
Statins as potential adjuvant therapy in lung cancer: a narrative review
Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide (1). It has two major clinically described subtypes: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), with NSCLC accounting for approximately 85% of all cases (2). In the past two decades, great progress has been made in multidisciplinary management of thoracic malignant tumors, including remarkable progress in chemotherapy, targeted therapy and immunotherapy (3,4). These innovations have thus reshaped the therapies for patients with lung cancer and improved clinical benefits. However, a considerable number of lung cancer patients still have poor response to the current standard treatment. The implementation of multi-line therapeutic strategies may potentially induce drug resistance, leading to tumor recurrence or refractory disease. Consequently, there is an urgent unmet need for the development of novel and more efficacious pharmacological interventions for lung cancer management.
Emerging evidence suggests the potential of repurposing non-oncological medications as anticancer agents. These include metformin, celecoxib, and statins, which have demonstrated varying degrees of antineoplastic activity (5-7). In particular, statins have been found to have potential inhibitory effects in lung cancer. Statins, widely used as lipid-lowering agents, function by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. Their long-term use is associated with a reduction in cardiovascular mortality (8). Recently, the potential antitumor properties of statins have garnered significant attention. In vitro studies have demonstrated that statins can induce apoptosis, inhibit invasion and metastasis, and suppress angiogenesis and tumor cell growth in lung cancer models (9,10). However, clinical research has yielded conflicting results, with some studies suggesting improved mortality rates in lung cancer patients receiving statins (11-13), while others have failed to demonstrate substantial clinical benefits (14,15). Several critical questions remain unresolved, including the potential benefit of adjunctive statin therapy in lung cancer patients, the differential effects of various statin types, and the optimal duration of statin treatment. This review aims to evaluate statins as adjunct therapy combined with conventional modalities of treatment like chemotherapy, targeted therapy, and immunotherapy, and also seeks to explain the molecular mechanisms underlying the antineoplastic effects of statin monotherapy and combination therapy in lung cancer. Furthermore, this review examines the role of statins across different stages of lung cancer and provides a comprehensive overview of their potential applications in both NSCLC and SCLC. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-66/rc).
Methods
Primarily, a literature search was conducted across databases including PubMed, Web of Science, and Google Scholar databases to identify relevant studies on the role of statins in lung cancer treatment. The search strategy is shown in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | Jan 2023 to Mar 2025 |
| Databases and other sources searched | PubMed, Web of Science, and Google Scholar databases |
| Search terms used | “Statin”, “lung cancer”, “adjuvant therapy”, “combination therapy” |
| Timeframe | From 1990 to 2025 |
| Inclusion criteria | Study type: original article, review, clinical trials; language restrictions: English only |
| Selection process | J.L. selected the studies, and all authors reviewed and approved the final list of studies included in the review |
The therapeutic potential of statins in lung cancer
Numerous preclinical studies using cell culture and animal models have demonstrated the potential of statin monotherapy to inhibit the growth and invasion of lung cancer (16,17). However, in most cases, statins are not utilized as single agents in treating lung cancer within clinical settings. Instead, they are commonly assessed in combination with the standard-of-care therapies that include chemotherapy, targeted agents, and immunotherapeutic approaches. The key information from the relevant clinical studies is summarized in Table 2 (18-31), while the study results are summarized in Table 3 and Table 4. The cumulative evidence extracted from these clinical trials indicates that combination regimens incorporating statins may potentially confer survival benefits, with hazard ratios (HRs) ranging from 0.44 to 1.38.
Table 2
| First author, year | Study design | Period | Total patients # | Histology | Stage | Statin type | Statin dosage | Combination therapy |
|---|---|---|---|---|---|---|---|---|
| Lee et al., 2017 (18) | Retrospective cohort | 2007–2012 | 7,298 | NSCLC (adenocarcinoma, squamous, other) | IIIB/IV | Simvastatin, lovastatin, pravastatin, | 40 mg/day | Carboplatin plus paclitaxel or pemetrexed or docetaxel |
| Lin et al., 2016 (19) | Retrospective cohort | 2007–2009 | 5,118 | NSCLC (adenocarcinoma, squamous, large cell carcinoma, other) | IV | Simvastatin, atorvastatin, lovastatin | NA | Chemotherapy |
| Maimon et al., 2012 (20) | Retrospective cohort | 2005–2011 | 107 | mNSCLC | IV | Atorvastatin | 40 mg/day | Erlotinib |
| Hung et al., 2017 (21) | Retrospective cohort | 1997–2013 | 8,535 | NSCLC (adenocarcinoma, squamous, other) | IIIB/IV | Simvastatin, lovastatin, pravastatin, fluvastatin, atorvastatin, rosuvastatin | NA | Gefitinib/erlotinib |
| Marrone et al.,2025 (22) | Retrospective cohort | 2007–2017 | 1,401 | NSCLC (adenocarcinoma, squamous, other) | I/II/III/IV | Simvastatin, rosuvastatin, lovastatin, pravastatin | NA | Nivolumab, pembrolizumab, atezolizumab, durvalumab |
| Rossi et al., 2021 (23) | Retrospective cohort | 2015–2020 | 122 | NSCLC (squamous, adenocarcinoma, other) | IV | NA | NA | Pembrolizumab, nivolumab, atezolizumab |
| Yu et al., 2018 (24) | Retrospective cohort | 2005–2013 | 301 | NSCLC (adenocarcinoma, squamous, other) | I/II/III/IV | NA | NA | Curative lung resection |
| Cortellini et al., 2020 (25) | Retrospective cohort | 2014–2020 | 528 | NSCLC (adenocarcinoma, squamous, other) | IV | NA | NA | Pembrolizumab, nivolumab, atezolizumab and other PD-1/PDL1 |
| Leighl et al., 2005 (26) | Prospective cohort | 2000–2002 | 774 | NSCLC (adenocarcinoma, squamous, undifferentiated, mixed, other) | IIIB with malignant effusion, IIIB without pleural effusion, IV | NA | NA | Carboplatin plus paclitaxel |
| Shepherd et al., 2005 (27) | Prospective cohort | 2001–2003 | 488 | NSCLC (adenocarcinoma, squamous, other) | IIIB/IV | NA | 40 mg/day | Erlotinib |
| Ramakrishna et al., 2012 (28) | Prospective cohort | 1998–2010 | 412 | NSCLC (adenocarcinoma, squamous, other) | I/II/III/IV | NA | NA | Curative lung resection |
| Lee et al., 2017 (29) | RCT | 2012–2015 | 68 | Nonadenocarcinomatous-NSCLC | IIIB/IV | Simvastatin | 40 mg/day | Afatinib |
| Seckl et al., 2017 (30) | RCT | 2007–2012 | 846 | SCLC | Limited disease, extensive disease | Pravastatin | 40 mg/day | Etoposide plus cisplatin or carboplatin |
| Han et al., 2011 (31) | RCT | 2006–2008 | 106 | NSCLC (adenocarcinoma, squamous, NOS) | IIIB/IV | Simvastatin | 40 mg/day | Gefitinib |
mNSCLC, metastatic NSCLC; NA, not available; NOS, not otherwise specified; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; RCT, randomized controlled trial; SCLC, small cell lung cancer.
