
Utilizing spectral detector computed tomography quantitative parameters via multimodality tumor tracking to predict occult lymph node metastasis in clinical stage 1 pure solid lung adenocarcinoma
Highlight box
Key findings
• Dual-layer spectral detector computed tomography (SDCT) quantitative parameters obtained via multimodal tumor tracking might offer new insights to help predict occult lymph node metastasis (OLNM) in clinical stage 1 (c1) pure solid lung adenocarcinoma (LAC) patients. The prediction model constructed with the multilayer perceptron (MLP) has higher diagnostic efficiency and may further aid in clinical decision-making.
What is known and what is new?
• Early predictions about the OLNM model were predominantly based on traditional clinical pathological information, routine computed tomography (CT) features of tumors and positron emission tomography-CT metabolic parameters. These models were typically constructed using Logistic regression statistical methods.
• SDCT quantitative parameters could help predict OLNM in c1 pure solid LAC. In particular, normalized enhancement fraction (NEF)40keV, NEF70keV and normalized iodine concentration parameters perform well, which provide a new perspective and method for the prediction of OLNM.
• The prediction model constructed based on the MLP algorithm has a higher predictive ability in OLNM prediction.
What is the implication, and what should change now?
• In clinical decision-making, doctors can use SDCT-related parameters to help assess whether patients have a risk of OLNM, thereby selecting more appropriate treatment plans and surgical methods. However, the sample size of this study is small and comes from a single center. Future research should include multicenter and large sample sizes to further verify the universality and reliability of the results of this study.
Introduction
Lung cancer is a malignant tumor with the highest morbidity and mortality in China and worldwide (1,2), and non-small cell lung cancer (NSCLC) is the main histological subtype, accounting for approximately 85% of lung cancer cases (3). The presence or absence of lymph node metastasis is crucial for the staging, treatment and prognosis of NSCLC patients. However, due to the existence of occult lymph node metastasis (OLNM), the clinical staging frequently proves inadequate, causing patients to miss the optimal treatment plan.
OLNM refers to the case in which lymph node metastasis is ignored in preoperative staging modalities and the clinical N stage (cN) =0; however, postoperative pathology confirms the existence of metastatic lymph nodes and pathological N staging (pN) ≠0 (4,5). When cN0 ≠ pN0, patients face an upgrade in postoperative staging. Therefore, when cN0 NSCLC patients undergo local lobectomy (wedge resection or segmentectomy) or nonsurgical treatment [stereotactic body radiotherapy (SBRT) or radiofrequency ablation (RFA)], the risk of local lymph node recurrence or inadequate treatment due to the existence of OLNM must be fully considered. Previous studies have further confirmed that in patients with cN0 NSCLC, the survival rate of OLNM(+) patients is significantly lower than that of OLNM(−) patients, and the recurrence rate is higher (6,7). Consequently, improving the detection rate of OLNM in NSCLC patients is vital.
In the field of predicting OLNM in NSCLC patients, early models were predominantly based on traditional clinical pathological information, routine computed tomography (CT) features of tumors and positron emission tomography-CT (PET-CT) metabolic parameters. These models were typically constructed using logistic regression (LR) statistical methods, with area under the curve (AUC) values ranging between 0.6–0.8 (8,9). Currently, non-invasive prediction models based on PET-CT radiomics and deep learning have shown great potential in the prediction of OLNM in NSCLC patients, achieving AUC values of 0.85 or higher (10,11). Although a PET-CT-based study has constructed prediction model with high diagnostic efficacy, the further popularization and application of PET-CT technology are limited by factors such as false positives due to infection and inflammation (12), high radiation doses, and expensive examination costs. Therefore, exploring new imaging methods to construct OLNM prediction models has always been a research focus.
Dual-layer spectral detector computed tomography (SDCT) is a new generation of dual-energy CT that can use substances with different X-ray energies to produce different energy information, from simple CT anatomic image conversion to functional imaging (13). Compared with conventional CT, SDCT can also provide multiple quantitative parameters that can objectively and accurately reflect the essential characteristics of substances and hemodynamic information (13). Early studies suggested that SDCT plays important roles in the identification of benign and malignant lung nodules, therapeutic effect evaluation, prediction of molecular markers and diagnosis of positive lymph nodes (14-17). However, research in the field of SDCT with multiple quantitative parameters and NSCLC OLNM is rare.
In this study, we selected clinical stage 1 [c1; cT1a–T2aN0M0, American Joint Committee on Cancer (AJCC) 9th Edition] pure solid lung adenocarcinoma (LAC) patients as the research object and explored the value of multiple SDCT parameters in the prediction of the OLNM of c1 pure solid LAC. Additionally, on the basis of clinicopathological characteristics and SDCT multi-quantitative parameters, we used and compared five different machine learning (ML) algorithms [extreme gradient boosting (XGBoost), random forest (RF), LR, multilayer perceptron (MLP) and support vector machine (SVM)] under this clinical scenario to construct a better OLNM prediction model and provide more accurate clinical decision-making. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1920/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional ethics committee of Jiangsu Cancer Hospital (Nanjing, Jiangsu, China) (No. KY-2023-048) and individual consent for this retrospective analysis was waived.
