
Evaluating long-term bronchoscopic outcomes of tracheobronchoplasty in patients with expiratory central airway collapse
Highlight box
Key findings
• Robotic and open tracheobronchoplasty (TBP) effectively reduced the expiratory central airway collapse (ECAC) severity score from severe to non-severe values 2 months post-surgery.
• Both surgical approaches showed similar benefits in bronchoscopic outcomes, pulmonary function tests (PFTs), and functional results.
What is known and what is new?
• ECAC is a condition involving mechanical airway obstruction, assessed via dynamic bronchoscopy, which evaluates the degree of collapse in the central airway. A score of 9 or more indicates severe disease. TBP is the preferred treatment for severe symptomatic ECAC, with open and robotic approaches available. While most studies show that TBP improves symptoms, quality of life, and functional status, few have compared bronchoscopic and long-term outcomes by surgical approach.
• This study found that both open and robotic TBP significantly improved the ECAC score from severe to non-severe values, even two years after surgery. PFTs showed minimal change over time, but functional outcomes improved significantly post-surgery. However, patients with obstructive sleep apnea may not experience the same level of improvement.
What is the implication and what should change now?
• Both open and robotic TBP are effective for treating severe ECAC. Clinicians should use dynamic bronchoscopy and functional measures to track patient progress and ensure the best outcomes. Additionally, managing coexisting conditions like obstructive sleep apnea is crucial for optimizing patient recovery and maximizing benefits.
Introduction
Expiratory central airway collapse (ECAC) is a condition that has gained recognition in recent years (1). It is estimated to affect around 13% of patients with known lung disease (2). ECAC encompasses two pathophysiologic processes: excessive dynamic airway collapse and tracheobronchomalacia (3). In excessive dynamic airway collapse, the posterior wall of the trachea displaces excessively forward, while tracheobronchomalacia involves an abnormal motion of the anterolateral portion of the tracheobronchial wall (1,3). Both conditions result in a mechanical outflow obstruction, which explains most patients’ complaints.
Common symptoms of ECAC include dyspnea, chronic cough, mucostasis, and recurrent respiratory infections (4). However, these symptoms are nonspecific and prevalent in other conditions such as asthma, chronic bronchitis, or bronchiectasis. Therefore, a high level of suspicion is necessary when patients with these conditions do not respond to appropriate treatments (1).
Dynamic bronchoscopy with conscious sedation is the gold standard test for diagnosing ECAC (1,5). This procedure allows real-time assessment of the extent of airway collapse across various segments of the central airway: cricoid/proximal trachea, mid trachea, distal trachea, right mainstem bronchus, bronchus intermedius, and left mainstem bronchus. An airway collapse exceeding 70% is considered pathologic, graded as mild (70% to 79%), moderate (80% to 89%), or severe (≥90%) (4). Recently, an overall severity score was developed by assigning numerical values to the different levels of segmental collapse: one for mild collapse, two for moderate collapse, and three for severe collapse (6). These values were then added to create an overall punctuation, with a score of nine or more efficiently predicting severe disease (6).
Tracheobronchoplasty (TBP) is the preferred surgery for patients with severe symptomatic ECAC (1,4,7). This procedure stabilizes the central airway by plicating and fixing a polypropylene mesh to the posterior membrane of the trachea and bilateral bronchi (7,8). TBP can be performed using a robotic-assisted approach or open technique, typically through a posterolateral thoracotomy (8-10). Studies have shown that TBP is a safe procedure that improves symptoms, quality of life, and functional status of patients with ECAC (7,11-14).
However, there is still a lack of studies exploring changes in bronchoscopy assessments and long-term outcomes after TBP, as well as the comparison of benefits between surgical approaches. Here, we evaluate changes in bronchoscopic outcomes, pulmonary function tests (PFTs), six-minute walk tests (6MWTs), quality-of-life indices, surgical outcomes and complications in patients with ECAC who underwent open or robotic-assisted TBP. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2139/rc).