Table 3
| First author, year | Total patients # | Study design | Outcome | HR (95% CI) | P value |
|---|---|---|---|---|---|
| Lee et al., 2017 (18) | 7,298 | Retrospective cohort | Overall survival | 0.80 (0.74–0.86) | <0.001 |
| Propensity scores case-control | Overall survival | 0.83 (0.73–0.95) | 0.007 | ||
| Lin et al., 2016 (19) | 5,118 | Retrospective cohort | Overall survival | 0.86 (0.81–0.91) | 0.001 |
| Lung cancer survival | 0.81 (0.75–0.87) | 0.001 | |||
| Propensity scores case-control | Overall survival | 0.86 (0.78–0.95) | 0.001 | ||
| Lung cancer survival | 0.79 (0.70–0.90) | 0.001 | |||
| Marrone et al., 2025 (22) | 1,401 | Retrospective cohort | Cancer-specific mortality | 0.59 (0.35–0.99) | <0.001 |
| Overall mortality | 0.62 (0.41–0.94) | <0.001 | |||
| Maimon et al., 2012 (20) | 107 | Retrospective cohort | Progression-free survival | 0.44 (0.22–0.88) | 0.02 |
| Overall survival | 0.63 (0.36–1.09) | 0.01 | |||
| Yu et al., 2018 (24) | 301 | Retrospective cohort | Cancer-specific survival | 0.468 (0.258–0.849) | 0.01 |
| Overall survival | 0.585 (0.378–0.904) | 0.02 | |||
| Cortellini et al., 2020 (25) | 528 | Retrospective cohort | Overall survival | 0.79 (0.62–1.01) | 0.06 |
| Progression-free survival | 0.87 (0.72–1.06) | 0.19 | |||
| Leighl et al., 2005 (26) | 774 | Prospective cohort | Progression-free survival | 1.02 (0.72–1.45) | 0.90 |
| Overall survival | 0.95 (0.44–1.07) | 0.75 | |||
| Shepherd et al., 2005 (27) | 488 | Prospective cohort | Progression-free survival | 0.72 (0.42–1.23) | 0.20 |
| Overall survival | 0.82 (0.51–1.34) | 0.43 | |||
| Ramakrishna et al., 2012 (28) | 412 | Prospective cohort | Overall survival | 0.66 (0.45–0.96) | 0.03 |
| Lee et al., 2017 (29) | 68 | RCT | Progression-free survival | 1.38 (0.84–2.29) | 0.90 |
| Seckl et al., 2017 (30) | 846 | RCT | Progression-free survival | 0.98 (0.85–1.13) | 0.81 |
| Overall survival | 1.01 (0.88–1.16) | 0.90 | |||
| Han et al., 2011 (31) | 106 | RCT | Progression-free survival | 0.89 (0.60–1.32) | 0.49 |
| Overall survival | 0.88 (0.57–1.35) | 0.49 |
CI, confidence interval; HR, hazard ratio; RCT, randomized controlled trial.
Table 4
| First author, year | No. of patients with statins | No. of patients without statins | Outcome | Statins group (months) | Non-statins group (months) | P value |
|---|---|---|---|---|---|---|
| Lee et al., 2017 (18) | 1,204 | 5,594 | Median OS | 14.5 | 9.9 | <0.001 |
| Hung et al., 2017 (21) | 1,707 | 6,828 | Median OS | 35.5 | 23.9 | <0.001 |
| Median PFS | 8.3 | 6.1 | <0.001 | |||
| Rossi et al., 2021 (23) | 70 | 52 | Median OS | 19.94 | 10.94 | <0.001 |
| Median PFS | 17.57 | 9.57 | <0.001 | |||
| Maimon et al., 2012 (20) | 51 | 56 | Median OS | 35.0 | 19.0 | 0.01 |
| Median PFS | 12.0 | 3.0 | 0.02 | |||
| Lee et al., 2017 (29) | 36 | 32 | Median OS | 10.7 | 7.0 | 0.93 |
| Median PFS | 1.0 | 3.6 | 0.24 | |||
| Seckl et al., 2017 (30) | 422 | 424 | Median OS | 10.7 | 10.6 | 0.90 |
| Median PFS | 7.7 | 7.3 | 0.81 | |||
| Han et al., 2011 (31) | 52 | 56 | Median OS | 13.6 | 12.0 | 0.49 |
| Median PFS | 3.3 | 1.9 | 0.49 |
OS, overall survival; PFS, progression-free survival.
Statins in combination with chemotherapy
Chemotherapy remains one of the cornerstones in the multimodal treatment of lung cancer, offering symptom palliation and improving quality of life in advanced disease, and decreasing recurrence risk in early-stage disease with adjuvant chemotherapy (32). Growing evidence indicates that the addition of statins may enhance the efficacy of chemotherapy. In vitro studies have shown that the combination of atorvastatin and carboplatin promotes apoptosis in lung cancer cells (33). Emerging clinical studies examining the combinations of statins and chemotherapy have produced varied results, likely due to differences in study design, patient characteristics, and the types of statins used (Table 3). Among the four studies reviewed, the randomized controlled trial (RCT) and one cohort study did not show notable improvements, whereas two other large-scale cohort studies indicated better survival outcomes. Progression-free survival (PFS) and overall survival (OS) remain two of the critical endpoints utilized to assess treatment efficacy in oncology. In a NSCLC cohort study, the addition of statin therapy did not significantly affect PFS [HR =1.02, 95% confidence interval (CI): 0.72–1.45] or OS (HR =0.95, 95% CI: 0.44–1.07) (26). Likewise, in SCLC patients receiving 40 mg/day pravastatin, an RCT did not find meaningful improvements in PFS (HR =0.98, 95% CI: 0.85–1.13) or OS (HR =1.01, 95% CI: 0.88–1.16) (30). However, a large-scale cohort study by Lee et al. reported improved OS in NSCLC patients receiving statins (HR =0.80, 95% CI: 0.74–0.86) (18). This was further corroborated by the propensity-matched cohort study that supported the potential beneficial role of statins in NSCLC treatment (HR =0.83, 95% CI: 0.73–0.95). Besides, a separate population-based study focusing on stage IV NSCLC found that among patients receiving systemic chemotherapy, statin use was associated with a 14% reduction in the risk of mortality (HR =0.86, 95% CI: 0.81–0.91), a benefit that persisted in the propensity-score matched analysis (HR =0.86, 95% CI: 0.78–0.95) (19). Lung cancer-specific survival was also improved (HR =0.81, 95% CI: 0.75–0.87), with a similar benefit observed in the propensity-score matched subgroup of patients who received chemotherapy (HR =0.79, 95% CI: 0.70–0.90). This aligns with the propensity-matched cohort study that further supported the potential beneficial role of statins in NSCLC treatment (HR =0.83, 95% CI: 0.73–0.95). Overall, while smaller-scale studies have not demonstrated a conclusive therapeutic advantage, large-scale cohort studies suggest that adding statins to chemotherapy may improve survival outcomes, underscoring their potential role in lung cancer treatment.
Statins in combination with targeted therapy
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are the standard-of-care in patients with advanced NSCLC with EGFR mutations (34,35). As illustrated in Tables 3,4, two out of five studies investigating the combination of statins with targeted therapy demonstrated considerable improvement in survival outcomes, while the other three showed no meaningful benefit. These included two RCTs and three cohort studies. Both RCTs consistently showed non-significant outcomes for statin-EGFR-TKI combination therapy: Han et al. showed numerically favorable PFS (HR =0.89, 95% CI: 0.60–1.32) and OS (HR =0.88, 95% CI: 0.57–1.35) (31), while Lee et al. demonstrated an unexpected trend toward increased PFS risk (HR =1.38, 95% CI: 0.84–2.29), with all 95% confidence intervals encompassing the null value (29). Two cohort studies revealed comparable outcomes: Maimon et al. reported a significant 56% reduction in PFS risk (HR =0.44, 95% CI: 0.22–0.88) without OS benefit (HR =0.63, 95% CI: 0.36–1.09) (20), while Shepherd et al. showed consistent but non-significant trends for both PFS (HR =0.72, 95% CI: 0.42–1.23) and OS (HR =0.82, 95% CI: 0.51–1.34) (27). Notably, all four of these studies had relatively small sample sizes, potentially limiting their statistical power. In contrast, a large-scale cohort study involving 8,535 patients provided compelling evidence supporting the use of statins in combination with targeted therapy. The patients were stratified into statin and non-statin groups, with results revealing significant improvements in median OS (35.5 vs. 23.9 months) and median PFS (8.3 vs. 6.1 months) favoring the statin group (Table 4) (21). These findings suggest that the addition of statins to EGFR-TKIs may potentiate the efficacy of targeted therapy and potentially improve survival outcomes in patients with advanced NSCLC harboring EGFR mutations.
