatous Pulmonary Diseases. Arch Pathol Lab Med 2022;146:233-51. [Crossref] [PubMed]


