
Comparison of neoadjuvant chemotherapy plus immunotherapy versus chemoradiotherapy for esophageal squamous cell carcinoma patients: efficacy and safety outcomes
Highlight box
Key findings
• This study compared the efficacy and safety of neoadjuvant chemotherapy plus immunotherapy (NICT) vs. neoadjuvant chemoradiotherapy (NCRT) in patients with locally advanced esophageal squamous cell carcinoma (ESCC) undergoing radical esophagectomy.
• We found that NCRT resulted in a significantly higher rate of pathological complete response (pCR) compared to NICT (44.57% vs. 16.00%, P<0.001).
• NICT was associated with fewer postoperative complications and a lower incidence of anastomotic leaks compared to NCRT.
What is known and what is new?
• It is established that NCRT is the standard neoadjuvant treatment for locally advanced ESCC, improving overall survival with manageable postoperative complications.
• This study contributes to existing knowledge by demonstrating that NICT, despite having a lower pCR rate, may provide better postoperative outcomes concerning complication rates and recovery.
What is the implication, and what should change now?
• The findings suggest that NICT could be a viable alternative to NCRT, particularly for patients who wish to avoid radiotherapy-related toxicities.
• Further large-scale, prospective, and randomized studies are needed to confirm these findings and guide clinical decision-making towards personalized treatment strategies for ESCC patients.
Introduction
Neoadjuvant therapy prior to radical esophagectomy is now recognized as the standard approach for treating locally advanced esophageal squamous cell carcinoma (ESCC). Research, including trials like CROSS and NEOCRTEC510, has shown that the combination of neoadjuvant chemoradiotherapy (NCRT) and surgical intervention can enhance overall survival (OS) while not notably raising the risk of postoperative complications compared with surgery performed alone (1-3). However, over 35% of patients still face tumor recurrence after receiving NCRT followed by surgical treatment (3).
With advancements in immunotherapy, integrating this treatment with chemotherapy, biologics, and radiation therapy has become a prevalent therapeutic strategy (4,5). Compared with traditional NCRT, neoadjuvant immunotherapy combined with chemotherapy (NICT), allows patients to avoid acute and long-term toxicities associated with radiotherapy, thereby improving postoperative recovery and quality of life. Additionally, omitting radiotherapy simplifies the neoadjuvant treatment process and reduces treatment time, as neoadjuvant radiotherapy typically requires patients to be hospitalized continuously for over a month. This is crucial for improving patient treatment adherence and satisfaction. Multiple clinical trials have validated the efficacy of NICT for locally advanced ESCC. For example, in the NICE study (6), 51 patients underwent neoadjuvant and surgical interventions, achieving an R0 resection rate of 98.0% and a pathological complete response (pCR) rate of 39.2% (20 out of 51). Furthermore, the overall postoperative complications noted were deemed manageable. The ESCORT-NEO/NCCES01 study (7) demonstrated that the combination of camrelizumab with nab-paclitaxel and cisplatin (group A: camrelizumab + nab-TP) and camrelizumab with paclitaxel and cisplatin (group B: camrelizumab + TP) both achieved significantly pCR rates than paclitaxel and cisplatin alone (group C: TP) (group A vs. group C: 28.0% vs. 4.7%, P<0.001; group B vs. group C: 15.4% vs. 4.7%, P=0.003). Both groups met the primary endpoint. Additionally, the addition of immunotherapy did not increase surgical risks, and the safety profile was manageable.
Although these investigations validated the effectiveness and safety of NICT, several studies (8-10) have addressed the pathological responses and surgical results of patients undergoing NCRT or NICT. Nonetheless, these researches (8-10) were performed solely at individual centers and included a limited cohort of patients. Consequently, we conducted this real-world study to evaluate the therapeutic efficacy and safety of NICT vs. NCRT followed by radical surgery in patients with locally advanced ESCC treated at three different hospitals. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2107/rc).