Statins in combination with immune checkpoint inhibitors (ICIs)
ICIs, such as antibodies targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (36), programmed cell death protein 1 (PD-1) (37), and programmed death receptor-ligand 1 (PD-L1) (38), have been used rather successfully in the treatment of advanced NSCLC. In this context, recent studies have investigated a multiplicity of combination strategies with immunotherapy combined with radiotherapy or chemoradiotherapy, or antiangiogenic therapy, with very promising clinical benefits shown (39-42). Clinical evidence suggests that concomitant use with statins may further increase therapeutic effectiveness. In a population-based study of 1,401 Medicare beneficiaries with advanced NSCLC, concurrent administration of statins and ICIs was associated with a 41% reduction in lung cancer-specific mortality (HR =0.59, 95% CI: 0.35–0.99) and a 38% decrease in overall mortality (HR =0.62, 95% CI: 0.41–0.94) (Table 3) (22). Similarly, Rossi et al. demonstrated significant improvements in survival outcomes in a cohort of 122 patients, with median OS increasing from 10.94 to 19.94 months and median PFS from 9.57 to 17.57 months (Table 4) (23). In contrast, a retrospective analysis of 528 patients revealed non-significant trends toward prolonged PFS (HR =0.79; 95% CI: 0.62–1.01) and OS (HR =0.87; 95% CI: 0.72–1.06) with statin-immunotherapy combination therapy (Table 3) (25). Collectively, these data indicate that statins may potentiate immunotherapy responses in NSCLC, though the magnitude of benefit appears inconsistent across studies. However, there are still a few outstanding issues related to creating optimal combination therapy regimens with statins. Determining optimal regimens and patient selection criteria presents a field for further research and study. Identifying potential biomarkers that could predict which patients would respond to statin-immunotherapy combinations might facilitate stratification of patients and creation of personalized treatment strategies. The incorporation of statins in immunotherapy regimens heralds an approach that is promising in regard to possibly better outcomes for patients with NSCLC. In this regard, further research calls for the full expounding of the clinical implications and optimization of the implementation of this combination approach.
Statins as adjuvant therapy in surgical management of NSCLC
Curative-intent surgical resection remains the gold standard of therapeutic approaches for patients with NSCLC, offering individuals the best chance of long-term survival and potential cure (43). However, there is growing consensus that surgery alone is inadequate treatment for patients with stage IB–IIIA NSCLC (44). Neo-adjuvant or adjuvant chemotherapy combined with surgery has a better prognosis (45). In this setting, statins are exciting adjunctive therapy, with recent studies indicating that patients who adhere to post-operative statin regimens have a significantly longer survival as in Table 3. In a retrospective analysis by Yu et al. of 301 patients treated with radical surgery for lung cancer, there were marked survival benefits with regular statin use. In particular, statin users exhibited markedly improved OS (HR =0.585, 95% CI: 0.378–0.904) and cancer-specific survival (HR =0.468, 95% CI: 0.258–0.849) (24). Ramakrishna et al. reported a similar finding within a cohort of 412 patients and noted prolonged OS (HR =0.66, 95% CI: 0.45–0.96) among the regular statin users (28). These data suggest that statins might serve as effective adjuvant therapy in patients who have undergone curative surgical resection of the tumor. The possible mechanism of the benefit with these drugs could be the reduction of the risk of recurrence and metastasis, therefore extending life with extended survival and quality of life. A survival advantage due to antitumor effects of statins, however, could not be definitively concluded since statins might also decrease some postoperative complications, thus enhancing survival (46).
Safety profile of statins in combination cancer therapies
Three RCTs and one cohort study have evaluated the safety profile of statins (23,29-31). Myalgia and myositis are the primary adverse effects associated with statin use. The incidence of any-grade myalgia and myositis was 18% in the statin group and 18.8% in the control group, while the incidence of grade 3 or higher events was 0.7% and 1.0%, respectively, with no statistically significant differences between the two groups (30). Furthermore, the use of statins did not increase the risk of adverse events in patients undergoing chemotherapy, TKIs, or ICIs. The combination of statins with chemotherapy showed a comparable incidence of grade 3–5 adverse events between the pravastatin group and the placebo group (81.2% vs. 81.4%, P=0.94) (30). When statins were used alongside TKIs, the occurrence of grade 3 or higher rash was also similar between the two groups, with 3 cases in the statin group and 2 in the placebo group (3/88 vs. 2/86) (29,31). For statins combined with immunotherapy, 26 patients in the statin group experienced any-grade toxicity (37.14%), including 5 cases of grade 3–4 toxicity (7.14%), and 4 patients (5.71%) required permanent discontinuation of treatment. In comparison, the non-statin group reported 18 cases of any-grade toxicity (34.61%), 4 cases of grade 3–4 toxicity (7.84%), and 2 patients (3.92%) requiring permanent discontinuation. Notably, no significant differences were observed between the two groups in the rates of any-grade toxicity (P=0.709) or grade 3–4 toxicity (P=0.851) (23).
In summary, statins demonstrate a favorable safety profile when used in combination with chemotherapy, EGFR-TKIs, or ICIs, without significantly increasing the incidence of severe adverse events. These findings suggest that statins can be safely incorporated as adjunctive agents in cancer treatment regimens. However, while the current evidence is encouraging, further large-scale, high-quality studies are needed to confirm the safety and potential benefits of statins across different therapeutic settings. Additionally, future research should focus on optimizing the integration of statins into clinical practice to maximize their therapeutic potential.
Mechanisms of statins in inhibiting NSCLC
One of the metabolic adaptations promoting rapid tumor growth is the upregulation of the mevalonate pathway. This pathway is associated with the occurrence, development, and phenotype of many human malignancies, including lung cancer (47). Statins exert their inhibitory effects on lung cancer through multiple distinct mechanisms, all of which are centered on the mevalonate pathway as the pivotal axis of action (Figure 1). In our study, these mechanisms have been categorized into two distinct groups for discussion: the antitumor mechanisms of statins as monotherapy, and the mechanisms by which statins enhance the efficacy of anti-lung cancer drugs. This approach to discussion facilitates a comprehensive understanding of the mechanisms by which statins exert their effects in both direct and adjuvant therapeutic strategies for lung cancer.
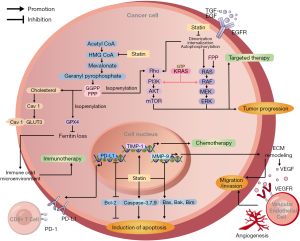
Intrinsic anti-tumor mechanisms of statins
Statins possess inherent antitumor properties, which are primarily manifested in two aspects: inhibition of tumor cell growth and metastasis, and induction of tumor cell apoptosis (48).
Inhibition of tumor cell growth and metastasis
One mechanism by which statins suppress growth and metastasis of tumors is through suppression of cholesterol synthesis, as cholesterol is regarded as the main structural component of the cell membrane (49). Moreover, high levels of cholesterol within cancer cells can promote tumor progression (50,51). Concurrently, statins their effects through the mevalonate pathway, which decreases the synthesis of isoprenoids such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) (52,53). Isoprenoids are crucial precursors for the formation of molecules controlling the cell cycle. Therefore, statins exert a profound influence on the expression and activities of cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitor (54). This regulation of cell cycle regulators results in cell cycle arrest at two key checkpoints: the G1/S and G2/M transitions (55,56). Most interestingly, tumor cells, compared to normal cells, are exhibited to be more sensitive to the antiproliferative action of statins. This may be related to differential growth factor-mediated signal transduction in the two cell types, resulting from accelerated proliferation and rapid growth known to be intrinsic to neoplastic cells. In addition to their effects on cholesterol biosynthesis and cell cycle regulation, statins can also downregulate the expression of matrix metalloproteinase-9 (MMP-9), a member of the matrix metalloproteinase (MMPs) family that plays a crucial role in tumor growth and metastasis (57). It has been demonstrated that MMP-9 degrades many proteins in the extracellular matrix (ECM) (58). This degradative action not only hydrolyzes histological barriers to the invasion of tumor cells but also releases growth factors and cytokines that are stored within the ECM, such as vascular endothelial growth factor (VEGF) (59,60). Thereafter, such factors promote the growth and migration of vascular endothelial cells and the formation of blood vessels associated with the tumor.