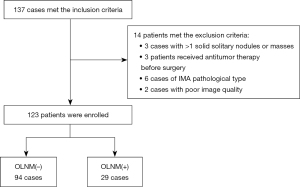
Patients who underwent SDCT examination in Jiangsu Cancer Hospital and were pathologically confirmed as LAC from September 2021 to May 2024 were included. The inclusion criteria for patients were as follows: (I) SDCT enhanced chest examination performed at Jiangsu Cancer hospital within 1 month before surgery; (II) preoperative CT revealed a solid nodule or mass with a diameter ≤4 cm; (III) cN =0 (lymph node short diameter <10 mm) and no distant metastasis in the clinical M stage (cM =0); (IV) postoperative pathology revealed invasive adenocarcinoma (IAC), and systematic lymph node dissection (SLND) was performed; and (V) complete clinical and pathological data. The exclusion criteria were as follows: (I) >1 solid solitary nodule or mass (n=3); (II) received antitumor therapy before surgery (n=3); (III) pathological type of invasive mucinous adenocarcinoma (IMA) (n=6); and (IV) poor image quality (n=2) affecting data analysis. In total, 137 patients met the inclusion criteria, and 14 patients met the exclusion criteria. Ultimately, 123 patients were enrolled, including 94 with OLNM(−) and 29 with OLNM(+). The flow chart of case inclusion and exclusion criteria is shown in Figure 1.
Acquisition of SDCT images
SDCT (IQon; Philips Healthcare, Best, the Netherlands) was used to perform enhanced chest examination following the same routine scanning protocol. Body position: supine; scanning range: from the thoracic inlet to the costophrenic angle; Scanning parameters: tube voltage, 120 kVp; tube current modulation; 3D modulation; collimator width, 64×0.625 mm; matrix, 512×512; scanning field of view, 372 mm; spacing, 0.90; rotational time, 0.50 s; scanning layer thickness, 5 mm; reconstructed layer thickness, 1 mm. The contrast agent (iodofol: 1.0–1.5 mL/kg, iodine: 350 mg/mL; Jiangsu Hengrui Medicine, Lianyungang, China) was injected into the antecubital vein at a flow rate of 2.5–3.0 mL/s, followed by 20 mL of normal saline at the same flow rate. After the injection, enhanced images of the chest were collected 50 s later.
Delineation of the full tumor volume via multimodal tumor tracking (MMTT)
Images were imported into the Philips workstation (IntelliSpace Portal, Philips Healthcare) for analysis and processed via 3D semiautomatic segmentation software MMTT. MMTT can quickly identify the target contour and obtain a smooth and neat target boundary. Meanwhile, the 2D manual tool can make layer-by-layer corrections to the results traced by the 3D tool to further obtain an accurate lesion area. The automatic registration of the lesion contour can be mapped to other sequences with one click, avoiding the repetitive work of repeatedly outlining the same lesion in different modal images. The c1 pure solid LAC lesions were delineated in the mediastinal window, and the area of interest of the aorta at a similar level was outlined to standardize the SDCT quantitative parameters. Specific operations were performed by a radiologist (with 5 years of experience in radiology) and guided by a senior radiologist (with 9 years of experience in radiology) who were blinded to the clinicopathological information.
Standardization of SDCT quantitative parameters
Quantitative parameters obtained by MMTT were recorded as follows: CT values of tumors at virtual noncontrast (VNC) and enhancement (40, 70 and 100 keV) were recorded as CTtumor-VNC, CTtumor-40keV, CTtumor-70keV and CTtumor-100keV, respectively; CT values of the aorta at VNC (CTaorta-VNC) and enhancement at 70 keV (CTaorta) were recorded and used as references. The following formulas were used for calculation: the tumor-to-aortal virtual plain scan ratio (SARVNC), tumor-to-aortal enhancement ratios (SAR40keV, SAR70keV and SAR100keV), differences between the tumor enhancement and virtual plain scan CT values (Δ40keV, Δ70keV and Δ100keV), contrast enhancement ratios (CER40keV, CER70keV and CER100keV), normalized enhancement fractions (NEF40keV, NEF70keV and NEF100keV), and λHU.
Iodine concentration (IC) (mg/mL) and effective atomic number (Zeff) values were normalized to the aorta to account for the hemodynamic and scanning time changes among individuals.
Construction of ML models
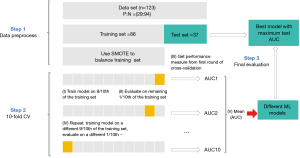
The data imbalance distribution problem between OLNM(+) and OLNM(−) was solved via the synthetic minority oversampling technique (SMOTE). It synthesizes new samples to increase the number of minority samples, which helps reduce the tendency of model over fitting and enhances the generalization ability and accuracy of the model. On the basis of the clinicopathological data and SDCT quantitative parameters, five different ML methods, namely, XGBoost, RF, LR, MLP and SVM, were used to construct the OLNM prediction model. The 10-fold cross-validation (CV) is used to evaluate and validate the model, and the AUC values of each model on the test set are compared to determine the best predictive model of the OLNM. In addition to the AUC, the indicators used to assess the predictive model include accuracy, precision, F1 score and recall (sensitivity). All five ML algorithms used for training the OLNM prediction model were implemented with Python, and the construction process is shown in Figure 2.