Methods
Patients
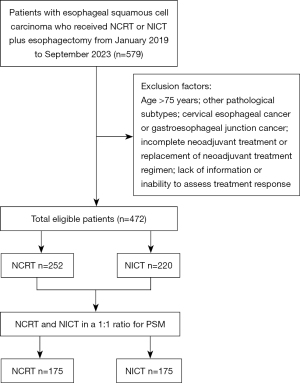
This retrospective study involved patients diagnosed with ESCC who received neoadjuvant therapy followed by radical esophagectomy at Cancer Hospital Affiliated to Shandong First Medical University, The First Affiliated Hospital of University of Science and Technology of China, and Anhui Cancer Hospital from January 2019 to September 2023. Patients eligible for inclusion were those between the ages of 18 and 75 years, possessing pathologically confirmed thoracic ESCC, and classified as clinical stage T1N+M0 or T2-4aNanyM0, according to the International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) tumor-node-metastasis (TNM) staging method, 8th edition. Those excluded from the study included individuals with additional tumors, patients who had not completed the prescribed neoadjuvant therapy, and those whose treatment information was incomplete. Ultimately, 472 patients were eligible for inclusion, with 252 patients in the NCRT group and 220 in the NICT group (Figure 1). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study has been approved by the Ethics Committee of Cancer Hospital Affiliated to Shandong First Medical University (SDTHEC2023004013). Given its retrospective nature, the committee has waived the informed consent requirement for this study. Additionally, all participating hospitals were informed of the study protocol and agreed to participate in the study. Each participating hospital ensured that the study was conducted in accordance with their local ethical standards and regulatory requirements. The confidentiality and privacy of all participants were strictly maintained throughout the study.
Neoadjuvant and surgical treatment options
Participants in this research were deemed suitable for radical esophagectomy after a thorough clinical evaluation. Those who underwent preoperative radiotherapy generally received radiation doses between 40.0 and 41.4 Gy, delivered in increments of 1.8 to 2.0 Gy. The radiotherapy approach employed was intensity-modulated radiotherapy (IMRT). The standard preoperative chemotherapy protocol included intravenous delivery of platinum-based agents, which were either given alongside fluorouracil (referred to as the PF regimen) or in combination with paclitaxel/albumin-paclitaxel (the TP regimen). The chemotherapy dosages were adjusted based on the specific tolerances of each patient (Table S1). Preoperative immunotherapy, involved the intravenous administration of Programmed cell death protein 1 (PD-1) immune checkpoint inhibitors (Table S2). Before surgery, all patients underwent pulmonary function tests (PFTs), electrocardiogram (ECG), echocardiography, chest computed tomography (CT) scans, and laboratory blood tests. Patients enrolled in this study were clinically evaluated and deemed suitable for radical esophagectomy. Patients were subjected to esophagectomy and/or lymph node dissection under general anesthesia approximately 4 to 6 weeks following the conclusion of neoadjuvant therapy.
Pathological examination
Pathology samples from each patient were evaluated by two experienced pathologists, who concentrated on the pathological type, resection margins, residual tumor characteristics and treatment response. R0 resection was identified as a curative procedure with negative margins (distal, proximal, and circumferential). The presence of pCR (ypT0N0) was characterized by the lack of viable tumor cells within both the primary tumor and the resected lymph nodes, based on the standard tumor regression grade (TRG) scoring criteria. The TRG includes four categories: TRG 0, indicating complete response where no cancer cells survive; TRG 1, representing moderate response with only small clusters or individual cancer cells present; TRG 2, showing mild response with remaining cancer foci amidst significant interstitial fibrosis; and TRG 3, indicating no response with minimal necrosis of cancer cells while a substantial number of cancer cells continue to exist (11).
Postoperative complications
Complications and adverse events that arose within 30 days post-surgery or during the hospital stay following esophagectomy were recorded. The identification of postoperative complications was based on the guidelines set forth by the Esophageal Complications Consensus Group (ECCG). Pulmonary complications included pneumonia treated with antibiotics during the hospital stay; pleural effusion necessitating an additional drainage procedure; pneumothorax needing intervention; and anastomotic leak, characterized as a full-thickness gastrointestinal defect involving the esophagus, anastomosis, staple line, or conduit, regardless of its presentation or method of detection. Additionally, cardiac complications that requiring intervention included atrial dysrhythmia, ventricular dysrhythmia, congestive heart failure, and pericarditis (12,13).