Induction of tumor cell apoptosis
Secondly, statins also exhibit an inducing effect on apoptosis in tumor cells. The mechanism is also mediated through the inhibition of isoprenoids production via the mevalonate pathway (61). These isoprenoids bind to cellular proteins, including G proteins, thereby activating isoprenylation of the proteins, leading to functional activation (62-64). Ras and Rho are the most well-known isoprenylated proteins. They play key roles in critical cellular signaling pathways, such as ERK/MAPK and PI3K/AKT, and promote the expression of genes associated with cell survival activities, including proliferation, differentiation, and apoptosis (65). Statins exert important cellular effects through their influence on the function of such proteins. Different experiments showed that statin therapy increases the expression of pro-apoptotic molecules such as Bak, Bim, and Bax while decreased that of anti-apoptotic molecules like Bcl-2, in addition to the activation of caspases (9,48,66). These observations indicate that statins might be able to induce apoptosis in tumor cells.
Synergistic mechanisms of statins
Besides their inherent antitumor properties, statins can enhance the efficacy of anti-lung cancer drugs through multiple mechanisms, resulting in synergistic effects between statins and anti-lung cancer agents.
Enhancing the efficacy of chemotherapeutic agents
Platinum-based drugs, which are major chemotherapeutic agents, exert their anti-invasive effects on NSCLC cells mainly by inhibiting AKT activity and increasing TIMP-1 expression (67). However, the inhibitory effect of carboplatin on AKT activity is transient. The addition of statins to chemotherapy has synergistic effects on the sustained inhibition of AKT activation. Given that aberrant AKT activation is associated with platinum resistance in NSCLC, statins may also help overcome carboplatin resistance. In addition, statins enhanced carboplatin-induced upregulation of TIMP-1 expression, and TIMP-1, as a metalloproteinase inhibitor, has been shown to inhibit tumor invasion (33,68).
Enhancing EGFR-TKI efficacy and overcoming resistance
Studies have shown that the synergistic effects of statins and EGFR-TKIs may be attributed to their multifaceted inhibition of the EGFR signaling pathway (69,70). The EGFR signal transduction system is an important cellular signal transduction that starts with the binding of upstream ligands like EGF and TGF-α to EGFR, which promotes it to form dimers, resulting in autophosphorylation and finally leading to the activation of the receptor. The activated EGFR subsequently triggers downstream cascades, primarily involving the RAS/RAF/MEK/ERK, PI3K/AKT, and JAK/STAT pathways (71). Abnormal EGFR activation may further cause dysregulation of these downstream signaling pathways, which contribute to tumor cell proliferation, growth, and metastasis. In this context, the main target of EGFR-TKIs is the intracellular domain of EGFR tyrosine kinase. EGFR-TKIs inhibit the autophosphorylation process of EGFR by competitively binding to the EGFR tyrosine kinase domain (72). Statins exert their effects through a distinct yet complementary mechanism. They prevent formation of GGPP, hence blocking the normal isoprenylation of Rho family proteins and make them inactive. Rho proteins inactivation can disassemble the actin cytoskeleton and impinge on EGFR dimerization, autophosphorylation, and internalization processes (73-75). Though the direct targets of EGFR-TKIs and statins are distinct, their effects eventually converge to strongly inhibit activation of EGFR. Importantly, statins can further attenuate EGFR downstream signaling by downregulating Ras and Rho proteins, leading to suppression of ERK and AKT activation (62,63). Consequently, the combination of statins and EGFR-TKIs showed better and more comprehensive inhibition of the EGFR signaling pathway.
K-Ras mutations represent a prevalent genetic alteration in NSCLC, occurring in 25–30% of adenocarcinomas (76). Constitutive activation of K-Ras signaling circumvents the requirement for EGFR-TKI activation, leading to primary resistance to EGFR-TKIs; in these cases, EGFR-TKIs monotherapy is usually ineffective (77). Combination therapy with statins and EGFR-TKI has shown potential to effectively combat this particular type of resistance (78). Statins reduce FPP and GGPP, which are both key precursors in post-translational modification of KRAS proteins. Without proper post-translational modifications, the KRAS protein cannot be properly localized to the cell membrane, thus affecting its signal transduction ability (79). This process attenuates the sustained signaling activation induced by the K-Ras mutation and may therefore re-sensitize tumor cells to EGFR-TKIs. Moreover, statins disrupt the KRAS/PI3K and KRAS/Raf complexes in KRAS-mutant NSCLC cells, thereby inhibiting AKT and ERK activity. This further enhances the efficacy of EGFR-TKIs (80,81). To validate this mechanism, the researchers conducted a series of experiments. The results showed that this synergistic effect was particularly evident in K-Ras mutant NSCLC cell lines such as Calu6 and A549 (81,82). These cell lines showed resistance when treated with EGFR-TKI monotherapy; however, growth was significantly inhibited by the addition of statins. Taken together, these experimental results support a mechanism by which statins overcome EGFR-TKI resistance by modulating the activities of AKT and ERK in KRAS mutant NSCLC cells.
This combined treatment strategy is not only effective against primary drug-resistant cells, but also has a pronounced synergistic effect on acquired drug-resistant cells. Although the underlying mechanisms are not identical, they both involve dual regulation of cholesterol metabolism and glucose uptake in neoplastic cells. In EGFR-TKI resistant NSCLC cells, cholesterol levels are markedly elevated, regardless of primary or acquired resistance. This elevation is attributed to EGFR-mediated upregulation of LDLR expression, which enhances cellular cholesterol uptake, thereby promoting cellular metabolism and proliferation. EGFR-TKIs can downregulate LDLR expression by inhibiting the SREBP-1-dependent EGFR signaling pathway, thereby reducing intracellular cholesterol content (70). Therefore, the combination of EGFR-TKIs and statins can simultaneously target cholesterol synthesis and cellular uptake pathways, synergistically reducing intracellular cholesterol levels. Statins also have the capacity to inhibit Caveolin-1 (Cav1) expression by reducing cellular cholesterol levels, thereby disrupting the Cav1-GLUT3-mediated glucose uptake and further restricting the growth of TKI-resistant NSCLC cells (83). Notably, this physical interaction between Cav1 and GLUT3 is much stronger in TKI-resistant cells than in TKI-sensitive cells, maybe due to changes in the physical properties of Cav1 itself. Amongst all glucose transporters, GLUT3 is characterized by the highest affinity and transport capacity for glucose and shows increased interaction with Cav1 in cells with acquired resistance to TKI, significantly increasing glucose uptake. This event is differential between the TKI-sensitive and-resistant cells and reflects differential metabolic requirements of cell growth and survival. In brief, statins would decrease cholesterol levels, but much more, they would inhibit the Cav1-GLUT3 axis, ultimately disrupting the metabolic equilibrium of neoplastic cells.
Enhancing immunotherapy efficacy
The efficacy of immunotherapy extremely depends on the immune status of the tumor microenvironment (TME) (84). Statins reshapes the components of the TME via multiple mechanisms, further enhancing the efficacy of immunotherapy (85). They can induce ferroptosis by downregulating GPX4 expression (86,87). The silencing of GPX4 makes a contribution to the reduction in levels of ferritin in NSCLC cells and helps to generate an immuno-cold microenvironment in which CD8+ T cells can exert an anti-tumor effect (88-90). Another mechanism involves statins transcriptionally suppressing PD-L1 expression in tumor cells, thereby reducing their capacity for immune evasion and enabling T cells to more effectively recognize and eliminate tumor cells (91). These synergistic effects converge to foster a microenvironment more amenable to immunotherapeutic intervention, thereby enhancing the efficacy of anti-PD-1/PD-L1 immunotherapy in NSCLC.