Methods
In this retrospective cohort study, we included patients diagnosed with ECAC who underwent TBP at a tertiary referral pulmonary center in the United States between January 2019 and June 2024. Patients with symptoms suggesting ECAC were evaluated using dynamic bronchoscopy, PFTs, 6MWTs, St. George’s Respiratory Questionnaire (SGRQ), and cough-specific quality of life questionnaire (CQLQ), for evaluation and diagnosis (15,16). As part of their diagnostic workup, patients were evaluated for other respiratory and gastrointestinal conditions that could contribute to their symptoms and received medical treatment as needed. Patients with a severe ECAC score, or those with a non-severe score but severe symptoms that did not improve with maximum medical therapy or an alternative diagnosis, underwent a stent trial to assess the potential benefits of surgery. Surgery could also be performed without a stent trial if patients had a severe ECAC score or persistent, troublesome cough and recurrent infections that did not respond to medical therapies. Our treatment algorithm is shown in Figure S1. As part of routine follow-up, patients were monitored with repeat assessments of PFTs, 6MWTs, SGRQ, CQLQ, and dynamic bronchoscopies after both the stent trial and surgery to monitor outcomes.
We collected sociodemographic, clinical, and procedural data through a review of medical records, including age, sex, body mass index (BMI), smoking history, comorbidities, past history of chest surgery, primary respiratory symptoms, PFTs results, 6MWTs results, dynamic bronchoscopy findings, the type of surgical approach, the duration of surgery, days with chest tubes, days in the intensive care unit (ICU), total hospital length of stay, 90-day survival, and complications based on the Clavien-Dindo (CD) classification (17). Data was collected and stored using a secure spreadsheet. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Institutional Review Board of Mayo Clinic Florida (#21-003543) and individual consent for this retrospective analysis was waived.
Outcomes of interest
We were interested in evaluating changes in the ECAC severity score, PFTs, 6MWTs, SGQR, and CQLQ over time. For PFTs, we followed established technical standards and evaluated changes in predicted values of forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) (18). The 6MWTs were conducted according to practical guidelines, measuring the distance covered in meters over six minutes and noting the requirement for supplemental oxygen (19). For SGRQ and CQLQ, delta values were calculated as mean values after surgery minus baseline values.
Surgical approaches
At Mayo Clinic Florida, we routinely perform open and robotic TBP. The decision to perform a robotic or open approach was based primarily on the surgeon’s preference.
The technique used for the open approach has been described in previous literature (14). Briefly, we used a standard posterior-lateral thoracotomy to access the chest cavity through the fourth intercostal space. After identifying vascular and nerve structures, we carefully dissected until exposing the posterior trachea. A ringed polytetrafluoroethylene mesh was cut into strips (two rings per strip) and sutured to the trachea with interrupted 4-0 Prolene sutures. We typically attached three to four strips to the trachea, two on the left mainstem bronchus, and three on the right mainstem bronchus, right upper lobe, and bronchus intermedius.
For the robotic approach, we used the da Vinci Xi robotic platform (Intuitive Surgical, Sunnyvale, CA). An 8-mm robotic port was inserted in the sixth intercostal space, along with three additional 8-mm ports in the same space and a 12-mm assistant port. After opening the right pleura over the posterior mediastinum and carefully identifying nerve structures, we exposed it until reaching the posterior wall of the trachea and both mainstem bronchi. Multiple composite splints were created using a strip of polypropylene mesh. The mesh was sewn to the posterior trachea using running 3-0 non-absorbable V-Loc sutures in a plicating fashion. Similarly, a mesh was placed for the left mainstem bronchus, anchored with a single felt strip and running 3-0 non-absorbable V-loc sutures. The right mainstem mesh was then placed over the bronchus intermedius, extending to the carina, and anchored in the same manner as the left mainstem.
Statistical analysis
Data were summarized using means and standard deviation (SD) or medians and interquartile range (IQR) for continuous variables, depending on data distribution. While frequencies and proportions were employed for categorical variables. To explore associations between variables, we employed the Chi-squared, Fisher’s Exact, and Wilcoxon Signed Rank tests.
Given the correlated nature of our data, we used linear mixed models to analyze changes in our outcomes of interest over time. In these models, we treated variables of interest as fixed effects, while patients were included as a random effect. We used random intercept and random slope models with linear, quadratic, or cubic time effects, selecting the best fit based on the Akaike information criterion and Bayesian information criterion. We created univariable and multivariable models to explore the association between specific variables of interest and outcome changes.