Follow-up
The patients who participated in the study were routinely monitored through various approaches, such as outpatient visit documentation, inpatient treatment admission records, and telephone follow-ups. For individuals whose most recent follow-up recorded in the case management system was more than 1 month beyond the study cutoff date, we performed phone interviews to gather information about their health progression and survival status. For patients whose case management system records exceeded 1 month and who could not be contacted by phone, their data were treated as censored, with the survival time recorded as the time of the last follow-up Standard assessments included a detailed physical examination, esophagography, enhanced CT scans and/or magnetic resonance imaging, positron emission tomography-CT scanning, and endoscopic ultrasound with biopsy. This research focused on postoperative survival analysis, with OS being measured from the treatment initiation date until death from any reason or the last follow-up appointment. Disease-free survival (DFS) was defined as the duration from the surgical procedure to either the occurrence of tumor recurrence, death, or the final follow-up visit.
Statistical analysis
In this research, propensity score matching (PSM) was utilized to establish a balanced cohort by taking into account various explanatory variables. The R language package (version 4.2.3) was employed to carry out 1:1 matching between the NICT and NCRT groups. A logistic regression model was used to calculate the propensity score, which incorporated possible confounding factors such as age, sex, smoking habits, alcohol consumption, comorbidities, Karnofsky Performance Status (KPS) score, tumor site, and clinical stage. Categorical variables were reported as total counts and percentages, with group comparisons analyzed using the chi-squared test, Fisher’s exact test, t-test, U test, and Z test. The statistical analysis was conducted using SPSS for Windows (version 27.0, IMB Corp., Armonk, NY, USA), and a P value of less than 0.05 was considered to indicate statistical significance.
Results
Baseline patient characteristics
In our research, the NCRT group consisted of 252 patients, while the NICT group included 220 patients; their initial characteristics are displayed in Table 1. Before PSM, noticeable differences were detected in age, KPS score, clinical T stage, and clinical N stage between the two groups. After applying PSM, statistical analysis indicated no significant differences in the other characteristics between the two groups (P>0.05).
Table 1
| Variables | Group | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NCRT (N=252) |
NICT (N=220) |
P value | SMD | NCRT (N=175) | NICT (N=175) |
P value | SMD | |||
| Sex | Male | 207 (82.14) | 177 (80.45) | 0.64 | −0.043 | 138 (78.86) | 139 (79.43) | 0.90 | 0.014 | |
| Female | 45 (17.86) | 43 (19.55) | 0.043 | 37 (21.14) | 36 (20.57) | −0.014 | ||||
| Age (years) | ≤60 | 113 (44.84) | 68 (30.91) | 0.002 | −0.301 | 61 (34.86) | 63 (36.00) | 0.57 | 0.024 | |
| >60 | 139 (55.16) | 152 (69.09) | 0.301 | 114 (65.14) | 112 (64.00) | −0.024 | ||||
| Smoking | Yes | 128 (50.79) | 128 (58.18) | 0.11 | 0.150 | 93 (53.14) | 95 (54.29) | 0.83 | 0.023 | |