Synergy with other drugs
It has been shown that statins can enhance the efficacy of other drugs against lung cancer treatment, including the ABL allosteric inhibitor ABL001 and the nonsteroidal anti-inflammatory drug (NSAID) celecoxib. Synergy between ABL allosteric inhibitors and statins is attained through their activities on the protein isoprenylation pathway, specifically in the process of protein geranylgeranylation. In a murine model study, researchers investigated the combination therapy of ABL001 and simvastatin. The results demonstrated that this combination therapy successfully inhibited the growth of lung cancer brain metastases and gefitinib-resistant metastatic tumors, while significantly improving mouse survival rates (92). Celecoxib, a NSAID, has shown potential in studies to reduce the risk of lung cancer (93-95). The study reported that simvastatin down-regulates survivin, an anti-apoptotic gene, by inhibiting the AKT signal pathway, which potentiated the celecoxib-induced apoptosis in lung cancer cells. Combination therapy with simvastatin and celecoxib also increases intracellular production of reactive oxygen species (ROS), leading to apoptosis (96). Therefore, even for some non-classical anti-tumor drugs, statins can also enhance their anti-tumor effects by different mechanisms.
The timing of statins in lung cancer treatment
Prognosis in patients diagnosed with lung cancer varies considerably over the different stages, with the efficacy of traditional treatment modalities mirroring this variation. The potential benefits of statins across various stages of lung cancer have garnered immense clinical interest, as these drugs have been recognized for their anti-neoplastic properties.
Statins in lung cancer prevention
Retrospective analyses have given exciting results about statin use in lung cancer prevention and treatment. Continuous statin use of up to 6 months has been related to a 53% reduced risk of lung cancer in patients with chronic conditions like cardiovascular diseases who had not developed lung cancer. The risk reduction was demonstrated to increase to 77% after 4 years of use (97). In another report, statin intake was found to have significantly decreased the risk of lung cancer in men with hypercholesterolemia (HR =0.64, 95% CI: 0.47–0.85), and the greatest effect noted among smokers (98). These studies indicate that regular use of statins may possibly be a preventive measure in lessening the risk of developing lung cancer. Despite their widespread application in managing hypercholesterolemia and cardiovascular diseases, the role of statins in lung cancer prevention has been substantially underestimated by researchers. This revelation could pave the way for promising future strategies in lung cancer prevention.
Statins in early-stage lung cancer
In patients with early-stage lung cancer, regardless of any previous chemotherapy, statin use has been associated with reduced 5-year mortality rates (99). This novel finding provides a completely new perspective on the treatment of early-stage lung cancer, as statins appear to have an adjuvant therapeutic role. However, it is essential to note that the observed survival benefits in early-stage patients might be attributed to statins’ positive impact on cardiovascular health. Given the already high 5-year survival rate for patients with early-stage lung cancer, the increase in survival is not necessarily due to the inhibitory effect of statins on lung cancer, but may be due to a reduction in cardiovascular disease mortality.
Statins in late-stage lung cancer
Conversely, in late-stage lung cancer patients with lower 5-year survival rates, statins demonstrate long-term survival effects primarily through their anti-tumor properties, with the influence of reducing cardiovascular disease mortality on study outcomes being relatively minimal. A large study of patients with stage IV NSCLC showed that the median survival was 7 months in the statin group and 4 months in the non-statin group (19). This suggests that statins also have a certain effect on patients with late-stage lung cancer. Preclinical researches have also claimed the survival benefits of fluvastatin for lung cancer patients with bone metastases (100,101), albeit in the animal experimental stage, offering valuable evidence for future clinical investigations.
The heterogeneity of statins and its impact on treatment outcomes
The role of statins in lung cancer remains controversial because a minority of studies presenting conflicting results regarding their survival benefits. The inconsistent results may be due to several factors: small sample size; experimental bias might take place; and most importantly, heterogeneity of statins.
Broadly, there are two classes of statins: hydrophilic and lipophilic. Hydrophilic statins, such as pravastatin and rosuvastatin, are poorly cell-membrane permeable and need active uptake by particular transport proteins on hepatocyte membranes for exerting the intracellular effects. Therefore, the action is majorly hepatic; very minimal in other organs. This selectivity confers an advantage in terms of reduced adverse muscle and central nervous system effects (102). In contrast, lipophilic statins, such as simvastatin, lovastatin, and atorvastatin, have wider tissue distribution and may thus exert effects beyond lipid-lowering, relevant to inhibition of lung cancer (103). However, this widespread distribution may also lead to neurological side effects, such as dizziness and headaches, as well as musculoskeletal symptoms like myalgia. Despite these potential adverse events, statins generally exhibit a favorable safety profile with good patient tolerability (104). In the context of lung cancer treatment, lipophilic statins may represent a more efficacious option due to their enhanced inhibitory effects on lung cancer cells (12). Such a selection of hydrophilic statins may explain less optimal treatment outcomes that were shown in some studies related to lung cancer.
Statins in overcoming chemoresistance in SCLC
SCLC constitutes approximately 15% of lung cancer cases and represents a neuroendocrine malignancy of highly aggressive nature (1). It is characterized by early dissemination and extensive metastasis, with the majority of patients presenting with advanced-stage disease at initial diagnosis, precluding surgical intervention (105). The current therapeutic landscape for SCLC includes radiotherapy, systemic chemotherapy with platinum drugs (e.g., cisplatin or carboplatin) combined with etoposide or irinotecan, and immunotherapy, mainly ICIs, with very few effective targeted therapies available. Despite initial high chemosensitivity and impressive response rates, SCLC rapidly develops chemoresistance after several treatment cycles, resulting in early recurrence in the majority of patients. Ineffective strategies to overcome chemoresistance have resulted in stagnation in the rate of patient survival over the past few decades. Therefore, new therapeutic strategies are now more urgently needed to meet the existing challenges in the management of SCLC.
To address chemoresistance in SCLC, it is imperative to elucidate the underlying mechanisms. One of the major studies in this regard indicated that high expression of GGPS1 induces chemoresistance in SCLC and that statins may overcome this resistance by suppressing GGPS1 expression (106). In this study, the clinical response following statin administration was analyzed in seven recurrent patients after first-line chemotherapy. Among these seven patients, three were treated with statin plus chemotherapy, and four were treated with chemotherapy only. Indeed, all three patients on simvastatin combined with chemotherapy showed appreciable responses: SP651 had a 40% reduction in the size of the primary tumor; in SP983, there was a 20% reduction in liver metastases; and SP772 had a 40–60% reduction in brain metastases, while the primary tumor shrank stably. Remarkably, two patients remained progression-free as long as 17 weeks. Comparatively, the PFS of those four patients who had chemotherapy alone ranged from 6 to 10 weeks. The findings suggest that statins may be able to overcome chemoresistance in SCLC, enhance response rates to chemotherapy, and maybe even prolong patient survival.
However, two clinical trials reported poor outcomes with statin and chemotherapy combinations (30,107). The discrepancies in research results may be attributed to heterogeneity in patient populations and failure to account for patient sensitivity to chemotherapeutic agents. When chemoresistance has not developed, conventional statin doses may fail to significantly inhibit SCLC growth. Conversely, when resistance occurs, the addition of statins may overcome chemoresistance (106). The efficacy of statins in SCLC is also intimately linked with the type of statin used. Based on previous discussions, lipophilic statins exhibited more powerful anticancer abilities; therefore, the use of a suitable statin type was another major determinant of efficacy in SCLC.