For specific surgical complications, such as mesh erosion and redo surgery, we calculated the time from the surgery date to the date of the complication. We employed the Kaplan-Meier estimate and compared times between surgical approaches using the log-rank test. Due to the exploratory nature of this analysis, no adjustment for multiplicity was undertaken. All statistical analyses were conducted using R Statistical Software version 4.4.1 (20). The statistical significance was defined as P<0.05.
Results
A total of 61 patients were included in the study. As shown in Table 1, most patients were female (n=42; 69%), had a median age of 63.0 years (IQR, 58.0–69.0 years), and had a median BMI of 30.8 kg/m2 (IQR, 28.1–32.6 kg/m2). Notably, most patients were non-smokers (n=35; 57%). The most common comorbidities at baseline included gastroesophageal reflux disease (n=37; 61%), hypertension (n=34; 56%), and asthma (n=32; 52%).
Table 1
| Variable | Overall (n=61) | Open (n=33) | Robotic (n=28) | P value |
|---|---|---|---|---|
| Age (years) | 63.0 (58.0–69.0) | 61.0 (52.0–69.0) | 65.0 (60.8–68.3) | 0.21 |
| Sex | 0.69 | |||
| Female | 42 [69] | 22 [67] | 20 [71] | |
| Male | 19 [31] | 11 [33] | 8 [29] | |
| Body mass index (kg/m2) | 30.8 (28.1–32.6) | 29.6 (27.4–31.7) | 31.7 (29.9–32.9) | 0.08 |
| Smoking history | 0.63 | |||
| Former | 26 [43] | 15 [45] | 11 [39] | |
| Never | 35 [57] | 18 [55] | 17 [61] | |
| Hypertension | 0.47 | |||
| No | 27 [44] | 16 [48] | 11 [39] | |
| Yes | 34 [56] | 17 [52] | 17 [61] | |
| Chronic heart failure | 0.89 | |||
| No | 58 [97] | 32 [97] | 26 [96] | |
| Yes | 2 [3] | 1 [3] | 1 [4] | |
| Chronic kidney disease | 0.35 | |||
| No | 60 [98] | 32 [97] | 28 [100] | |
| Yes | 1 [2] | 1 [3] | 0 [0] | |
| Diabetes mellitus | 0.54 | |||
| No | 48 [79] | 25 [76] | 23 [82] | |
| Yes | 13 [21] | 8 [24] | 5 [18] | |
| Asthma | 0.87 | |||
| No | 29 [48] | 16 [48] | 13 [46] | |
| Yes | 32 [52] | 17 [52] | 15 [54] | |
| Chronic obstructive pulmonary disorder | 0.53 | |||
| No | 50 [82] | 28 [85] | 22 [79] | |
| Yes | 11 [18] | 5 [15] | 6 [21] | |
| Obstructive sleep apnea | 0.32 | |||
| No | 35 [57] | 17 [52] | 18 [64] | |
| Yes | 26 [43] | 16 [48] | 10 [36] | |
| Gastroesophageal reflux disease | 0.61 | |||
| No | 24 [39] | 12 [36] | 12 [43] | |
| Yes | 37 [61] | 21 [64] | 16 [57] | |
| Prior chest surgery | 0.47 | |||
| No | 60 [98] | 33 [100] | 27 [96] | |
| Yes | 1 [2] | 0 | 1 [4] |
Data are presented as median (interquartile range) or n [%].
At the time of diagnosis, patients reported various symptoms: chronic cough (n=56; 92%), shortness of breath (n=52; 85%), respiratory secretions (n=22; 36%), and recurrent respiratory tract infections (n=17; 28%).
Before undergoing surgery, 55 (90%) patients underwent a stent trial. The median stent duration was 5 days (IQR, 3–7 days), with only 4 (7%) patients having the stent for more than 7 days. Upon stent removal, granulation tissue was noted in 37 (67%) patients, 8 (15%) had secretions within the stent, 5 (9%) patients experienced stent migration or coughing of the stent, 2 (3%) showed signs of inflammation, and 4 (7%) had bleeding during the removal process.