| No | 124 (49.21) | 92 (41.82) | −0.150 | 82 (46.86) | 80 (45.71) | −0.023 | ||||
| Drinking | Yes | 144 (57.14) | 132 (60.00) | 0.53 | 0.058 | 103 (58.86) | 103 (58.86) | >0.99 | 0.000 | |
| No | 108 (42.86) | 88 (40.00) | −0.058 | 72 (41.14) | 103 (58.86) | 0.000 | ||||
| Comorbidity | Yes | 188 (74.60) | 148 (67.27) | 0.08 | −0.156 | 125 (71.43) | 123 (70.29) | 0.81 | −0.025 | |
| No | 64 (25.40) | 72 (32.73) | 0.156 | 50 (28.57) | 52 (29.71) | 0.025 | ||||
| KPS | ≤80 | 157 (62.30) | 166 (75.45) | 0.002 | 0.306 | 126 (72.00) | 124 (70.86) | 0.81 | −0.025 | |
| >80 | 95 (37.70) | 54 (24.55) | −0.306 | 49 (28.00) | 51 (29.14) | 0.025 | ||||
| Tumor location | Upper-thoracic | 14 (5.56) | 13 (5.91) | 0.89 | 0.015 | 11 (6.29) | 11 (6.29) | 0.86 | 0.000 | |
| Middle-thoracic | 104 (41.27) | 95 (43.18) | 0.039 | 70 (40.00) | 75 (42.86) | 0.058 | ||||
| Lower-thoracic | 134 (53.17) | 112 (50.91) | −0.045 | 94 (53.71) | 89 (50.86) | −0.057 | ||||
| Clinical stage T | T2 | 13 (5.16) | 30 (13.64) | 0.006 | 0.247 | 9 (5.14) | 11 (6.29) | 0.53 | 0.047 | |
| T3 | 227 (90.08) | 179 (81.36) | −0.224 | 159 (90.86) | 153 (87.43) | −0.103 | ||||
| T4 | 12 (4.76) | 11 (5.00) | 0.011 | 7 (4.00) | 11 (6.29) | 0.094 | ||||
| Clinical stage N | N0 | 84 (33.33) | 54 (24.55) | 0.04 | −0.204 | 55 (31.43) | 48 (27.43) | 0.41 | −0.090 | |
| N+ | 168 (66.67) | 166 (75.45) | 0.204 | 120 (68.57) | 127 (72.57) | 0.090 | ||||
| Clinical stage TNM | II | 87 (34.52) | 66 (30.00) | 0.29 | −0.099 | 54 (30.86) | 52 (29.71) | 0.50 | −0.025 | |
| III | 153 (60.71) | 137 (62.27) | 0.032 | 114 (65.14) | 111 (63.43) | −0.036 | ||||
| IV | 12 (4.76) | 17 (7.73) | 0.111 | 7 (4.00) | 12 (6.86) | 0.113 | ||||
Comorbidities: including hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and coronary atherosclerotic heart disease. KPS, Karnofsky Performance Status; NCRT, neoadjuvant chemoradiotherapy; NICT, neoadjuvant immunotherapy combined with chemotherapy; PSM, propensity score matching; SMD, standardized mean difference; TNM, tumor-node-metastasis.
Comparison of treatment efficacy between the NCRT and NICT groups
All patients underwent R0 resection, and the findings are presented in Table 2. The NCRT group demonstrated a more favorable pathological response than the NICT group, both before and after PSM. After PSM, the pCR rate of the NCRT group was significantly greater than that of the NICT group (44.57% vs. 16.00%, P<0.001). Moreover, the NCRT group exhibited greater primary tumor regression than the NICT group, with a notably higher proportion of patients achieving TRG grade 0 (57.71% vs. 29.14%, P<0.001). In addition, a significantly smaller number of patients in the NCRT group were categorized as having TRG grades 2 and 3, in contrast to those in the NICT group. Statistically significant disparities were noted between the NCRT and NICT groups regarding positive lymph node responses following neoadjuvant therapy, with the NCRT group realizing a higher response rate (71.67% vs. 55.12%, P=0.007). Furthermore, when assessing lymph node yield in both cohorts, the NCRT group displayed a notably reduced lymph node yield compared with the NICT group (P<0.001).