Discussion
Statins have demonstrated preclinical potential as adjunctive agents in lung cancer therapy, but clinical evidence remains inconclusive, with significant discrepancies between observational studies and RCTs. While large-scale cohort studies consistently demonstrate significant survival benefits (18,19,21,22), all three RCTs failed to show significant efficacy (29-31). This divergence arises from six interrelated factors:
First, healthy user bias in cohort studies is particularly significant. For example, research by Hung et al. based on the National Health Insurance database in China’s Taiwan region revealed that statin users had higher rates of comorbidities such as diabetes (52.8% vs. 23.4%) and hypertension (78.4% vs. 55.6%) compared to non-users and were more likely to receive chemotherapy (30.9% vs. 28.8%) (21). This suggests that statin users might engage in better health management due to cardiovascular comorbidities, indirectly influencing survival outcomes. In contrast, RCTs balance such confounders through randomization. Second, immortal time bias is prevalent in cohort studies. For instance, Hung et al. required cumulative statin use ≥28 days, inherently excluding early mortality cases post-diagnosis from the exposure group (21). RCTs avoid this bias by randomizing patients at diagnosis. Third, dose and duration differences are critical. Observational studies often involve long-term statin use (>5 years) with diverse agents, potentially accumulating antitumor effects or drug-specific benefits. In contrast, RCTs (e.g., pravastatin 40 mg/day until disease progression) that employ short-term, single-agent regimens are unlikely to achieve antitumor plasma concentrations (30). Fourth, statistical power disparities amplify differences: Lin et al.’s cohort (n=5,118) could detect subtle effects (19), whereas underpowered RCTs such as the Afatinib-Simvastatin trial (n=68) lack capacity to identify modest effect sizes or subgroup-specific benefits (29). Fifth, population heterogeneity: cohort studies (e.g., Marrone et al.) broadly include NSCLC patients (even PD-1/PD-L1 inhibitor users), potentially capturing sensitive subgroups (e.g., KRAS-mutated or metabolic syndrome patients) (22), while RCTs often restrict eligibility (e.g., small-cell lung cancer only) or enforce strict exclusions (e.g., hepatic/renal dysfunction), limiting generalizability (30). Finally, unmeasured confounding persists in observational designs-despite statistical adjustments for comorbidities and treatments, factors like EGFR mutation status or TME (unavailable in Hung et al.’s claims data) could bias results (21), whereas RCTs minimize such bias through randomization.
In summary, observational studies reporting positive associations may reflect systemic biases, subgroup heterogeneity, long-term cumulative effects, or the statistical power of large populations to detect marginal effects, suggesting a potential efficacy of statins within specific clinical settings, whereas RCTs’ negative outcomes highlight the challenges of translating preclinical mechanisms into clinical practice under conventional trial designs. To resolve this paradox, future research must prioritize biomarker-driven RCTs with optimized dosing (e.g., lipophilic statins at higher doses), molecularly enriched populations (e.g., KRAS-activated subgroups), and combination strategies (e.g., with immunotherapy). Simultaneously, mechanistic studies should clarify statins’ stage-specific roles in lung carcinogenesis, and comparative trials should evaluate differential effects between hydrophilic/lipophilic subtypes. Only through such precision-oriented approaches can we determine whether statins hold genuine therapeutic value or merely serve as proxies for unmeasured health behaviors in observational data.
Conclusions
This comprehensive review elucidates the current challenges in lung cancer management, evaluates the efficacy of statin therapy in conjunction with standard treatments, delineates the mechanisms underlying the antineoplastic effects of statins, addresses the timing of statin intervention in lung cancer, and examines their role in SCLC. Our findings suggest that statins may have potential in both the prevention and treatment of lung cancer. The data indicate that statins could potentially exert additive effects when combined with chemotherapy, targeted therapy, and immunotherapy. Despite the promising outlook, numerous challenges and unresolved issues remain to be addressed. This is an excellent and very opportune time to conduct well-designed and adequately powered clinical trials to evaluate statins as a novel adjuvant drug for lung cancer.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-66/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-66/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-66/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Li MSC, Mok KKS, Mok TSK. Developments in targeted therapy & immunotherapy-how non-small cell lung cancer management will change in the next decade: a narrative review. Ann Transl Med 2023;11:358. [Crossref] [PubMed]
- Araghi M, Mannani R, Heidarnejad Maleki A, et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int 2023;23:162. [Crossref] [PubMed]
- Yin M, Zhou J, Gorak EJ, et al. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist 2013;18:1248-55. [Crossref] [PubMed]
- Zappavigna S, Cossu AM, Grimaldi A, et al. Anti-Inflammatory Drugs as Anticancer Agents. Int J Mol Sci 2020;21:2605. [Crossref] [PubMed]
- Ung MH, MacKenzie TA, Onega TL, et al. Statins associate with improved mortality among patients with certain histological subtypes of lung cancer. Lung Cancer 2018;126:89-96. [Crossref] [PubMed]
- Ference BA, Kastelein JJP, Ginsberg HN, et al. Association of Genetic Variants Related to CETP Inhibitors and Statins With Lipoprotein Levels and Cardiovascular Risk. JAMA 2017;318:947-56. [Crossref] [PubMed]
- Yu X, Pan Y, Ma H, et al. Simvastatin inhibits proliferation and induces apoptosis in human lung cancer cells. Oncol Res 2013;20:351-7. [Crossref] [PubMed]
- Wong WW, Dimitroulakos J, Minden MD, et al. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 2002;16:508-19. [Crossref] [PubMed]
- Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer 2017;104:52-7. [Crossref] [PubMed]
- Cardwell CR, Mc Menamin Ú, Hughes CM, et al. Statin use and survival from lung cancer: a population-based cohort study. Cancer Epidemiol Biomarkers Prev 2015;24:833-41. [Crossref] [PubMed]
- Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2012;367:1792-802. [Crossref] [PubMed]
- Jacobs EJ, Newton CC, Thun MJ, et al. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res 2011;71:1763-71. [Crossref] [PubMed]
- Lohinai Z, Dome P, Szilagyi Z, et al. From Bench to Bedside: Attempt to Evaluate Repositioning of Drugs in the Treatment of Metastatic Small Cell Lung Cancer (SCLC). PLoS One 2016;11:e0144797. [Crossref] [PubMed]
- Fernández LP, Merino M, Colmenarejo G, et al. Metabolic enzyme ACSL3 is a prognostic biomarker and correlates with anticancer effectiveness of statins in non-small cell lung cancer. Mol Oncol 2020;14:3135-52. [Crossref] [PubMed]
- Roudier E, Mistafa O, Stenius U. Statins induce mammalian target of rapamycin (mTOR)-mediated inhibition of Akt signaling and sensitize p53-deficient cells to cytostatic drugs. Mol Cancer Ther 2006;5:2706-15. [Crossref] [PubMed]
- Lee YG, Lee JH, Jang JS, et al. Prognostic benefit of statin with or without metformin in elderly patients with advanced non-small cell lung cancer: A nationwide population-based outcome study. J Clin Oncol 2017;35:e20661.
- Lin JJ, Ezer N, Sigel K, et al. The effect of statins on survival in patients with stage IV lung cancer. Lung Cancer 2016;99:137-42. [Crossref] [PubMed]
- Maimon N, Keizman D, Gottfried M. Statins (ASIs) may improve the outcome of erlotinib as second line treatment (tx) in patients (pts) with metastatic non-small cell lung cancer (mNSCLC). J Clin Oncol 2012;30:e18108.
- Hung MS, Chen IC, Lee CP, et al. Statin improves survival in patients with EGFR-TKI lung cancer: A nationwide population-based study. PLoS One 2017;12:e0171137. [Crossref] [PubMed]
- Marrone MT, Reuss JE, Crawford A, et al. Statin Use With Immune Checkpoint Inhibitors and Survival in Nonsmall Cell Lung Cancer. Clin Lung Cancer 2025;26:201-9. [Crossref] [PubMed]
- Rossi A, Filetti M, Taurelli Salimbeni B, et al. Statins and immunotherapy: Togetherness makes strength The potential effect of statins on immunotherapy for NSCLC. Cancer Rep (Hoboken) 2021;4:e1368. [Crossref] [PubMed]
- Yu WS, Lee CY, Park SY, et al. Prognostic factors for resected non-small cell lung cancer in patients with type 2 diabetes mellitus. J Surg Oncol 2018;117:985-93. [Crossref] [PubMed]
- Cortellini A, Tucci M, Adamo V, et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer 2020;8:e001361. [Crossref] [PubMed]
- Leighl NB, Paz-Ares L, Douillard JY, et al. Randomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: National Cancer Institute of Canada-Clinical Trials Group Study BR.18. J Clin Oncol 2005;23:2831-9. [Crossref] [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Ramakrishna S, Andrei AC, Varlotto J, et al. Statin Use Is Associated With Decreased Local Recurrence and Improved Overall Survival in Resectable Non-small Cell Lung Cancer (NSCLC). Chest 2012;142:925A.