In terms of surgical intervention, most patients underwent open TBP (n=33; 54%), while the remainder had a robotic approach (n=28; 46%). There were no significant differences in baseline characteristics between the two surgical groups.
ECAC severity score
In the overall group, there was a significant improvement in the median ECAC severity score from the initial values before surgery to three months after surgery [12 (IQR, 10–14) vs. 1 (IQR, 0–3), P<0.001] (see Figure S2). The changes in the ECAC severity score over time, categorized by the type of surgery, are shown in Figure 1.
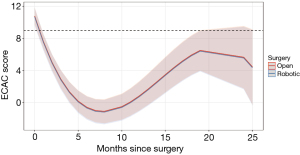
Patients who underwent open TBP initially had a mean score of 10.79, which decreased markedly at 3 months (2.94) and 12 months (0.83) after the surgery. However, by 24 months, the scores deteriorated to a predicted value of 5.66. Similarly, patients who underwent robotic TBP had an initial mean score of 10.69, which also showed notable decreases at 3 months (2.84) and 12 months (0.73), followed by a deterioration to a predicted score of 5.56 at 24 months.
Univariable linear mixed models showed no statistically significant differences between the types of surgery regarding changes in the ECAC severity score over time (P=0.89) (Table 2). However, older patients [0.08, 95% confidence interval (CI): 0.02–0.15, P=0.01], as well as those with hypertension (1.71, 95% CI: 0.44–2.96, P=0.009) and obstructive sleep apnea (1.44, 95% CI: 0.13–2.76, P=0.03), exhibited significantly worse scores over time compared to those without these characteristics at baseline. In a multivariable model (Table 3), the only statistically significant predictor of worsening ECAC severity scores over time was the presence of obstructive sleep apnea at diagnosis (1.33, 95% CI: 0.09–2.60, P=0.03).
Table 2
| Variable | Estimate | 95% CI | P value |
|---|---|---|---|
| Robotic vs. open TBP | −0.10 | −1.41 to 1.23 | 0.89 |
| Male vs. female | 1.07 | −0.32 to 2.45 | 0.13 |
| Age (years) | 0.08 | 0.02 to 0.15 | 0.01 |
| Body mass index (kg/m2) | −0.05 | −0.24 to 0.13 | 0.57 |
| Non-smoker vs. former | 0.45 | −0.91 to 1.77 | 0.51 |
| Hypertension vs. no | 1.71 | 0.44 to 2.96 | 0.009 |
| Chronic heart failure vs. no | −2.56 | −6.11 to 1.00 | 0.16 |
| Chronic kidney disease vs. no | 2.83 | −2.02 to 7.78 | 0.25 |
| Diabetes mellitus vs. no | 0.97 | −0.62 to 2.60 | 0.23 |
| Asthma vs. no | 1.05 | −0.29 to 2.34 | 0.11 |
| Chronic obstructive pulmonary disorder vs. no | 0.89 | −0.86 to 2.65 | 0.30 |
| Obstructive sleep apnea vs. no | 1.44 | 0.13 to 2.76 | 0.03 |
| Gastroesophageal reflux disease vs. no | 1.21 | −0.12 to 2.51 | 0.07 |
| Baseline SGRQ | 0.02 | −0.02 to 0.07 | 0.29 |
| Baseline CQLQ | 0.03 | −0.03 to 0.08 | 0.35 |
| Baseline FEV1% | −0.01 | −0.05 to 0.02 | 0.38 |
| Baseline FVC% | 0.00 | −0.04 to 0.03 | 0.89 |
| Baseline SMWT (m) | 0.00 | −0.01 to 0.01 | 0.89 |
CI, confidence interval; CQLQ, cough-specific quality of life questionnaire; ECAC, expiratory central airway collapse; FEV1%, forced expiratory volume in 1 second percentage of predicted; FVC%, forced vital capacity percentage of predicted; SGRQ, St. George’s respiratory questionnaire; SMWT, six-minute walk test; TBP, tracheobronchoplasty.