Table 2
| Group | Level | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|---|
| NCRT (N=252) | NICT (N=220) | P value | NCRT (N=175) | NICT (N=175) | P value | |||
| pCR | Yes | 122 (48.41) | 42 (19.09) | <0.001 | 78 (44.57) | 28 (16.00) | <0.001 | |
| No | 130 (51.59) | 178 (80.91) | 97 (55.43) | 147 (84.00) | ||||
| R0 resection | Yes | 252 (100.00) | 220 (100.00) | N/A | 175 (100.00) | 175 (100.00) | N/A | |
| No | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||||
| pCR of LNM | Yes | 122 (72.62) | 97 (58.43) | 0.006 | 86 (71.67) | 70 (55.12) | 0.007 | |
| No | 46 (27.38) | 69 (41.57) | 34 (28.33) | 57 (44.88) | ||||
| ypT stage | 0 | 145 (57.54) | 52 (23.64) | <0.001 | 96 (54.86) | 38 (21.71) | <0.001 | |
| Tis | 5 (1.98) | 16 (7.27) | 5 (2.86) | 16 (9.14) | ||||
| I | 20 (7.94) | 33 (15.00) | 12 (6.86) | 27 (15.43) | ||||
| II | 37 (14.68) | 42 (19.09) | 29 (16.57) | 32 (18.29) | ||||
| III | 44 (17.46) | 73 (33.18) | 32 (18.29) | 58 (33.14) | ||||
| IV | 1 (0.40) | 4 (1.82) | 1 (0.57) | 4 (2.29) | ||||
| ypN stage | N0 | 197 (78.17) | 133 (60.45) | <0.001 | 136 (77.71) | 104 (59.43) | <0.001 | |
| N+ | 55 (21.83) | 87 (39.55) | 39 (22.29) | 71 (40.57) | ||||
| TRG | 0 | 151 (59.92) | 64 (29.09) | <0.001 | 101 (57.71) | 51 (29.14) | <0.001 | |
| 1 | 42 (16.67) | 28 (12.73) | 30 (17.14) | 23 (13.14) | ||||
| 2 | 43 (17.06) | 65 (29.55) | 31 (17.71) | 51 (29.14) | ||||
| 3 | 16 (6.35) | 63 (28.64) | 13 (7.43) | 50 (28.57) | ||||
| Lymph node yield | n | 17 [13–22] | 26 [21–35] | <0.001 | 18 [13–22] | 26 [21–35] | <0.001 | |
Data are presented as n (%) or median [interquartile range]. The total number of pCR of LNM is the number of cases with clinical stage N+. LNM, lymph node metastasis; NCRT, neoadjuvant chemoradiotherapy; NICT, neoadjuvant immunotherapy combined with chemotherapy; pCR, pathological complete response; PSM, propensity score matching; TRG, tumor regression grade.
Comparison of postoperative complications between the NCRT and NICT groups
The postoperative complications that arose following radical surgery after neoadjuvant treatment are outlined in Table 3. The most commonly observed complications included pulmonary issues, such as infections, atelectasis, and pulmonary edema. A statistically significant difference in complication prevalence was found between the NICT and NCRT groups (31.43% vs. 45.14%, P=0.008). Additionally, the rate of anastomotic leak was significantly different between the NCRT and NICT groups (10.29% vs. 0.57%, P<0.001). Other reported complications included wound infections, the necessity for postoperative blood transfusions, and thrombosis. The need of cardiac complications, notably pericardial effusion, arrhythmias, angina pectoris, and myocardial infarctions, did not show a significant difference between the two groups. Furthermore, we evaluated the hospitalization durations for both groups. It was found that the NCRT group experienced a significantly longer postoperative hospital stay than the NICT group (P<0.001); nonetheless, there were no statistically significant differences in readmission rates to the intensive care unit, overall readmission rates, or mortality rates at 90 days.
Table 3
| Group | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| NCRT (N=252) | NICT (N=220) | P value | NCRT (N=175) | NICT (N=175) | P value | ||
| Total postoperative complications | 108 (42.86) | 69 (31.36) | 0.01 | 79 (45.14) | 55 (31.43) | 0.008 | |
| Pulmonary complications | 75 (29.76) | 61 (27.73) | 0.63 | 54 (30.86) | 48 (27.43) | 0.48 | |
| Anastomotic leak | 27 (10.71) | 2 (0.91) | <0.001 | 18 (10.29) | 1 (0.57) | <0.001 | |
| Wound infection | 11 (4.37) | 6 (2.73) | 0.34 | 8 (4.57) | 5 (2.86) | 0.57 | |
| Cardiac complication | 20 (7.94) | 10 (4.55) | 0.13 | 16 (9.14) | 9 (5.14) | 0.15 | |
| POLS (day) | 13.5 [12–16] | 11 [10–13] | <0.001 | 13 [11–16] | 11 [10–13] | <0.001 | |
| ICU readmission | 7 (2.78) | 7 (3.18) | 0.80 | 3 (1.71) | 4 (2.29) | >0.99 | |
| Readmission rate | 13 (5.16) | 8 (3.64) | 0.42 | 9 (5.14) | 7 (4.00) | 0.61 | |
| 90-day mortality rate | 1 (0.40) | 5 (2.27) | 0.10 | 1 (0.57) | 5 (2.86) | 0.24 | |
Data are presented as n (%) or median [interquartile range]. ICU, intensive care unit; NCRT, neoadjuvant chemoradiotherapy; NICT, neoadjuvant immunotherapy combined with chemotherapy; POLS, postoperative length of stay; PSM, propensity score matching.