- Lee Y, Lee KH, Lee GK, et al. Randomized Phase II Study of Afatinib Plus Simvastatin Versus Afatinib Alone in Previously Treated Patients with Advanced Nonadenocarcinomatous Non-small Cell Lung Cancer. Cancer Res Treat 2017;49:1001-11. [Crossref] [PubMed]
- Seckl MJ, Ottensmeier CH, Cullen M, et al. Multicenter, Phase III, Randomized, Double-Blind, Placebo-Controlled Trial of Pravastatin Added to First-Line Standard Chemotherapy in Small-Cell Lung Cancer (LUNGSTAR). J Clin Oncol 2017;35:1506-14. [Crossref] [PubMed]
- Han JY, Lee SH, Yoo NJ, et al. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res 2011;17:1553-60. [Crossref] [PubMed]
- Cheng Y, Chen J, Zhang W, et al. Benmelstobart, anlotinib and chemotherapy in extensive-stage small-cell lung cancer: a randomized phase 3 trial. Nat Med 2024;30:2967-76. [Crossref] [PubMed]
- Chen J, Lan T, Hou J, et al. Atorvastatin sensitizes human non-small cell lung carcinomas to carboplatin via suppression of AKT activation and upregulation of TIMP-1. Int J Biochem Cell Biol 2012;44:759-69. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Yi JS, Ready N, Healy P, et al. Immune Activation in Early-Stage Non-Small Cell Lung Cancer Patients Receiving Neoadjuvant Chemotherapy Plus Ipilimumab. Clin Cancer Res 2017;23:7474-82. [Crossref] [PubMed]
- Kimura H, Araya T, Yoneda T, et al. Long-lasting responses after discontinuation of nivolumab treatment for reasons other than tumor progression in patients with previously treated, advanced non-small cell lung cancer. Cancer Commun (Lond) 2019;39:78. [Crossref] [PubMed]
- Ryu R, Ward KE. Atezolizumab for the First-Line Treatment of Non-small Cell Lung Cancer (NSCLC): Current Status and Future Prospects. Front Oncol 2018;8:277. [Crossref] [PubMed]
- Upadhaya S, Neftelino ST, Hodge JP, et al. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat Rev Drug Discov 2021;20:168-9. [Crossref] [PubMed]
- Cheng Y, Spigel DR, Cho BC, et al. Durvalumab after Chemoradiotherapy in Limited-Stage Small-Cell Lung Cancer. N Engl J Med 2024;391:1313-27. [Crossref] [PubMed]
- Tsukita Y, Tozuka T, Kushiro K, et al. Immunotherapy or Chemoimmunotherapy in Older Adults With Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2024;10:439-47. [Crossref] [PubMed]
- Padda SK, Reckamp KL. Combination of Immunotherapy and Antiangiogenic Therapy in Cancer-a Rational Approach. J Thorac Oncol 2021;16:178-82. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines® Insights: Non-Small Cell Lung Cancer, Version 2.2023. J Natl Compr Canc Netw 2023;21:340-50. [Crossref] [PubMed]
- Muthusamy B, Patil PD, Pennell NA. Perioperative Systemic Therapy for Resectable Non-Small Cell Lung Cancer. J Natl Compr Canc Netw 2022;20:953-61. [Crossref] [PubMed]
- Jia XH, Xu H, Geng LY, et al. Efficacy and safety of neoadjuvant immunotherapy in resectable nonsmall cell lung cancer: A meta-analysis. Lung Cancer 2020;147:143-53. [Crossref] [PubMed]
- Amar D, Park B, Zhang H, et al. Beneficial effects of perioperative statins for major pulmonary resection. J Thorac Cardiovasc Surg 2015;149:1532-8. [Crossref] [PubMed]
- Mullen PJ, Yu R, Longo J, et al. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer 2016;16:718-31. [Crossref] [PubMed]
- Ahmadi M, Amiri S, Pecic S, et al. Pleiotropic effects of statins: A focus on cancer. Biochim Biophys Acta Mol Basis Dis 2020;1866:165968. [Crossref] [PubMed]
- Ahn J, Lim U, Weinstein SJ, et al. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2814-21. [Crossref] [PubMed]
- Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab 2020;2:132-41. [Crossref] [PubMed]
- Merino Salvador M, Gómez de Cedrón M, Moreno Rubio J, et al. Lipid metabolism and lung cancer. Crit Rev Oncol Hematol 2017;112:31-40. [Crossref] [PubMed]
- Maciejak A, Leszczynska A, Warchol I, et al. The effects of statins on the mevalonic acid pathway in recombinant yeast strains expressing human HMG-CoA reductase. BMC Biotechnol 2013;13:68. [Crossref] [PubMed]
- Qi XF, Zheng L, Lee KJ, et al. HMG-CoA reductase inhibitors induce apoptosis of lymphoma cells by promoting ROS generation and regulating Akt, Erk and p38 signals via suppression of mevalonate pathway. Cell Death Dis 2013;4:e518. [Crossref] [PubMed]
- Chen MJ, Cheng AC, Lee MF, et al. Simvastatin induces G(1) arrest by up-regulating GSK3β and down-regulating CDK4/cyclin D1 and CDK2/cyclin E1 in human primary colorectal cancer cells. J Cell Physiol 2018;233:4618-25. [Crossref] [PubMed]
- Fu CH, Lee TJ, Huang CC, et al. Simvastatin inhibits the proliferation of HL-60 clone 15- derived eosinophils by inducing the arrest of the cell cycle in the G1/S phase. Eur J Pharmacol 2019;856:172400. [Crossref] [PubMed]
- Liang YW, Chang CC, Hung CM, et al. Preclinical Activity of Simvastatin Induces Cell Cycle Arrest in G1 via Blockade of Cyclin D-Cdk4 Expression in Non-Small Cell Lung Cancer (NSCLC). Int J Mol Sci 2013;14:5806-16. [Crossref] [PubMed]
- Augoff K, Hryniewicz-Jankowska A, Tabola R, et al. MMP9: A Tough Target for Targeted Therapy for Cancer. Cancers (Basel) 2022;14:1847. [Crossref] [PubMed]
- Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, et al. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 2020;11:5120. [Crossref] [PubMed]
- Quintero-Fabián S, Arreola R, Becerril-Villanueva E, et al. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front Oncol 2019;9:1370. [Crossref] [PubMed]
- Wang X, Khalil RA. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv Pharmacol 2018;81:241-330. [Crossref] [PubMed]
- Mo H, Jeter R, Bachmann A, et al. The Potential of Isoprenoids in Adjuvant Cancer Therapy to Reduce Adverse Effects of Statins. Front Pharmacol 2018;9:1515. [Crossref] [PubMed]
- Thurnher M, Nussbaumer O, Gruenbacher G. Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin Cancer Res 2012;18:3524-31. [Crossref] [PubMed]
- Gazzerro P, Proto MC, Gangemi G, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev 2012;64:102-46. [Crossref] [PubMed]
- Hooff GP, Wood WG, Müller WE, et al. Isoprenoids, small GTPases and Alzheimer's disease. Biochim Biophys Acta 2010;1801:896-905. [Crossref] [PubMed]
- Perurena N, Situ L, Cichowski K. Combinatorial strategies to target RAS-driven cancers. Nat Rev Cancer 2024;24:316-37. [Crossref] [PubMed]
- Weis M, Heeschen C, Glassford AJ, et al. Statins have biphasic effects on angiogenesis. Circulation 2002;105:739-45. [Crossref] [PubMed]
- Hanai J, Doro N, Sasaki AT, et al. Inhibition of lung cancer growth: ATP citrate lyase knockdown and statin treatment leads to dual blockade of mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J Cell Physiol 2012;227:1709-20. [Crossref] [PubMed]
- Yang X, Fraser M, Moll UM, et al. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res 2006;66:3126-36. [Crossref] [PubMed]
- Nguyen PA, Chang CC, Galvin CJ, et al. Statins use and its impact in EGFR-TKIs resistance to prolong the survival of lung cancer patients: A Cancer registry cohort study in Taiwan. Cancer Sci 2020;111:2965-73. [Crossref] [PubMed]
