
The value of the monocyte to high-density lipoprotein cholesterol ratio in predicting the thrombosis burden and long-term prognosis of acute myocardial infarction patients: a retrospective cohort study
Introduction
Acute myocardial infarction (AMI) is a major cause of mortality in the adult population. A study of 10,651 AMI patients, with a median follow-up of 6.7 years, reported that 26.01% (2,770/10,651) of the patients died, and that 51.8% of the deaths were due to cardiovascular disease, and 37.6% were due to ischemic heart disease (1). Percutaneous coronary intervention has an irreplaceable role in the treatment of AMI patients, but it may exacerbate distal microthrombosis, lead to the further impairment of myocardial perfusion and microcirculation, and ultimately lead to a poor prognosis (2). The thrombosis burden refers to the degree of thrombosis, and the thrombosis burden is closely related to the area of the AMI, the severity of the lesions, and the degree of myocardial injury. Bai et al. reported that the higher the coronary thrombosis burden, the higher the risk of major adverse cardiovascular events (MACEs) (3). Therefore, using biomarkers to estimate thrombosis burden can help enhance our understanding of the long-term prognosis of AMI patients.
The monocyte-to-high-density lipoprotein cholesterol ratio (MHR) is closely related to the development of cardiovascular disease. A cross-sectional study showed that the MHR was an independent risk factor for carotid plaque formation in postmenopausal women [odds ratio =1.795, 95% confidence interval (CI): 1.198–2.689, P=0.005] (4). A study of diabetic populations also showed that the MHR was associated with increased carotid intima-media thickness (5). Li et al. reported that the MHR was significantly correlated with the SYNTAX score (r=0.216, P<0.001) (6). Ya et al. also reported that the MHR was associated with a high risk of coronary artery disease in type 2 diabetes mellitus patients (7).
Therefore, we hypothesized that the MHR may be associated with the thrombosis burden and long-term prognosis of AMI patients. However, to date, few studies have focused on the relationship between the MHR, and the thrombosis burden and long-term prognosis of AMI patients. The present study aimed to investigate the value of the MHR in predicting the thrombosis burden and long-term prognosis of AMI patients. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-517/rc).
Methods
General information
From January 2018 to December 2019, the data of 324 AMI patients admitted to the Affiliated Jinhua Hospital, Zhejiang University School of Medicine were retrospectively collected. All the patients underwent percutaneous coronary intervention. To be eligible for inclusion in the study, the patients had to meet the following inclusion criteria: (I) have AMI; (II) be aged ≥18 years; and (III) have complete clinical data available; (IV) no infectious diseases or autoimmune diseases. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had AMI combined with other types of heart disease; (II) had dysfunction of important organs such as the liver and kidney; (III) had malignant tumors; (IV) had leukemia or other hematologic diseases; and/or (V) were lost to follow-up. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the Affiliated Jinhua Hospital, Zhejiang University School of Medicine (No. 20250122) and informed consent was taken from all the patients.
Sample size
According to the requirements of multiple regression analysis, one independent variable corresponds to at least 10 samples, and it was expected to include 3–5 variables. The estimated 5-year mortality rate and MACE rate were 20% and 30%. Thus, the minimum sample size was calculated to be 250.
Data collection
Patient data were collected, including age, sex, body mass index, smoking history, alcoholism history, hypertension, diabetes, hyperlipidemia, chronic lung disease, medication use (lipid lowering drugs, hypoglycemic drugs and antihypertensive drugs), glomerular filtration rate, myocardial infarction site, course, left ventricular ejection fraction (LVEF), N-terminal pro-B-type natriuretic peptide (NT-proBNP), interleukin-6, C reactive protein, types of myocardial infarction, monocytes, high density lipoprotein-cholesterol, MHR, D-dimer, thrombolysis in myocardial infarction (TIMI) grade, number of stents inserted during coronary intervention, type of stent, surgical success rate, duration of dual antiplatelet therapy, the rate of 5-year MACE (If a patient experienced recurrent angina, revascularization surgery, heart failure, severe arrhythmia, stroke, etc., MACE was diagnosed), and 5-year mortality.