Survival
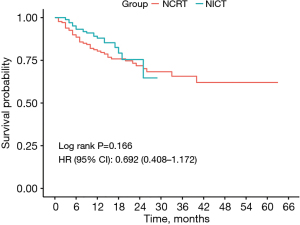
The patients were monitored for a median period of 28.5 months (ranging from 1 to 68 months) following PSM, as illustrated in Figure 2. For the NCRT group, the median duration of follow-up was 28.5 months (with a range of 1 to 68 months), whereas the NICT group had a median follow-up of 14.5 months (ranging from 1 to 26 months). Given the limited follow-up duration, which falls significantly short of the median OS time following neoadjuvant treatment for ESCC (2), we did not analyze OS. Instead, we focused solely on analyzing DFS. The follow-up results showed no significant difference in DFS between the NCRT group and the NICT group at 6 and 12 months (6-month DFS rate: 90.4% vs. 93.2%, P=0.47; 12-month DFS rate: 82.2% vs. 88.0%, P=0.25).
Discussion
This retrospective investigation, which was conducted across multiple centers in real-world settings, examined the pathological responses and postoperative complications associated with NICT and NCRT for ESCC patients undergoing radical surgery. Following PSM, the findings revealed that the NCRT cohort had superior pathological responses than the NICT cohort. Almost 50% of patients within the NCRT group attained pCR. More than half of the patients achieved a TRG score of 0, indicating complete regression of the primary tumor. In contrast, the NICT group reported fewer postoperative complications and a reduced incidence of anastomotic leaks compared to the NCRT group. Additionally, we found that the number of lymph nodes dissected in the NCRT group was lower than that in the NICT group. However, according to the consensus (14), the majority of patients in the NCRT group still had more than 15 lymph nodes dissected. Therefore, we believe that this difference may not be clinically significant.
Because the favorable outcomes demonstrated in trials such as CROSS, NCRT has become the standard neoadjuvant treatment for ESCC. According to findings from the NEOCRTEC5010 trial (2), a pCR rate of 43.2% was achieved with NCRT, aligning with the outcomes observed in our current study. Prior research has suggested that chemotherapy may positively influence immune regulation, potentially enhancing the immune system’s capacity to fight tumors (15,16). Chemotherapy combined with immunotherapy avoids the long-term toxicity and side effects of radiotherapy and may be an ideal neoadjuvant treatment modality. Recent NICT trials, including NICE (6) and KEEP-G03 (17), reported pCR rates of 39.2% (20 out of 51 patients) and 20.0% (6 out of 30 patients), respectively, which correlate with our results. The multicenter, randomized, parallel-controlled phase III ESCORT-NEO/NCCES01 trial (7) demonstrated that the pCR rates were 28.0% in the camrelizumab + nab-paclitaxel + cisplatin (Cam + nab-TP) group and 15.4% in the camrelizumab + paclitaxel + cisplatin (Cam + TP) group. Our NICT group exhibited a similar pCR rate to those observed in these trials.
Previous single-center retrospective comparative studies have also observed the same results as our trial, namely that NCRT can achieve better pathological responses than NICT. Yu et al. (8) assessed a total of 202 patients, with 121 receiving conventional NCRT and 81 treated with NICT. After adjusting for the inverse probability of treatment weighting, it was found that the NCRT group had a significantly lower pathological T stage than the NICT group (P=0.01) and also recorded reduced TRG scores (TRG 1: 49.0% vs. 28.6%, TRG 4–5: 15.5% vs. 22.3%). Hong et al. (10) found pCR rates of 18.8% (6/32) for the NICT group and 43.8% (14/32) for the NCRT group. The pCR rate was significantly higher in the NCRT group than in the NICT group (43.8% vs. 18.8%, P=0.03). Zhao et al. (18) reported that the NCRT group had a significantly higher probability of TRG 0 and pCR rate than the NICT group (TRG 0: 57.3% vs. 32.7%, P=0.003; pCR: 48.2% vs. 29.1%, P=0.03).