Statistical analysis
The data were statistically analyzed via SPSS 22 (SPSS, Inc., Chicago, IL, USA). Categorical data, expressed as counts and percentages, were compared via Pearson’s chi square test. Continuous data were assessed for normality via the Shapiro-Wilk test. When normally distributed, the data were expressed as the means ± standard deviations () and were compared via a t-test; otherwise, the data were expressed as the medians (interquartile ranges) [M (P25, P75)] and were compared via the Mann-Whitney U test. MedCalc15 (MedCalc Software, Mariakerke, Belgium) software was used to draw the receiver operating characteristic (ROC) curves, compare the diagnostic efficiency and determine the best cutoff value. A value of P<0.05 (two tailed) was considered statistically significant.
Results
Comparison of clinicopathological characteristics between the OLNM(+) and OLNM(−) groups
In total, 123 patients were enrolled in this study, including 29 OLNM(+) and 94 OLNM(−) patients, and the incidence rate of OLNM was 23.6%. The clinicopathological features of the two groups are shown in Table 1. Tumor volume (3.868 vs. 1.236 cm3) and the proportion of patients with carcinoembryonic antigen (CEA) >5 ng/mL (41.4% vs. 10.6%) were significantly greater in the OLNM(+) group than in the OLNM(−) group (both P<0.001). No significant differences were found in age, sex, smoking history, tumor diameter, lobe site, tumor distribution, degree of differentiation or high-risk factors for IAC prognosis, including visceral pleural invasion (VPI), spread through the air space (STAS) and lymphovascular invasion (LVI), between the OLNM(+) and OLNM(−) groups.
Table 1
| Characteristics | OLNM(+) (n=29) | OLNM(−) (n=94) | P value |
|---|---|---|---|
| Age (years) | 59 (54.5–68.0) | 63 (55.75–69.0) | 0.35 |
| Gender | 0.75 | ||
| Male | 13 (44.8) | 39 (41.5) | |
| Female | 16 (55.2) | 55 (58.5) | |
| Smoking | 0.83 | ||
| Yes | 12 (41.4) | 41 (43.6) | |
| No | 17 (58.6) | 53 (56.4) | |
| Diameter (mm) | 23.141±9.574 | 20.993±6.147 | 0.16 |
| Volume (cm3) | 3.868 (1.233–5.239) | 1.236 (0.624–2.654) | <0.001 |
| Lobe site | 0.38 | ||
| Upper left | 6 (20.7) | 20 (21.3) | |
| Lower left | 9 (31.0) | 18 (19.1) | |
| Upper right | 8 (27.6) | 31 (33.0) | |
| Middle right | 0 (0) | 5 (5.3) | |
| Lower right | 6 (20.7) | 20 (21.3) | |
| Tumor distribution | 0.06 | ||
| Central | 20 (69.0) | 46 (48.9) | |
| Peripheral | 9 (31.0) | 48 (51.1) | |
| Differentiation | 0.19 | ||
| Poorly | 17 (58.6) | 42 (44.7) | |
| Moderately | 12 (41.4) | 52 (55.3) | |
| VPI | 0.23 | ||
| Yes | 20 (69.0) | 53 (56.4) | |
| No | 9 (31.0) | 41 (43.6) | |
| STAS | 0.34 | ||
| Yes | 9 (31.0) | 21 (22.3) | |
| No | 20 (69.0) | 73 (77.7) | |
| LVI | 0.09 | ||
| Yes | 7 (24.1) | 9 (9.6) | |
| No | 22 (75.9) | 85 (90.4) | |
| CEA | <0.001 | ||
| ≤5 ng/mL | 17 (58.6) | 84 (89.4) | |
| >5 ng/mL | 12 (41.4) | 10 (10.6) |
Data are presented as n (%), mean ± standard deviation (x±s) and the medians (interquartile ranges). CEA, carcinoembryonic antigen; LVI, lymphovascular invasion; OLNM, occult lymph node metastasis; STAS, spread through air spaces; VPI, visceral pleural invasion.
Diameter, pathological subtype and pN of 29 patients with IAC with OLNM(+)
Compared with those of the lesions stratified by diameter, the frequencies of OLNM in stages T1a (D ≤10 mm), T1b (10 mm < D ≤20 mm), T1c (20 mm < D ≤30 mm) and T2a (30 mm < D ≤40 mm) were 0% (0/2), 10.0% (5/50), 28.1% (16/57) and 57.14% (8/14), respectively. Among the 29 OLNM(+) patients, 58.62% (17/29) had solid/micropapillary predominant IAC, 41.38% (12/29) had acinar/papillary predominant IAC, and 0% (0/29) had lepidic predominant IAC. Among the 29 patients who were OLNM(+), 13 with cN0 before the operation were upstaged to pN1, and 16 with cN0 were upstaged to pN2 (11 pN2a and 5 pN2b) (Table 2).