Thrombosis burden assessment
The thrombosis burden of each patient was assessed by TIMI grading preoperatively (8). Under the TIMI grading system, the thrombus was graded as follows: grade 0: no thrombus; grade 1: probable thrombosis; grade 2: definite thrombus with a maximum diameter <50% of the diameter of the vessel; grade 3: definite thrombus with a maximum vessel diameter of 50–200% of the diameter of the vessel; grade 4: definite thrombus with a maximum thrombus diameter ≥2 times the diameter of the blood vessel; and grade 5: definite thrombus leading to the complete occlusion of blood vessels. Patients with TIMI grades of 4–5 were classified as having a high thrombosis burden, while those with TIMI grades of 0–3 were classified as having a low thrombosis burden.
Grouping
The monocytes and high-density lipoprotein-cholesterol of the patients were detected on admission, and the MHR was then calculated. The MHR was calculated by an independent investigator who was blinded to the patient’s other clinical features. According to the median of MHR, the patients were assigned to the high MHR group (n=162), and low MHR group (n=162).
Statistical analysis
SPSS 26.0 (IBM, Chicago, IL, USA) software was used for the statistical analysis. The continuous variables are expressed as the mean ± standard deviation, and differences between the two groups were analyzed using the independent sample t-test; the count data are expressed as the number (percentage), and differences between the two groups were analyzed using the Chi-squared test. The receiver operating characteristic (ROC) curve was used to analyze the diagnostic value of the MHR for MACE and mortality. Multivariate logistics regression and COX regression were used to analyze risk factors.
Results
Comparison of the clinical features between the two groups
Figure 1 shows the patient inclusion flowchart. Significant differences were found between the two groups in terms of the LVEF, TIMI grade, 5-year MACE rate, and 5-year mortality (P<0.05) (Table 1).
Table 1
| Variables | High MHR group (N=162) | Low MHR group (N=162) | t/χ2 value | P value |
|---|---|---|---|---|
| MHR | 0.32±0.04 | 0.20±0.03 | 29.294 | <0.001 |
| Age (years) | 62.63±13.26 | 64.73±13.71 | 1.401 | 0.16 |
| Gender | 0.014 | 0.91 | ||
| Male | 111 (68.52) | 110 (67.90) | ||
| Female | 51 (31.48) | 52 (32.10) | ||
| Body mass index (kg/m2) | 25.51±4.09 | 25.99±4.29 | 1.037 | 0.30 |
| Smoking history | 35 (21.60) | 27 (16.67) | 1.277 | 0.26 |
| Alcoholism history | 21 (12.96) | 24 (14.81) | 0.232 | 0.63 |
| Hypertension | 72 (44.44) | 82 (50.62) | 1.238 | 0.27 |
| Diabetes | 56 (34.57) | 51 (31.48) | 0.349 | 0.56 |
| Hyperlipidemia | 113 (69.75) | 115 (70.99) | 0.059 | 0.81 |
| Chronic lung disease | 18 (11.11) | 16 (9.88) | 0.131 | 0.72 |
| Lipid lowering drugs | 56 (34.57) | 51 (31.48) | 0.349 | 0.56 |
| Hypoglycemic drugs | 56 (34.57) | 51 (31.48) | 0.349 | 0.56 |