Table 3
| Variable | Estimate | 95% CI | P value |
|---|---|---|---|
| Age (years) | 0.07 | −0.01 to 0.13 | 0.06 |
| Hypertension vs. no | 0.91 | −0.42 to 2.23 | 0.18 |
| Obstructive sleep apnea vs. no | 1.33 | 0.09 to 2.60 | 0.03 |
| Gastroesophageal reflux disease vs. no | 0.83 | −0.42 to 2.07 | 0.18 |
CI, confidence interval; ECAC, expiratory central airway collapse.
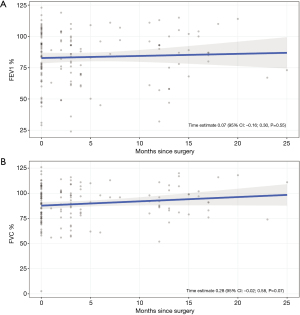
PFTs
Figure 2 shows the changes in predicted values for FEV1% and FVC% over time. While there was a slight numerical improvement in both measures, these differences were not statistically significant. For FEV1%, the scores remained stable across the time points: baseline (83.32%), 3 months (83.53%), 12 months (84.14%), and 24 months (84.95%). Similarly, FVC% scores also showed a slight improvement: baseline (88.28%), 3 months (89.12%), 12 months (91.64%), and 24 months (95%).
6MWTs
In the overall population, there was a significant improvement in median 6MWTs values in meters from before surgery to 6 months after [336.8 (IQR, 270.4–391.2) vs. 366.4 (IQR, 339.6–431.0), P=0.01] (see Figure S3). After adjusting for the fraction of inspired oxygen, predictive models indicated a greater improvement in 6MWTs for patients who underwent robotic surgery (12.31, 95% CI −20.82 to 45.83, P=0.46), although this was not statistically significant (Figure 3).

Patients who had open TBP exhibited initial 6MWT values of 327.89, with notable improvements observed at 3 months (375.17), 12 months (386.91), and 24 months (404.31) post-surgery. Similarly, patients undergoing robotic TBP had initial values of 340.20, showing marked improvements at 3 months (387.49), 12 months (399.22), and 24 months (416.52) after surgery.
SGRQ
Among 25 patients with baseline, stent, and post-surgery SGRQ scores, the mean baseline score was 67.0 (SD: 19.7), improving to 38.7 (SD: 20.9) with the stent and 39 (SD: 21.8) post-surgery. In the open approach (n=15), scores improved from 64.8 (SD: 18.3) to 41.4 (SD: 21.1) with the stent (P=0.007) and 42.6 (SD: 23.9) post-surgery (P=0.004), with no significant difference between stent and surgery (P=0.88). In the robotic approach (n=10), scores improved from 70.3 (SD: 22.2) to 34.6 (SD: 21.0) with the stent (P=0.001) and 33.6 (SD: 18.0) post-surgery (p<0.001), with no significant difference between stent and surgery (P=0.91).
When comparing patients with the open (n=19) and robotic (n=13) approach who had scores at baseline and after surgery, the robotic group showed a greater numerical improvement (68.9 to 35.9; delta: −33.0) than the open group (66.3 to 39.8, delta: −26.5), but this was not statistically significant (P=0.41).
CQLQ
Among 25 patients with baseline, stent, and post-surgery CQLQ scores, the mean baseline score was 71.8 (SD: 15.2), improving to 60.4 (SD: 17.7) with the stent and 51.5 (SD: 18.7) post-surgery. In the open approach (n=15), scores improved from 70.7 (SD: 12.3) to 64.3 (SD: 19.6) with the stent (P=0.10) and 52.1 (SD: 22.3) post-surgery (P=0.001), with no significant difference between stent and surgery (P=0.10). In the robotic approach (n=10), scores improved from 73.3 (SD: 19.1) to 54.6 (SD: 13.1) with the stent (P=0.007) and 50.7 (SD: 12.7) post-surgery (P=0.005), with no significant difference between stent and surgery (P=0.51).
When comparing patients with the open (n=18) and robotic (n=13) approach who had scores at baseline and after surgery, the open group showed a greater numerical improvement (72.2 to 48.9; delta: −23.2) than the robotic group (74.5 to 52.8, delta: −21.7), but this was not statistically significant (P=0.82).