- Luo Y, Yang Y, Peng P, et al. Cholesterol synthesis disruption combined with a molecule-targeted drug is a promising metabolic therapy for EGFR mutant non-small cell lung cancer. Transl Lung Cancer Res 2021;10:128-42. [Crossref] [PubMed]
- Wee P, Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel) 2017;9:52. [Crossref] [PubMed]
- Liu Q, Yu S, Zhao W, et al. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer 2018;17:53. [Crossref] [PubMed]
- Zhao TT, Le Francois BG, Goss G, et al. Lovastatin inhibits EGFR dimerization and AKT activation in squamous cell carcinoma cells: potential regulation by targeting rho proteins. Oncogene 2010;29:4682-92. [Crossref] [PubMed]
- Marcianò G, Palleria C, Casarella A, et al. Effect of Statins on Lung Cancer Molecular Pathways: A Possible Therapeutic Role. Pharmaceuticals (Basel) 2022;15:589. [Crossref] [PubMed]
- Pelaia G, Gallelli L, Renda T, et al. Effects of statins and farnesyl transferase inhibitors on ERK phosphorylation, apoptosis and cell viability in non-small lung cancer cells. Cell Prolif 2012;45:557-65. [Crossref] [PubMed]
- Burns TF, Borghaei H, Ramalingam SS, et al. Targeting KRAS-Mutant Non-Small-Cell Lung Cancer: One Mutation at a Time, With a Focus on KRAS G12C Mutations. J Clin Oncol 2020;38:4208-18. [Crossref] [PubMed]
- Reita D, Pabst L, Pencreach E, et al. Direct Targeting KRAS Mutation in Non-Small Cell Lung Cancer: Focus on Resistance. Cancers (Basel) 2022;14:1321. [Crossref] [PubMed]
- Fiala O, Pesek M, Finek J, et al. Statins augment efficacy of EGFR-TKIs in patients with advanced-stage non-small cell lung cancer harbouring KRAS mutation. Tumour Biol 2015;36:5801-5. [Crossref] [PubMed]
- Wang WH, Yuan T, Qian MJ, et al. Post-translational modification of KRAS: potential targets for cancer therapy. Acta Pharmacol Sin 2021;42:1201-11. [Crossref] [PubMed]
- Park IH, Kim JY, Jung JI, et al. Lovastatin overcomes gefitinib resistance in human non-small cell lung cancer cells with K-Ras mutations. Invest New Drugs 2010;28:791-9. [Crossref] [PubMed]
- Chen J, Bi H, Hou J, et al. Atorvastatin overcomes gefitinib resistance in KRAS mutant human non-small cell lung carcinoma cells. Cell Death Dis 2013;4:e814. [Crossref] [PubMed]
- Otahal A, Aydemir D, Tomasich E, et al. Delineation of cell death mechanisms induced by synergistic effects of statins and erlotinib in non-small cell lung cancer cell (NSCLC) lines. Sci Rep 2020;10:959. [Crossref] [PubMed]
- Ali A, Levantini E, Fhu CW, et al. CAV1 - GLUT3 signaling is important for cellular energy and can be targeted by Atorvastatin in Non-Small Cell Lung Cancer. Theranostics 2019;9:6157-74. [Crossref] [PubMed]
- Sadeghi Rad H, Monkman J, Warkiani ME, et al. Understanding the tumor microenvironment for effective immunotherapy. Med Res Rev 2021;41:1474-98. [Crossref] [PubMed]
- Zhu PF, Wang MX, Chen ZL, et al. Targeting the Tumor Microenvironment: A Literature Review of the Novel Anti-Tumor Mechanism of Statins. Front Oncol 2021;11:761107. [Crossref] [PubMed]
- Shimada K, Skouta R, Kaplan A, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol 2016;12:497-503. [Crossref] [PubMed]
- Viswanathan VS, Ryan MJ, Dhruv HD, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 2017;547:453-7. [Crossref] [PubMed]
- Lin Z, Zou S, Wen K. The crosstalk of CD8+ T cells and ferroptosis in cancer. Front Immunol 2023;14:1255443. [Crossref] [PubMed]
- Sun LL, Linghu DL, Hung MC. Ferroptosis: a promising target for cancer immunotherapy. Am J Cancer Res 2021;11:5856-63.
- Jiang Z, Lim SO, Yan M, et al. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest 2021;131:e139434. [Crossref] [PubMed]
- Mao W, Cai Y, Chen D, et al. Statin shapes inflamed tumor microenvironment and enhances immune checkpoint blockade in non-small cell lung cancer. JCI Insight 2022;7:e161940. [Crossref] [PubMed]
- Luttman JH, Hoj JP, Lin KH, et al. ABL allosteric inhibitors synergize with statins to enhance apoptosis of metastatic lung cancer cells. Cell Rep 2021;37:109880. [Crossref] [PubMed]
- Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer 2006;6:130-40. [Crossref] [PubMed]
- Zhang P, Song E, Jiang M, et al. Celecoxib and Afatinib synergistic enhance radiotherapy sensitivity on human non-small cell lung cancer A549 cells. Int J Radiat Biol 2021;97:170-8. [Crossref] [PubMed]
- Kim J, Noh MH, Hur DY, et al. Celecoxib upregulates ULBP-1 expression in lung cancer cells via the JNK/PI3K signaling pathway and increases susceptibility to natural killer cell cytotoxicity. Oncol Lett 2020;20:279. [Crossref] [PubMed]
- Kim YS, Seol CH, Jung JW, et al. Synergistic Effect of Sulindac and Simvastatin on Apoptosis in Lung Cancer A549 Cells through AKT-Dependent Downregulation of Survivin. Cancer Res Treat 2015;47:90-100. [Crossref] [PubMed]
- Khurana V, Bejjanki HR, Caldito G, et al. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest 2007;131:1282-8. [Crossref] [PubMed]
- Kwon YJ, You NY, Lee JW, et al. High Receipt of Statins Reduces the Risk of Lung Cancer in Current Smokers With Hypercholesterolemia: The National Health Insurance Service-Health Screening Cohort. Clin Lung Cancer 2019;20:e177-85. [Crossref] [PubMed]
- Chou CW, Lin CH, Hsiao TH, et al. Therapeutic effects of statins against lung adenocarcinoma via p53 mutant-mediated apoptosis. Sci Rep 2019;9:20403. [Crossref] [PubMed]
- Yang Z, Su Z, DeWitt JP, et al. Fluvastatin Prevents Lung Adenocarcinoma Bone Metastasis by Triggering Autophagy. EBioMedicine 2017;19:49-59. [Crossref] [PubMed]
- Sarkar D. Statins as Inhibitors of Lung Cancer Bone Metastasis. EBioMedicine 2017;19:6-7. [Crossref] [PubMed]
- Hague WE, Simes J, Kirby A, et al. Long-Term Effectiveness and Safety of Pravastatin in Patients With Coronary Heart Disease: Sixteen Years of Follow-Up of the LIPID Study. Circulation 2016;133:1851-60. [Crossref] [PubMed]
- Huang WY, Li CH, Lin CL, et al. Long-term statin use in patients with lung cancer and dyslipidemia reduces the risk of death. Oncotarget 2016;7:42208-15. [Crossref] [PubMed]
- Howard JP, Wood FA, Finegold JA, et al. Side Effect Patterns in a Crossover Trial of Statin, Placebo, and No Treatment. J Am Coll Cardiol 2021;78:1210-22. [Crossref] [PubMed]
- Li S, Lv J, Li Z, et al. Overcoming multi-drug resistance in SCLC: a synergistic approach with venetoclax and hydroxychloroquine targeting the lncRNA LYPLAL1-DT/BCL2/BECN1 pathway. Mol Cancer 2024;23:243. [Crossref] [PubMed]
- Guo C, Wan R, He Y, et al. Therapeutic targeting of the mevalonate-geranylgeranyl diphosphate pathway with statins overcomes chemotherapy resistance in small cell lung cancer. Nat Cancer 2022;3:614-28. [Crossref] [PubMed]
- Han JY, Lim KY, Yu SY, et al. A phase 2 study of irinotecan, cisplatin, and simvastatin for untreated extensive-disease small cell lung cancer. Cancer 2011;117:2178-85. [Crossref] [PubMed]