| Antihypertensive drugs | 72 (44.44) | 82 (50.62) | 1.238 | 0.27 |
| Glomerular filtration rate (mL/min) | 109.84±11.94 | 110.94±12.04 | 0.826 | 0.41 |
| Myocardial infarction site | 2.482 | 0.29 | ||
| Anterior wall | 72 (44.44) | 61 (37.65) | ||
| Inferior wall | 49 (30.25) | 62 (38.27) | ||
| Others | 41 (25.31) | 39 (24.07) | ||
| Course (h) | 4.57±2.14 | 4.34±2.34 | 0.942 | 0.35 |
| Types of myocardial infarction | 0.554 | 0.46 | ||
| STEMI | 42 (25.93) | 48 (29.63) | ||
| NSTEMI | 120 (74.07) | 114 (70.37) | ||
| LVEF (%) | 49.48±4.10 | 54.38±3.80 | 11.154 | <0.001 |
| LVEF (%) <50% | 79 (48.77) | 25 (15.43) | 41.293 | <0.001 |
| NT-proBNP (pg/mL) | 1,526.72±537.43 | 1,515.56±534.89 | 0.187 | 0.85 |
| NT-proBNP >1,508 pg/mL | 85 (52.47) | 77 (47.53) | 0.790 | 0.37 |
| D-dimer (mg/L) | 2.87±1.09 | 2.90±1.09 | 0.260 | 0.80 |
| Interleukin-6 (pg/mL) | 18.04±8.93 | 17.72±8.53 | 0.330 | 0.74 |
| Hypersensitive C-reactive protein (mg/L) | 4.83±1.98 | 5.02±1.89 | 0.883 | 0.38 |
| Number of stents | 2.62±1.16 | 2.42±1.10 | 1.626 | 0.11 |
| Type of stents | 0.391 | 0.53 | ||
| Drug-eluting stents | 22 (13.58) | 26 (16.05) | ||
| Metal stents | 140 (86.42) | 136 (83.95) | ||
| Surgical success rate | 162 (100.00) | 162 (100.00) | – | – |
| Duration of dual antiplatelet therapy (months) | 15.09±2.08 | 14.74±1.95 | 1.546 | 0.12 |
| Antihypertensive medications | 72 (44.44) | 82 (50.62) | 1.238 | 0.27 |
| Hypoglycemic drugs | 56 (34.57) | 51 (31.48) | 0.349 | 0.56 |
| Statins | 113 (69.75) | 115 (70.99) | 0.059 | 0.81 |
| TIMI grading | 28.571 | <0.001 | ||
| 0–3 grade | 106 (65.43) | 146 (90.12) | ||
| 4–5 grade | 56 (34.57) | 16 (9.88) | ||
| MACE | 72 (44.44) | 25 (15.43) | 32.504 | <0.001 |
| Mortality | 52 (32.10) | 14 (8.64) | 27.476 | <0.001 |
Data are presented as mean ± standard deviation or n (%). MACE, major adverse cardiovascular events; MHR, monocyte-to-high-density lipoprotein cholesterol ratio; NT-proBNP, N-terminal pro-brain natriuretic peptide; NSTEMI, Non-ST-segment elevation myocardial infarction; STEMI, ST-elevated myocardial infarction; TIMI, thrombolysis in myocardial infarction; LVEF, left ventricular ejection fraction.
Predictive value of the MHR for TIMI grade in AMI patients
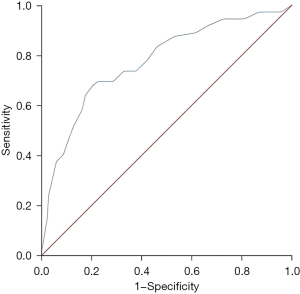
The MHR was valuable in predicting the TIMI grade of the AMI patients, and the area under the curve (AUC) of the ROC curve was 0.776 (95% CI: 0.712–0.840, P<0.001) (Figure 2). The best cut-off value was 0.295, and the sensitivity and specificity were 0.681 and 0.794, the Youden index was 0.475.
Predictive value of the MHR for the 5-year MACE rate in AMI patients
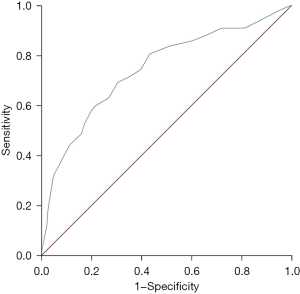
The MHR was valuable in predicting the 5-year MACE rate in AMI patients, and the AUC of the ROC curve was 0.743 (95% CI: 0.681–0.805, P<0.001) (Figure 3). The best cut-off value was 0.265, and the sensitivity and specificity were 0.691 and 0.696, the Youden index was 0.387.