Table 2
| Variables | OLNM(−) | OLNM(+) | PR |
|---|---|---|---|
| Diameter: stratification | |||
| D ≤10 mm | 2 | 0 | 0% |
| 10 mm < D ≤20 mm | 45 | 5 | 10.00% |
| 20 mm < D ≤30 mm | 41 | 16 | 28.10% |
| 30 mm < D ≤40 mm | 6 | 8 | 57.10% |
| Pathological subtype: classification | – | – | |
| Lepidic predominant | 0/29 | ||
| Acinar/papillary predominant | 12/29 | ||
| Solid/micropapillary predominant | 17/29 | ||
| Pathological lymph nodes: stage | – | – | |
| pN1 | 13/29 | ||
| pN2 | 16/29 | ||
| pN2a | 11/16 | ||
| pN2b | 5/16 |
IAC, invasive adenocarcinoma; OLNM, occult lymph node metastasis; PR, positive ratio; pN1, ipsilateral peribronchial and/or hilar nodes and intrapulmonary nodes; pN2, ipsilateral mediastinal and/or subcarinal nodes; pN2a, single-station lymph node metastasis; pN2b, multi-station lymph node metastasis.
Differences in the SDCT quantitative parameters between the OLNM(+) and OLNM(−) groups
The quantitative parameters and schematic diagram of the delineation of the target primary lesion acquired by MMTT in the OLNM(+) and OLNM(−) groups are shown in Figures 3,4, respectively. All the SDCT quantitative parameters were normalized uniformly. A total of 17 quantitative and derived quantitative indicators were included for statistical analysis. Table 3 shows the analysis results of the differences in the quantitative parameters related to the primary lesions between the OLNM(+) and OLNM(−) groups. Except for SARVNC, SAR70keV and SAR100keV (P=0.25, 0.11, 0.98), the remaining 14 quantitative parameters [SAR40keV, Δ40keV, Δ70keV, Δ100keV, CER40keV, CER70keV, CER100keV, NEF40keV, NEF70keV, NEF100keV, λ40–70keV, λ40–100keV, normalized iodine concentration (NIC), normalized effective atomic number (NZeff)] in the OLNM(+) group were significantly lower than those in the OLNM(−) group (P<0.05).
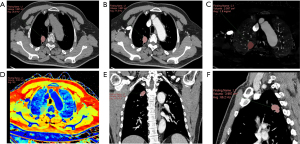
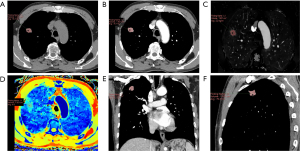
Table 3
| Parameters | OLNM(+) (n=29) | OLNM(−) (n=94) | P value |
|---|---|---|---|
| SARVNC | 0.691±0.197 | 0.621±0.307 | 0.25 |
| SAR40keV | 0.757 (0.628–0.863) | 0.898 (0.756–1.073) | 0.002 |
| SAR70keV | 0.311 (0.270–0.361) | 0.340 (0.290–0.417) | 0.11 |
| SAR100keV | 0.216 (0.175–0.244) | 0.204 (0.174–0.268) | 0.98 |
| Δ40keV | 145.842±42.913 | 174.003±43.315 | 0.003 |
| Δ70keV | 43.000±13.391 | 51.468±13.299 | 0.003 |
| Δ100keV | 18.728±6.042 | 22.143±6.188 | 0.01 |
| CER40keV | 4.214 (3.469–6.144) | 6.371 (4.227–10.760) | 0.001 |
| CER70keV | 1.176 (1.014–1.829) | 1.901 (1.265–3.205) | 0.001 |
| CER100keV | 0.536 (0.453–0.790) | 0.827 (0.549–1.391) | 0.002 |
| NEF40keV | 0.780 (0.613–0.902) | 0.967 (0.809–1.156) | 0.001 |
| NEF70keV | 0.229 (0.183–0.269) | 0.284 (0.237–0.344) | 0.001 |
| NEF100keV | 0.101±0.034 | 0.128±0.043 | 0.003 |
| λ40-70keV | 3.428±0.989 | 4.085±1.007 | 0.003 |
| λ40-100keV | 2.121±0.620 | 2.531±0.626 | 0.002 |
| NIC | 0.240±0.069 | 0.289±0.076 | 0.002 |
| NZeff | 0.798 (0.767–0.839) | 0.818 (0.787–0.861) | 0.03 |
Data were presented as mean ± standard deviation (x±s) and the medians (interquartile ranges). Δ, difference between the tumor enhancement and virtual plain scan CT value; CER, contrast enhancement ratio; NEF, standardized reinforcement score; NIC, normalized iodine concentration; NZeff, normalized effective atomic number; OLNM, occult lymph node metastasis; SAR, tumor-to-aortal enhancement ratio; SDCT, spectral detector computed tomography; VNC, virtual noncontrast.