In total, the rates of complications have been documented in over 50% of both open and minimally invasive esophagectomy studies (19), with reported incidences ranging from 17% to 74% (19,20). Prior research (9,10) has investigated the occurrence of postoperative complications in these groups, indicating that adding immunotherapy did not increase the complication risk. Our research revealed that the NICT group experienced fewer postoperative complications than the NCRT group, with NICT exhibiting notable advantages over NCRT concerning the frequency of anastomotic leaks. This may also account for why the postoperative hospital stay was significantly shorter in the NICT group than in the NCRT group. Various studies have suggested that preoperative neoadjuvant radiotherapy is a significant risk factor for anastomotic leaks due to its ability to cause esophageal fibrosis and microvascular disease in the submucosal layer, which can hinder tissue healing. Furthermore, numerous investigations (21,22) have shown that administering neoadjuvant radiotherapy before surgery is an important factor affecting anastomotic leakage. As an emerging neoadjuvant treatment modality, NICT has demonstrated significant advantages in reducing postoperative complications and shortening the length of hospital stay, offering a safer and more effective treatment option for patients with ESCC.
Although studies have shown that patients achieving pCR may have more favorable prognoses, an increasing number of studies indicate that pCR obtained from various neoadjuvant treatment strategies can lead to different survival outcomes. pCR associated with radiotherapy does not necessarily confer an OS advantage. In the JCOG1109 NExT trial (23), the combination of doublet chemotherapy and radiotherapy (NeoCF + RT) achieved a higher pCR rate than doublet chemotherapy alone. However, there was no significant difference in OS or progression-free survival (PFS) between the NeoCF + RT group and the doublet chemotherapy group. The NeoRes I study (24) further explored the effectiveness of preoperative chemotherapy as opposed to preoperative concurrent chemoradiotherapy in esophageal and esophagogastric junction cancer, finding no notable differences in 5-year PFS, OS, or recurrence patterns between the two treatment approaches. Furthermore, Tang et al. (25) evaluated the safety and efficacy of NCRT vs. neoadjuvant chemotherapy followed by minimally invasive esophagectomy (MIE) for locally advanced ESCC. They found that although the NCRT group achieved a significantly higher pCR rate (31/112, 27.7%) than the neoadjuvant chemotherapy group (3/104, 2.9%; P<0.001), there was no difference in PFS [hazard ratio (HR) 0.83, 95% confidence interval (CI): 0.59–1.16; P=0.27]. These results suggest that a high pCR rate alone should not be the sole focus; long-term follow-up is essential to assess the efficacy and safety of different treatment modalities.
Our research has some limitations due to its retrospective design, which may introduce possible confounding factors. Although we used PSM to minimize their impact, it cannot eliminate all confounding factors. Moreover, we did not include the year of treatment, the surgeon, or the institution in the PSM analysis. Notably, each group of patients received a variety of chemotherapies and PD-1 immune checkpoint inhibitors, potentially impacting the effectiveness of neoadjuvant treatments. Nevertheless, such variability is anticipated as treatment modalities are continuously advancing. Subsequent research could examine the long-term effects, such as OS and DFS, of NCRT compared with NICT for locally advanced thoracic ESCC patients. In addition, larger-scale prospective randomized controlled trials are necessary to confirm the results of this study and to enhance the evidence base for clinical decision-making.