Predictive value of the MHR for 5-year mortality in AMI patients
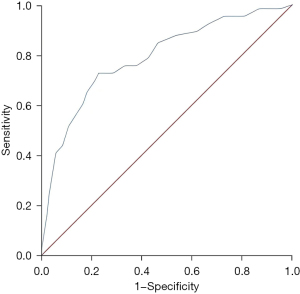
The MHR was valuable in predicting 5-year mortality in the AMI patients, and the AUC of the ROC curve was 0.790 (95% CI: 0.726–0.854, P<0.001) (Figure 4). The best cut-off value was 0.285, and the sensitivity and specificity were 0.727 and 0.771, the Youden index was 0.498.
The risk factors for TIMI grade in AMI patients
A high MHR and LVEF <50% were independent risk factors for grade 4–5 TIMI in the AMI patients, and the relative risks were 2.367 (95% CI: 1.165–4.807) and 13.568 (95% CI: 6.800–27.072), respectively (Table 2).
Table 2
| Variables | B value | Standard error | Wald value | P value | Relative risk (95% CI) |
|---|---|---|---|---|---|
| High MHR | 0.861 | 0.361 | 5.679 | 0.02 | 2.367 (1.165–4.807) |
| LVEF <50% | 2.608 | 0.352 | 54.748 | <0.001 | 13.568 (6.800–27.072) |
| NT-proBNP >1,508 pg/mL | 0.518 | 0.334 | 2.411 | 0.12 | 1.679 (0.873–3.231) |
AMI, acute myocardial infarction; CI, confidence interval; LVEF, left ventricular ejection fraction; MHR, monocyte-to-high-density lipoprotein cholesterol ratio; NT-proBNP, N-terminal pro-brain natriuretic peptide; TIMI, thrombolysis in myocardial infarction.
The risk factors for the 5-year MACE rate in AMI patients
A high MHR, LVEF <50%, and NT-proBNP >1,508 pg/mL were risk factors for the 5-year MACE rate in the AMI patients, and the relative risks were 2.035 (95% CI: 1.270–3.262), 11.044 (95% CI: 6.536–18.662), and 3.271 (95% CI: 2.048–5.224) (Table 3).
Table 3
| Variables | B value | Standard error | Wald value | P value | Relative risk (95% CI) |
|---|---|---|---|---|---|
| High MHR | 0.711 | 0.241 | 8.713 | 0.003 | 2.035 (1.270–3.262) |
| LVEF <50% | 2.402 | 0.268 | 80.529 | <0.001 | 11.044 (6.536–18.662) |
| NT-proBNP >1,508 pg/mL | 1.185 | 0.239 | 24.625 | <0.001 | 3.271 (2.048–5.224) |
AMI, acute myocardial infarction; CI, confidence interval; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular event; MHR, monocyte-to-high-density lipoprotein cholesterol ratio; NT-proBNP, N-terminal pro-brain natriuretic peptide.
The risk factors for the 5-year mortality in AMI patients
High MHR, LVEF <50%, and NT-proBNP >1,508 pg/mL were risk factors for 5-year mortality in the AMI patients, and the relative risks were 2.163 (95% CI: 1.168–4.006), 8.150 (95% CI: 4.303–15.439), and 1.702 (95% CI: 1.010–2.869), respectively (Table 4).