Comparison of SDCT quantitative parameters in predicting OLNM efficacy
The diagnostic efficacy of the quantitative SDCT parameters with significant differences between OLNM(+) and OLNM(−) for predicting OLNM in c1 pure solid LAC patients is shown in Table 4. Among the multiple quantitative SDCT parameters, NEF40keV, NEF70keV and NIC achieved relatively high diagnostic efficiency, and their AUCs were 0.710, 0.705 and 0.701, respectively. NEF40keV had the highest AUC =0.710; when the cutoff value was 0.907, its sensitivity was 79.3% and its specificity was 61.7%.
Table 4
| Parameters | AUC | Cut-off value | Sensitivity (%) | Specificity (%) | P value |
|---|---|---|---|---|---|
| SAR40keV | 0.694 | 0.893 | 86.200 | 51.100 | <0.001 |
| Δ40keV | 0.689 | 158.800 | 72.400 | 64.900 | 0.003 |
| Δ70keV | 0.679 | 47.300 | 72.400 | 64.900 | 0.004 |
| Δ100keV | 0.667 | 20.400 | 69.000 | 64.900 | 0.007 |
| CER40keV | 0.699 | 6.175 | 79.300 | 52.100 | <0.001 |
| CER70keV | 0.697 | 1.176 | 51.700 | 80.900 | <0.001 |
| CER100keV | 0.687 | 0.792 | 79.300 | 53.200 | <0.001 |
| NEF40keV | 0.710 | 0.907 | 79.300 | 61.700 | <0.001 |
| NEF70keV | 0.705 | 0.270 | 79.300 | 60.600 | <0.001 |
| NEF100keV | 0.693 | 0.117 | 79.300 | 61.700 | <0.001 |
| λ40-70keV | 0.690 | 3.717 | 72.400 | 67.000 | 0.002 |
| λ40-100keV | 0.693 | 2.060 | 62.100 | 76.600 | 0.002 |
| NIC | 0.701 | 0.267 | 79.300 | 66.000 | <0.001 |
| NZeff | 0.631 | 0.776 | 41.400 | 83.000 | 0.03 |
Δ, difference between the tumor enhancement and virtual plain scan CT value; CER, contrast enhancement ratio; NEF, standardized reinforcement score; NIC, normalized iodine concentration; NZeff, normalized effective atomic number; SAR, tumor-to-aortal enhancement ratio; SDCT, spectral detector computed tomography; OLNM, occult lymph node metastasis.
Diagnostic performance of the five different ML models
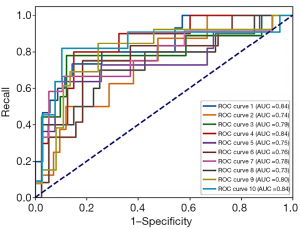
On the basis of the statistically significant clinical data (volume, CEA) and SDCT quantitative parameters (SAR40keV, Δ40keV, Δ70keV, Δ100keV, CER40keV, CER70keV, CER100keV, NEF40keV, NEF70keV, NEF100keV, λ40–70keV, λ40–100keV, NIC, NZeff), 5 different ML methods including: XGBoost, RF, LR, MLP and SVM were used to further construct the OLNM prediction model. Our study revealed that the OLNM prediction model for c1 pure solid LAC built on the MLP algorithm had the highest average AUC =0.778 on the test set, and the accuracy =0.708, precision =0.726, F1 score =0.662 and recall =0.851 (Table 5). Figure 5 shows the ROC curve diagram of the 10-fold CV of the MLP model.
Table 5
| Models | AUC | Accuracy | Precision | F1 | Recall |
|---|---|---|---|---|---|
| XGB | 0.759 | 0.772 | 0.859 | 0.541 | 0.835 |
| RF | 0.727 | 0.756 | 0.845 | 0.522 | 0.821 |
| LR | 0.731 | 0.704 | 0.752 | 0.507 | 0.835 |
| MLP | 0.778 | 0.708 | 0.726 | 0.662 | 0.851 |
| SVM | 0.736 | 0.700 | 0.743 | 0.506 | 0.831 |
LR, logistic regression; ML, machine learning; MLP, multilayer perceptron; RF, random forest; SVM, support vector machine; XGB, extreme gradient boosting.
Discussion
As the most common subtype of NSCLC, LAC has a greater probability of developing OLNM than other types of lung cancer (18). For c1 LAC patients, the existence of OLNM has an important impact on the selection of surgical methods, the decision-making of radiotherapy and chemotherapy regimen and the prognosis (5,6). Therefore, actively exploring and predicting OLNM for c1 LAC are highly important. On the basis of the quantitative SDCT parameters, the characteristics of the primary lesions and the clinicopathological data of the patients, we used five different ML algorithms to establish the optimal OLNM prediction model for c1 LAC, which has rarely been reported in previous studies.