Surgical outcomes
As shown in Table S1, median surgery time was slightly longer in the open approach (352 min) than in the robotic (325 min) (P=0.55). However, the open approach had significantly longer chest tube duration (4 vs. 1 day, P<0.001), days in the ICU (1 vs. 0 day, P<0.001), and hospital stay (6 vs. 2 days, P<0.001).
For the overall cohort, the most common complications were CD I (85%), primarily related to the increased need for pharmacologic pain management, as well as the need for vigilance for pneumothorax or subcutaneous emphysema. CD IIIb complications occurred in 20% of patients related to redo surgery or mesh erosion requiring bronchoscopy intervention, respectively. No CDIV complications or mortality occurred within 90 days (Table S2). CD IIIb complications were more frequent in the open approach (30% vs. 7%, P=0.02). Mesh erosion occurred in 3 open cases (at 5.18, 46.39, and 57.67 months) and 1 robotic case (11.87 months, P=0.76). Redo surgery due to symptom recurrence was required more often in the open group (6 cases at 0.17, 6.35, 6.84, 7.74, 13.90, 24.29 months) than in the robotic group (1 case at 44.19 months) (P=0.10).
Discussion
In this study, we conducted a long-term assessment of changes in bronchoscopic outcomes, PFTs, and exercise capacity in patients with ECAC who underwent TBP. Regardless of the surgical approach, patients showed an improvement in the ECAC severity score, with values remaining below severe levels over time. Additionally, there was a significant improvement in the quality-of-life indices and 6MWT distance, while PFTs remained stable over time.
To the best of our knowledge, this is the first report to explore objective changes in bronchoscopic outcomes over time after TBP. We noted a substantial improvement in baseline ECAC severity scores from 12 to 1 at three months post-surgery. However, scores at two years started to rise, albeit remaining below the severity threshold of nine. From a surgical perspective, several factors may explain the recurrence of symptoms during long-term follow-up, including the continued collapse of smaller airways or bronchioles, which are not amenable to surgical correction, and the presence of tracheal malacia beyond the cricoid in some patients—an area that cannot be addressed by this surgery.
Additionally, regarding comorbidities, we found that most of our patients had concomitant conditions, such as gastroesophageal reflux disease, hypertension, and asthma, which aligns with previous literature (7,12). It is of utmost importance to note that older patients and those with hypertension or obstructive sleep apnea may not respond as well to surgery as younger patients or those without these comorbidities. While we did not assess BMI changes over time, we suspect that an increase in BMI could contribute to symptom deterioration, and the natural progression of the disease may also influence long-term outcomes. Attributing the lack of long-term improvement to a single factor would not be ideal; rather, we believe that a combination of all these factors likely contributes to the deterioration in ECAC scores observed over time.
In our study, we found that patients undergoing robotic TBP had shorter ICU stays, reduced hospital stays, and fewer days with chest tubes than those undergoing the open approach. Interestingly, these results were more favorable than those in a recent study comparing open versus robotic TBP, where the operative time was 6.2 hours for the open approach and 8.4 hours for the robotic approach, compared to our study’s 5.8 hours for open and 5.4 hours for robotic (21). While the length of hospital stay was similar for the open approach in both studies, our robotic approach had a shorter average stay (2 days) compared to 5 days in the other study. We also observed shorter ICU stays for both approaches—1 day for open and 0 days for robotic—compared to the other study’s durations of 3 and 1 day, respectively.
Regarding surgical complications, in our cohort, we found a lower rate of major complications, such as mesh erosion and the need for reoperation, in the robotic approach compared to the open approach. However, when comparing data on major surgical complications (CD ≥ IIIa: 19% for robotic and 21% for open) from the abovementioned study, we observed a higher proportion in our cohort (21). It is important to note, though, that the follow-up period in the other study was only 90 days, which may not be long enough to detect long-term complications like reoperations or mesh erosion. Therefore, these rates are not directly comparable.
Several studies have evaluated changes in PFT after TBP, but with inconclusive results (11,12,22-24). In one of the largest robotic-TBP series, the authors noted a significant improvement in both predicted FEV1 and FVC values at 4 months after surgery (12). However, after including more patients and conducting longer follow-up, they found a statistically significant but not clinically important difference in FEV1% at 16 months post-surgery (77% vs. 83%) (22). In line with other studies, we observed a numerical improvement in predicted FEV1 and FVC values, but this was not statistically or clinically important (11,23). It is paramount to remember that PFTs are not typically used to diagnose ECAC, and patients often present without substantial airflow limitation or with normal PFT values (1,13). Thus, it is recommended that patients are not followed up based solely on these tests.