Table 4
| Variables | B value | Standard error | Wald value | P value | Relative risk (95% CI) |
|---|---|---|---|---|---|
| High MHR | 0.772 | 0.314 | 6.026 | 0.01 | 2.163 (1.168–4.006) |
| LVEF <50% | 2.098 | 0.326 | 41.434 | <0.001 | 8.150 (4.303–15.439) |
| NT-proBNP >1,508 pg/mL | 0.532 | 0.266 | 3.982 | 0.046 | 1.702 (1.010–2.869) |
AMI, acute myocardial infarction; CI, confidence interval; LVEF, left ventricular ejection fraction; MHR, monocyte-to-high-density lipoprotein cholesterol ratio; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Discussion
The main outcomes of the present study
The MHR may be associated with the thrombosis burden and long-term prognosis of AMI patients. However, few studies have focused on the relationship between the MHR, and the thrombosis burden and long-term prognosis of AMI patients. The present study investigated the value of the MHR in predicting the thrombosis burden and long-term prognosis of AMI patients, and found that the MHR was valuable in predicting the 4–5 TIMI grade, 5-year MACE rate, and 5-year mortality in AMI patients, and the AUCs of the ROC curves were 0.776 (95% CI: 0.712–0.840, P<0.001), 0.743 (95% CI: 0.681–0.805, P<0.001), and 0.790 (95% CI: 0.726–0.854, P<0.001), respectively. A high MHR was an independent risk factor for TIMI grade 4–5, the 5-year MACE rate, and 5-year mortality in the AMI patients, and the relative risks were 2.367 (95% CI: 1.165–4.807), 2.259 (95% CI: 1.088–4.690), and 2.524 (95% CI: 1.214–5.245), respectively.
The innate immune system, which is mediated by innate immune cells such as monocytes and neutrophils, plays an important role in the pathogenesis of cardiovascular diseases (9). Vascular endothelial cells in patients with cardiovascular disease are activated by mechanical stimulation, resulting in an increased release of interleukin-6 and hydrogen peroxide, which promotes the differentiation of human monocytes into intermediate monocytes, which significantly increases the expression of inflammatory factors, which in turn results in impaired endothelial cell function and increased oxidative stress, and promotes chronic inflammation, vascular wall fibrosis, and extracellular matrix remodeling (10). Monocyte activation plays an important role in chronic inflammation and cardiovascular disease, and its differentiated macrophages are involved in regulating inflammatory cytokine release and myocardial tissue remodeling. High-density lipoprotein cholesterol can reduce platelet-monocyte aggregate-induced integrin αM expression, thereby inhibiting monocyte activation, exerting anti-inflammatory effects, and acting as a protective factor for coronary heart disease, reducing the occurrence of MACE (11,12). The MHR is a composite inflammatory biomarker based on monocytes and high-density lipoprotein cholesterol, and the MHR reflects the body’s inflammatory status and anti-inflammatory capacity, and is correlated with the severity of coronary artery disease (13). The MHR can contribute to the progression of cardiovascular disease, which in turn leads to increased MACE and mortality in AMI patients.
Comparison with previous studies
Atherosclerosis and the coronary thrombosis burden play important roles in the pathophysiology of AMI. Platelet activation and the inflammatory response are the key factors of atherosclerosis and the coronary thrombosis burden. A study of 375 patients who underwent coronary angiography for suspected “chest pain” found that the MHR was significantly increased in AMI patients, and the MHR was significantly positively correlated with the Gensini score and the Grace score (14). Xu et al. also found that the MHR was valuable in predicting the development of AMI (15). Moreover, a meta-analysis of 11 studies with a total of 7,421 patients found a significant increase in the in-hospital mortality (5.5% vs. 0.9%, P<0.001), and long-term mortality (13.7% vs. 7.5%, P<0.001) of patients with acute coronary syndromes with high MHRs (16). A study in the United States also confirmed that the MHR was independently significantly associated with all-cause mortality and cardiovascular mortality in the general population (17). It has also been shown that an elevated MHR is associated with restenosis after coronary artery stenting, which increases the risk of MACE in patients (18). Previous studies have also shown that an elevated MHR is correlated with the development of atherosclerosis (4,19-21). The findings of these previous studies support the findings of the present study, which showed that an elevated MHR was associated with an increased 5-year MACE rate and mortality in AMI patients.