MMTT allows automatic extraction and quantitative analysis of tumors. It is an efficient post processing software package capable of monitoring the development of and changes in tumors and evaluating radiotherapy efficacy. In this study, we used MMTT to outline the full volume of tumors and then outlined the aorta in the corresponding layers for normalization processing, which could help us obtain more objective and stable data. Some subsolid nodules of c1 LAC have no or a low proportion of solid components, and they cannot meet the delineation requirements. Additionally, a previous study reported that there was no lymph node metastasis in pure ground glass nodules (19). Moreover, Lee et al. (20) and Haruki et al. (21) reported that no lymph node metastasis was present in patients whose solid proportion was ≤50%. Therefore, in this study, we did not include the subsolid nodules of c1-stage LAC but focused on the pure solid nodules, which are more prone to OLNM.
In this study, the incidence of OLNM was 23.6%, which was slightly higher than the 8.18–23.1% reported in previous studies (4,9,22,23). This may be closely related to the fact that all the subjects we included were pure solid lesions. Our study revealed no significant differences in age, sex, smoking history, tumor diameter, lobe site, tumor distribution, degree of differentiation or high-risk factors (VPI, STAS, LVI) related to prognosis between OLNM(+) and OLNM(−) patients. The proportion of patients with CEA >5 ng/mL (41.4% vs. 10.6%) in the OLNM(+) group was greater than that in the OLNM(−) group. Those with abnormal CEA levels were prone to lymph node metastasis, which was consistent with the findings of previous studies (4,8,22,24). The tumor volume of OLNM(+) patients was greater than that of OLNM(−) patients (3.868 vs. 1.236 cm3). On the one hand, a larger tumor volume indicates a more rapid growth rate and a greater degree of malignancy, which increases the likelihood of developing OLNM. On the other hand, we retrospectively observed the SDCT images of the tumors and found that, in the case of similar diameters, the OLNM(+) lesions were more full in the reconstructed images than the OLNM(−) lesions were, so they had a larger volume. Some studies have shown that the occurrence of OLNM is related to the tumor diameter (19,24,25). However, no significant difference in diameter (23.141 vs. 20.993 mm) was found in our study between the OLNM(+) and OLNM(−) patients. This may be caused by the small and unevenly distributed sample size, but it highlights the advantages of the volume obtained via MMTT. Furthermore, when the 29 OLNM(+) lesions were stratified by diameter, we found that the frequency of OLNM in c1 pure solid LAC increased with increasing clinical T stage. The frequencies of OLMM in stages T1a (D ≤10 mm), T1b (10 mm < D ≤20 mm), T1c (20 mm < D ≤30 mm) and T2a (30 mm < D ≤40 mm) were 0% (0/2), 10.0% (5/50), 28.1% (16/57) and 57.14% (8/14), respectively. No OLNM were found in stage T1a. Moreover, among the 29 patients with OLNM(+), those with solid/micropapillary predominant IAC (17/29) were more prone to OLNM, followed by those with acinar/papillary predominant IAC (12/29), and no OLNM was found with lepidic predominant IAC, which was consistent with previous results (19).
Enhanced CT-derived parameters such as the CT enhancement value and enhancement ratio can reduce the background effects caused by machine and individual differences and are considered useful tools for assessing tumor angiogenesis. Moreover, the CT enhancement value and enhancement ratio are closely related to microvascular and lymphatic infiltration within the tumor and can be used as alternative markers for the preoperative detection of lymphatic vessel invasion (26,27). Inspired by this, we emphasize the application of SDCT quantitative parameters and their derived quantitative parameters after normalization to predict the OLNM of c1 pure solid LAC more objectively and accurately. In this study, NEF40keV, NEF70keV and NIC performed better in predicting OLNM, with AUCs of 0.710, 0.705 and 0.701, respectively. The NIC is a relatively stable quantitative parameter that is an objective and quantitative reflection of the intake of a tumor-iodized contrast agent. Its intake is directly proportional to the density of tumor blood vessels (28,29). In the present study, the NIC of the primary lesion in the OLNM(+) group was lower than that in the OLNM(−) group, which is consistent with the findings of previous studies (30). This result may be due to the greater degree of malignancy of the primary focus in OLNM(+) patients and the invasion and destruction of adjacent blood vessels as well as the immature development and low functional efficiency of new tumor blood vessels.
In addition, during the process of exploring the SDCT quantitative parameters to predict the OLNM in c1 pure solid LAC, we found that NZeff also has a certain value. Compared with OLNM(−) patients, OLNM(+) patients had a lower NZeff (0.798 vs. 0.818). Zeff reflects the atomic number of a compound material; the denser the material is, the greater the Zeff (31). Previous studies have reported that Zeff has good differential diagnostic efficiency in distinguishing benign and malignant orbital tumors from ovarian tumors (32,33). Wang et al. (34) applied Zeff to predict Ki-67 expression in laryngeal squamous cell carcinoma, suggesting that the Zeff value can be used as an indirect predictor of survival and prognosis, thereby determining the ability of tumor recurrence. Lv et al. (35) reported that Zeff was also highly sensitive in differentiating between glioma recurrence and treatment-related changes. The value of NZeff in predicting the OLNM of c1 LAC has not been reported before and provides a new way to forecast OLNM research in the oncology field.