Unlike PFTs, most studies have reported improvements in exercise capacity after surgery (7,11,23). Our study found a marked increase in the distance of 6MWTs at 2 years of follow-up, with a mean improvement of 76 m for both open and robotic TBP approaches. This value is higher than the established threshold of 35 m for clinically important improvement (25). This simple assessment of functional status is a feasible test to evaluate improvements in exercise capacity and indirectly assess improvements in symptomatology (13).
Regarding quality-of-life indices, both the open and robotic approaches demonstrated significant improvements in the SGRQ, with improvements of 26.5 and 33.0 points, respectively, from baseline to post-surgery scores. These improvements exceed the clinically meaningful threshold of 12 points, indicating very effective treatment (26). Additionally, both approaches showed improvements in the CQLQ, with the open approach improving by 23.2 points and the robotic approach by 21.7 points—both surpassing the minimally important difference of 13 points (27). While there are risks such as migration, accumulation of secretions, and bleeding, the improvement in quality of life observed after the stent trial was comparable to that seen after surgery. This suggests that clinicians could use this procedure to assess potential benefits for patients considering TBP.
Future studies should focus on long-term outcomes for patients with ECAC to assess how the disease evolves over time. Additionally, exploring factors such as changes in BMI or other interventions patients undergo after surgery could help identify their role in the progression of symptoms. It would also be valuable to investigate characteristics of patients who required reintervention. Furthermore, a prospective study that periodically assesses patients after surgery using clinically relevant metrics and compares dynamic bronchoscopy with dynamic computed tomography scans should be prioritized.
Our study has some limitations to consider when interpreting the results. First, the retrospective design makes it prone to selection bias and limits our ability to account for unmeasured variables, such as treatment for other comorbidities, which may have influenced some of our outcomes. Additionally, there was a high proportion of missing data for all variables of interest, particularly at later time points, which could have affected our estimates (see Figure S4, which shows the dynamic bronchoscopies performed during follow-up). Additionally, the single-center nature of our study may limit the generalizability of our findings. Moreover, although there were no significant differences in baseline characteristics between groups, a prospective study with a larger population would be advisable to confirm our results.
Conclusions
In conclusion, both open and robotic TBP are viable treatment options for patients with ECAC. TBP improves anatomic obstruction in the central airways, leading to symptom improvement in the short term and potentially offering long-term benefits for some patients. Robotic TBP offers equivalent surgical outcomes but lower complication rates compared to open TBP. Moreover, we believe a multidisciplinary team of primary care providers, interventional pulmonologists, and thoracic surgeons should evaluate patients with symptoms suggestive of ECAC to promptly identify those with severe disease and facilitate timely referrals for appropriate surgical management.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2139/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2139/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2139/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2139/coif). S.F.B. serves as an unpaid editorial board member of Journal of Thoracic Disease from September 2024 to August 2026. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Institutional Review Board of Mayo Clinic Florida (#21-003543) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abia-Trujillo D, Majid A, Johnson MM, et al. Central Airway Collapse, an Underappreciated Cause of Respiratory Morbidity. Mayo Clin Proc 2020;95:2747-54. [Crossref] [PubMed]
- Carden KA, Boiselle PM, Waltz DA, et al. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest 2005;127:984-1005. [Crossref] [PubMed]
- Kheir F, Fernandez-Bussy S, Gangadharan SP, et al. Excessive Dynamic Airway Collapse or Tracheobronchomalacia: Does It Matter? Arch Bronconeumol (Engl Ed) 2019;55:69-70. [Crossref] [PubMed]
- Kheir F, Majid A. Tracheobronchomalacia and Excessive Dynamic Airway Collapse: Medical and Surgical Treatment. Semin Respir Crit Care Med 2018;39:667-73. [Crossref] [PubMed]
- Funes-Ferrada R, Yu Lee-Mateus A, Barrios-Ruiz A, et al. Expiratory Central Airway Collapse and Pneumatic Stenting With Continuous Positive Pressure Titration: A Technique Description. Mayo Clin Proc 2024;99:1913-20. [Crossref] [PubMed]
- Abia-Trujillo D, Yu Lee-Mateus A, Hernandez-Rojas D, et al. Excessive Dynamic Airway Collapse Severity Scoring System: A Call Out for an Overall Severity Determination. J Bronchology Interv Pulmonol 2023;30:200-6. [Crossref] [PubMed]
- Buitrago DH, Majid A, Alape DE, et al. Single-Center Experience of Tracheobronchoplasty for Tracheobronchomalacia: Perioperative Outcomes. Ann Thorac Surg 2018;106:909-15. [Crossref] [PubMed]
- Lazzaro RS, Bahroloomi D, Wasserman GA, et al. Robotic Tracheobronchoplasty: Technique. Oper Tech Thorac Cardiovasc Surg 2022;27:218-26.