The present study also investigated the relationship between the MHR and the thrombosis burden in AMI patients, which has seldomly been examined in previous studies. A study of patients with deep vein thrombosis showed that an elevated MHR is associated with an increased thrombotic burden, which supports the findings of the present study (22). An elevated MHR, which may be related to vascular endothelial cell injury caused by chronic inflammation, may be the main reason for the poor prognosis of and incidence of thrombosis in AMI patients (23-25).
Limitations of the present study
The present study was a retrospective study, and it failed to investigate the mechanisms of the MHR in promoting the development of AMI. Moreover, a high and a low MHR in this study is only applicable to this study, it cannot be generalized as a high and low can be different for another population. Finally, the present study restricted to a single hospital which limits the applicability and generalizability of the findings to a broader population, a prospectively multicenter cohort study was further needed.
Conclusions
The MHR can be used to predict the thrombosis burden and long-term prognosis of AMI patients.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-517/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-517/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-517/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-517/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the Affiliated Jinhua Hospital, Zhejiang University School of Medicine (No. 20250122) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schmitz T, Freuer D, Raake P, et al. Association between BMI and cause-specific long-term mortality in acute myocardial infarction patients. Am J Prev Cardiol 2025;21:100899. [Crossref] [PubMed]
- Del Portillo JH, Echeverri D, Cabrales J. Association of the use of manual thrombus aspiration with intracoronary thrombotic burden in patients with ST segment elevation myocardial infarction in the real world. Int J Cardiol Heart Vasc 2020;26:100436. [Crossref] [PubMed]
- Bai J, Chen L, Xu L, et al. The value of serum Sema4D level in predicting the prognosis of patients with acute ST-segment elevation myocardial infarction and with high thrombus burden. BMC Cardiovasc Disord 2023;23:230. [Crossref] [PubMed]
- Guo J, Qin H, Li X. Association between monocyte/high-density lipoprotein cholesterol ratio and carotid plaque in postmenopausal women: A cross-sectional study. Medicine (Baltimore) 2024;103:e37425. [Crossref] [PubMed]
- Amouzegar A, Mirzaasgari Z, Mehrabi A, et al. Association of monocyte/high-density lipoprotein cholesterol ratio and the carotid intima-media thickness in diabetic patients. BMC Endocr Disord 2022;22:323. [Crossref] [PubMed]
- Li Y, Li S, Ma Y, et al. Relationship between non-high-density lipoprotein cholesterol/apolipoprotein A-I and monocyte/high-density lipoprotein cholesterol ratio and coronary heart disease. Coron Artery Dis 2020;31:623-7. [Crossref] [PubMed]
- Ya G, Qiu Z, Tianrong P. Relation of Monocyte/High-Density Lipoprotein Cholesterol Ratio with Coronary Artery Disease in Type 2 Diabetes Mellitus. Clin Lab 2018;64:901-6. [Crossref] [PubMed]
- Çınar T, Karabağ Y, Burak C, et al. A simple score for the prediction of stent thrombosis in patients with ST elevation myocardial infarction: TIMI risk index. J Cardiovasc Thorac Res 2019;11:182-8. [Crossref] [PubMed]
- Aldarondo D, Wayne E. Monocytes as a convergent nanoparticle therapeutic target for cardiovascular diseases. Adv Drug Deliv Rev 2022;182:114116. [Crossref] [PubMed]
- Dregoesc MI, Țigu AB, Bekkering S, et al. Intermediate monocytes are associated with the first major adverse cardiovascular event in patients with stable coronary artery disease. Int J Cardiol 2024;400:131780. [Crossref] [PubMed]