ML has the advantages of nonlinearity, strong fault tolerance, and retention through the updating of databases. ML is suitable for disease diagnosis and has been frequently applied in research and exploration of medical imaging in recent years (36,37). Given that different ML models have different characteristics and different scopes of application, this study used five different ML algorithms to establish and compare the prediction models. The study showed that the OLNM prediction model for c1 pure solid LAC built on the MLP algorithm achieved the highest average AUC =0.778, indicating that the proposed MLP model exhibited greater predictive ability for OLNM than the other commonly used models did. The main advantage of the MLP, also referred to as the artificial neural network (ANN), is that complex models can be constructed by adjusting the number of hidden layers and the number of hidden units within each hidden layer to learn various types of information contained in the data (37).
There are some limitations in this study. First, for NSCLC subsolid nodules, there is also a risk of OLNM when the solid component is over 0.5, and these patients were not included in the current study. Second, the interpretation of conventional CT signs is subjective, but it is still the easiest resource for us to access. Later, the CT signs of the primary lesion are adopted to further improve the OLNM prediction model. Third, although the incidence of OLNM is relatively low, the sample size of this study is small, and the patients were from a single center; thus, external verification is lacking. Therefore, multicenter and large-sample studies are needed.
Conclusions
In conclusion, SDCT quantitative parameters derived through MMTT have a certain significance in predicting OLNM in c1 pure solid LAC. The OLNM prediction model constructed with the MLP model, which is based on clinicopathological data and quantitative SDCT parameters, has higher diagnostic efficacy and may further assist in clinical decision-making.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1920/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1920/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1920/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1920/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional ethics committee of Jiangsu Cancer Hospital (Nanjing, Jiangsu, China) (No. KY-2023-048) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Zheng RS, Chen R, Han BF, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi 2024;46:221-31. [Crossref] [PubMed]
- Testa U, Castelli G, Pelosi E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers (Basel) 2018;10:248. [Crossref] [PubMed]
- Moon Y, Choi SY, Park JK, et al. Risk Factors for Occult Lymph Node Metastasis in Peripheral Non-Small Cell Lung Cancer with Invasive Component Size 3 cm or Less. World J Surg 2020;44:1658-65. [Crossref] [PubMed]
- Deng J, Zhong Y, Wang T, et al. Lung cancer with PET/CT-defined occult nodal metastasis yields favourable prognosis and benefits from adjuvant therapy: a multicentre study. Eur J Nucl Med Mol Imaging 2022;49:2414-24. [Crossref] [PubMed]
- Rusch VW, Hawes D, Decker PA, et al. Occult metastases in lymph nodes predict survival in resectable non-small-cell lung cancer: report of the ACOSOG Z0040 trial. J Clin Oncol 2011;29:4313-9. [Crossref] [PubMed]
- Bille A, Woo KM, Ahmad U, et al. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg 2017;51:674-9. [Crossref] [PubMed]
- He XQ, Luo TY, Li X, et al. Clinicopathological and computed tomographic features associated with occult lymph node metastasis in patients with peripheral solid non-small cell lung cancer. Eur J Radiol 2021;144:109981. [Crossref] [PubMed]
- Ouyang ML, Xia HW, Xu MM, et al. Prediction of occult lymph node metastasis using SUV, volumetric parameters and intratumoral heterogeneity of the primary tumor in T1-2N0M0 lung cancer patients staged by PET/CT. Ann Nucl Med 2019;33:671-80. [Crossref] [PubMed]
- Qiao J, Zhang X, Du M, et al. (18)F-FDG PET/CT radiomics nomogram for predicting occult lymph node metastasis of non-small cell lung cancer. Front Oncol 2022;12:974934. [Crossref] [PubMed]
- Zhong Y, Cai C, Chen T, et al. PET/CT based cross-modal deep learning signature to predict occult nodal metastasis in lung cancer. Nat Commun 2023;14:7513. [Crossref] [PubMed]
- Girvin F, Ko JP. Pulmonary nodules: detection, assessment, and CAD. AJR Am J Roentgenol 2008;191:1057-69. [Crossref] [PubMed]
- Rassouli N, Etesami M, Dhanantwari A, et al. Detector-based spectral CT with a novel dual-layer technology: principles and applications. Insights Imaging 2017;8:589-98. [Crossref] [PubMed]