- Seastedt KP, Wilson JL, Gangadharan SP. Robotic Surgery for Tracheobronchomalacia. Thorac Surg Clin 2023;33:61-9. [Crossref] [PubMed]
- Bakhos CT, Magarinos J, Bent D, et al. Tracheobronchoplasty for tracheobronchomalacia. J Vis Surg 2022;8:15. [Crossref] [PubMed]
- Majid A, Guerrero J, Gangadharan S, et al. Tracheobronchoplasty for severe tracheobronchomalacia: a prospective outcome analysis. Chest 2008;134:801-7. [Crossref] [PubMed]
- Lazzaro R, Patton B, Lee P, et al. First series of minimally invasive, robot-assisted tracheobronchoplasty with mesh for severe tracheobronchomalacia. J Thorac Cardiovasc Surg 2019;157:791-800. [Crossref] [PubMed]
- McGinn J, Herbert B, Maloney A, et al. Quality of life outcomes in tracheobronchomalacia surgery. J Thorac Dis 2020;12:6925-30. [Crossref] [PubMed]
- Castillo-Larios R, Yu Lee-Mateus A, Hernandez-Rojas D, et al. Clinical Outcomes After Tracheobronchoplasty With Ringed Polytetrafluoroethylene Vascular Graft. Ann Thorac Surg Short Rep 2023;1:553-7. [Crossref] [PubMed]
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med 1991;85 Suppl B:25-31; discussion 33-7.
- French CT, Irwin RS, Fletcher KE, et al. Evaluation of a cough-specific quality-of-life questionnaire. Chest 2002;121:1123-31. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J 2005;26:153-61. [Crossref] [PubMed]
- ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [Crossref] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing 2024. Available online: https://www.R-project.org/
- Cho JM, Carpenter SL, Mathew F, et al. The first comparative analysis of open and robotic tracheobronchoplasty for excessive Central airway collapse. Eur J Cardiothorac Surg 2025;67:ezaf026. [Crossref] [PubMed]
- Inra ML, Wasserman GA, Karp J, et al. Improvement in postoperative lung function in patients with moderate to severe airway obstruction after robotic-assisted thoracoscopic tracheobronchoplasty. J Thorac Cardiovasc Surg 2023;165:876-85. [Crossref] [PubMed]
- Gangadharan SP, Bakhos CT, Majid A, et al. Technical aspects and outcomes of tracheobronchoplasty for severe tracheobronchomalacia. Ann Thorac Surg 2011;91:1574-80; discussion 1580-1. [Crossref] [PubMed]
- Wright CD, Grillo HC, Hammoud ZT, et al. Tracheoplasty for expiratory collapse of central airways. Ann Thorac Surg 2005;80:259-66. [Crossref] [PubMed]
- Puhan MA, Mador MJ, Held U, et al. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J 2008;32:637-43. [Crossref] [PubMed]
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J 2002;19:398-404. [Crossref] [PubMed]
- Fletcher KE, French CT, Irwin RS, et al. A prospective global measure, the Punum Ladder, provides more valid assessments of quality of life than a retrospective transition measure. J Clin Epidemiol 2010;63:1123-31. [Crossref] [PubMed]