- Kjeldsen EW, Luo J, Nordestgaard LT, et al. Reevaluating the Role of High-Density Lipoprotein Cholesterol: New Perspectives on Cardiovascular Disease and Alzheimer Disease. Clin Chem 2023;69:1329-32. [Crossref] [PubMed]
- Yang Y, Zhang J, Jia L, et al. Uric acid to high-density lipoprotein cholesterol ratio predicts adverse cardiovascular events in patients with coronary chronic total occlusion. Nutr Metab Cardiovasc Dis 2023;33:2471-8. [Crossref] [PubMed]
- Wu Y, Meng Y, Yi W, et al. The ratio of monocyte count and high-density lipoprotein cholesterol mediates the association between urinary tungsten and cardiovascular disease: a study from NHANES 2005-2018. Environ Sci Pollut Res Int 2023;30:85930-9. [Crossref] [PubMed]
- Cao J, Li R, He T, et al. Role of combined use of mean platelet volume-to-lymphocyte ratio and monocyte to high-density lipoprotein cholesterol ratio in predicting patients with acute myocardial infarction. J Cardiothorac Surg 2023;18:172. [Crossref] [PubMed]
- Xu L, Tian L, Yan Z, et al. Diagnostic and prognostic value of miR-486-5p, miR-451a, miR-21-5p and monocyte to high-density lipoprotein cholesterol ratio in patients with acute myocardial infarction. Heart Vessels 2023;38:318-31. [Crossref] [PubMed]
- Pruc M, Kubica J, Banach M, et al. Prognostic value of the monocyte-to-high-density lipoprotein-cholesterol ratio in acute coronary syndrome patients: A systematic review and meta-analysis. Kardiol Pol 2025;83:52-61. [Crossref] [PubMed]
- Jiang M, Yang J, Zou H, et al. Monocyte-to-high-density lipoprotein-cholesterol ratio (MHR) and the risk of all-cause and cardiovascular mortality: a nationwide cohort study in the United States. Lipids Health Dis 2022;21:30. [Crossref] [PubMed]
- Meng H, Zhou X, Li L, et al. Monocyte to high-density lipoprotein cholesterol ratio predicts restenosis of drug-eluting stents in patients with unstable angina pectoris. Sci Rep 2024;14:30175. [Crossref] [PubMed]
- Zhang Z, Gao Y, Li Z, et al. Association of carotid atherosclerotic plaque and intima-media thickness with the monocyte to high-density lipoprotein cholesterol ratio among low-income residents of rural China: a population-based cross-sectional study. BMC Public Health 2023;23:2541. [Crossref] [PubMed]
- Xi J, Men S, Nan J, et al. The blood monocyte to high density lipoprotein cholesterol ratio (MHR) is a possible marker of carotid artery plaque. Lipids Health Dis 2022;21:130. [Crossref] [PubMed]
- Kohsari M, Moradinazar M, Rahimi Z, et al. New inflammatory biomarkers (lymphocyte and monocyte percentage to high-density lipoprotein cholesterol ratio and lymphocyte to monocyte percentage ratio) and their association with some cardiometabolic diseases : Results from a large Kurdish cohort study in Iran. Wien Klin Wochenschr 2022;134:626-35. [Crossref] [PubMed]
- Doğan Z, Bektaşoğlu G, Dümür Ş, et al. Evaluation of the relationship between monocyte to high-density lipoprotein cholesterol ratio and thrombus burden in patients with deep vein thrombosis. Rev Assoc Med Bras (1992) 2023;69:e20221211.
- Yang W, Zhong Y, Zhou P, et al. Monocyte to high-density lipoprotein cholesterol ratio as a marker of the presence and progression of diabetic kidney disease. Ren Fail 2025;47:2438846. [Crossref] [PubMed]
- Shu H, Han S, Qiu W, et al. Association of the Monocyte to High-Density Lipoprotein Cholesterol Ratio and Neutrophil to High-Density Lipoprotein Cholesterol Ratio With the Severity of New-Onset Coronary Artery Disease. J Inflamm Res 2025;18:463-76. [Crossref] [PubMed]
- Wang L, Liu Y, Shi W, et al. Value of the monocyte-to-high-density lipoprotein cholesterol ratio in refining the detection of prevalent heart failure: Insights from the NHANES 1999-2018. Lipids 2024;59:93-100. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