- Liu K, Wang M, Xu Y, et al. Value of spectral computed tomography-derived quantitative parameters based on full volume analysis in the diagnosis of benign/malignant and pathological subtypes of solitary pulmonary nodules. Quant Imaging Med Surg 2023;13:3827-40. [Crossref] [PubMed]
- Guo S, Wang D, Zhao Q, et al. Dual-layer detector spectral computed tomography quantitative parameters for predicting pathological complete remission after neoadjuvant treatment of breast cancer. Quant Imaging Med Surg 2025;15:149-63. [Crossref] [PubMed]
- Wu F, Zhou H, Li F, et al. Spectral CT Imaging of Lung Cancer: Quantitative Analysis of Spectral Parameters and Their Correlation with Tumor Characteristics. Acad Radiol 2018;25:1398-404. [Crossref] [PubMed]
- Ren X, Song Z, Zhang D, et al. Differentiation of benign and malignant lesions in Bethesda III and IV thyroid nodules via dual-energy computed tomography quantitative parameters and morphologic features. Quant Imaging Med Surg 2024;14:4567-78. [Crossref] [PubMed]
- Gómez-Caro A, Garcia S, Reguart N, et al. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computer tomography scan. Eur J Cardiothorac Surg 2010;37:1168-74. [Crossref] [PubMed]
- Wang S, Bao X, Yang F, et al. Multiparametric evaluation of mediastinal lymph node metastases in clinical T0-T1c stage non-small-cell lung cancers. Eur J Cardiothorac Surg 2024;65:ezae059. [Crossref] [PubMed]
- Lee SM, Park CM, Paeng JC, et al. Accuracy and predictive features of FDG-PET/CT and CT for diagnosis of lymph node metastasis of T1 non-small-cell lung cancer manifesting as a subsolid nodule. Eur Radiol 2012;22:1556-63. [Crossref] [PubMed]
- Haruki T, Aokage K, Miyoshi T, et al. Mediastinal nodal involvement in patients with clinical stage I non-small-cell lung cancer: possibility of rational lymph node dissection. J Thorac Oncol 2015;10:930-6. [Crossref] [PubMed]
- Gu Y, She Y, Xie D, et al. A Texture Analysis-Based Prediction Model for Lymph Node Metastasis in Stage IA Lung Adenocarcinoma. Ann Thorac Surg 2018;106:214-20. [Crossref] [PubMed]
- Beyaz F, Verhoeven RLJ, Schuurbiers OCJ, et al. Occult lymph node metastases in clinical N0/N1 NSCLC; A single center in-depth analysis. Lung Cancer 2020;150:186-94. [Crossref] [PubMed]
- Song CY, Kimura D, Sakai T, et al. Novel approach for predicting occult lymph node metastasis in peripheral clinical stage I lung adenocarcinoma. J Thorac Dis 2019;11:1410-20. [Crossref] [PubMed]
- Zhong Y, Yuan M, Zhang T, et al. Radiomics Approach to Prediction of Occult Mediastinal Lymph Node Metastasis of Lung Adenocarcinoma. AJR Am J Roentgenol 2018;211:109-13. [Crossref] [PubMed]
- Li Y, Su H, Yang L, et al. Can lymphovascular invasion be predicted by contrast-enhanced CT imaging features in patients with esophageal squamous cell carcinoma? A preliminary retrospective study. BMC Med Imaging 2022;22:93. [Crossref] [PubMed]
- Ma Z, Liang C, Huang Y, et al. Can lymphovascular invasion be predicted by preoperative multiphasic dynamic CT in patients with advanced gastric cancer? Eur Radiol 2017;27:3383-91. [Crossref] [PubMed]
- Marcon J, Graser A, Horst D, et al. Papillary vs clear cell renal cell carcinoma. Differentiation and grading by iodine concentration using DECT-correlation with microvascular density. Eur Radiol 2020;30:1-10. [Crossref] [PubMed]
- Kim TM, Lee JM, Yoon JH, et al. Prediction of microvascular invasion of hepatocellular carcinoma: value of volumetric iodine quantification using preoperative dual-energy computed tomography. Cancer Imaging 2020;20:60. [Crossref] [PubMed]
- Xie X, Yan H, Liu K, et al. Value of dual-layer spectral detector CT in predicting lymph node metastasis of non-small cell lung cancer. Quant Imaging Med Surg 2024;14:749-64. [Crossref] [PubMed]
- Garcia LI, Azorin JF, Almansa JF. A new method to measure electron density and effective atomic number using dual-energy CT images. Phys Med Biol 2016;61:265-79. [Crossref] [PubMed]
- Luo S, Sha Y, Wu J, et al. Differentiation of malignant from benign orbital tumours using dual-energy CT. Clin Radiol 2022;77:307-13. [Crossref] [PubMed]
- Elsherif SB, Zheng S, Ganeshan D, et al. Does dual-energy CT differentiate benign and malignant ovarian tumours? Clin Radiol 2020;75:606-14. [Crossref] [PubMed]
- Wang P, Tang Z, Xiao Z, et al. Dual-energy CT in predicting Ki-67 expression in laryngeal squamous cell carcinoma. Eur J Radiol 2021;140:109774. [Crossref] [PubMed]
- Lv Y, Zhou J, Lv X, et al. Dual-energy spectral CT quantitative parameters for the differentiation of Glioma recurrence from treatment-related changes: a preliminary study. BMC Med Imaging 2020;20:5. [Crossref] [PubMed]
- Gore JC. Artificial intelligence in medical imaging. Magn Reson Imaging 2020;68:A1-4. [Crossref] [PubMed]
- Currie G, Hawk KE, Rohren E, et al. Machine Learning and Deep Learning in Medical Imaging: Intelligent Imaging. J Med Imaging Radiat Sci 2019;50:477-87. [Crossref] [PubMed]