Conclusions
In individuals suffering from locally advanced thoracic ESCC, the percentages of patients who achieved pCR, TRG0, and positive lymph node pCR, were markedly higher in those who underwent NCRT than in those treated with NICT. Conversely, NICT correlated with a decreased rate of postoperative complications and a lower frequency of anastomotic leaks when set against NCRT. Further large-scale, prospective, and randomized studies are needed to evaluate this conclusion, with a particular focus on long-term follow-up results.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2107/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2107/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2107/prf
Funding: This study was supported in part by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2107/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Worrell SG, Goodman KA, Altorki NK, et al. The Society of Thoracic Surgeons/American Society for Radiation Oncology Updated Clinical Practice Guidelines on Multimodality Therapy for Locally Advanced Cancer of the Esophagus or Gastroesophageal Junction. Ann Thorac Surg 2024;117:15-32. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Kojima T, Doi T. Immunotherapy for Esophageal Squamous Cell Carcinoma. Curr Oncol Rep 2017;19:33. [Crossref] [PubMed]
- Doki Y, Ajani JA, Kato K, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 2022;386:449-62. [Crossref] [PubMed]
- Liu J, Yang Y, Liu Z, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e004291. [Crossref] [PubMed]
- Qin J, Xue L, Hao A, et al. Neoadjuvant chemotherapy with or without camrelizumab in resectable esophageal squamous cell carcinoma: the randomized phase 3 ESCORT-NEO/NCCES01 trial. Nat Med 2024;30:2549-57. [Crossref] [PubMed]
- Yu YK, Meng FY, Wei XF, et al. Neoadjuvant chemotherapy combined with immunotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2024;168:417-428.e3. [Crossref] [PubMed]
- Xu L, Wei XF, Li CJ, et al. Pathologic responses and surgical outcomes after neoadjuvant immunochemotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. Front Immunol 2022;13:1052542. [Crossref] [PubMed]
- Hong ZN, Gao L, Weng K, et al. Safety and Feasibility of Esophagectomy Following Combined Immunotherapy and Chemotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma: A Propensity Score Matching Analysis. Front Immunol 2022;13:836338. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers (Version 5) [J]. NCCN, 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Bolger JC, Castro PP, Marwah A, et al. Nodal yield <15 is associated with reduced survival in esophagectomy and is a quality metric. Ann Thorac Surg 2023;116:130-6. [Crossref] [PubMed]
- Jackaman C, Majewski D, Fox SA, et al. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer Immunol Immunother 2012;61:2343-56. [Crossref] [PubMed]
- Galluzzi L, Zitvogel L, Kroemer G. Immunological Mechanisms Underneath the Efficacy of Cancer Therapy. Cancer Immunol Res 2016;4:895-902. [Crossref] [PubMed]
- Chen X, Xu X, Wang D, et al. Neoadjuvant sintilimab and chemotherapy in patients with potentially resectable esophageal squamous cell carcinoma (KEEP-G 03): an open-label, single-arm, phase 2 trial. J Immunother Cancer 2023;11:e005830. [Crossref] [PubMed]
- Zhao J, Hao S, Tian J, et al. Comparison of Neoadjuvant Immunotherapy Plus Chemotherapy versus Neoadjuvant Chemoradiotherapy for Patients with Esophageal Squamous Cell Carcinoma: A Propensity Score Matching Study. J Inflamm Res 2023;16:3351-63. [Crossref] [PubMed]
- Dunst CM, Swanström LL. Minimally invasive esophagectomy. J Gastrointest Surg 2010;14:S108-14. [Crossref] [PubMed]
- Courrech Staal EF, Aleman BM, Boot H, et al. Systematic review of the benefits and risks of neoadjuvant chemoradiation for oesophageal cancer. Br J Surg 2010;97:1482-96. [Crossref] [PubMed]
- Park JS, Choi GS, Kim SH, et al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg 2013;257:665-71. [Crossref] [PubMed]
- Kobayashi M, Mohri Y, Ohi M, et al. Risk factors for anastomotic leakage and favorable antimicrobial treatment as empirical therapy for intra-abdominal infection in patients undergoing colorectal surgery. Surg Today 2014;44:487-93. [Crossref] [PubMed]
- Kato K, Machida R, Ito Y, et al. Doublet chemotherapy, triplet chemotherapy, or doublet chemotherapy combined with radiotherapy as neoadjuvant treatment for locally advanced oesophageal cancer (JCOG1109 NExT): a randomised, controlled, open-label, phase 3 trial. Lancet 2024;404:55-66. [Crossref] [PubMed]
- von Döbeln GA, Klevebro F, Jacobsen AB, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus 2019;32: [Crossref] [PubMed]
- Tang H, Wang H, Fang Y, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol 2023;34:163-72. [Crossref] [PubMed]


