
Efficacy evaluation of tyrosine kinase inhibitors for advanced non-small cell lung cancer patients with leptomeningeal metastasis
Highlight box
Key findings
• Targeted agents represent a promising therapeutic option for non-small cell lung cancer (NSCLC) patients with leptomeningeal metastases (LM), particularly in those receiving concurrent radiotherapy or chemotherapy for LM lesions.
What is known and what is new?
• Targeted therapy has significant efficacy in driver gene mutated NSCLC. However, due to the rarity of LM, and the barrier role of the blood brain barrier, targeted therapy for treating patients with non-small cell LM lung cancer has not been explored in detail.
• This is the largest sample size of study focusing on targeted therapy combined with local leptomeningeal therapy in NSCLC patients.
What is the implication, and what should change now?
• Targeted therapy showed promising outcomes in the treatment of advanced NSCLC patients with LM, especially in those with concurrent leptomeningeal lesions undergoing radiotherapy or chemotherapy but this needs to be supported by more prospective studies.
Introduction
Leptomeningeal metastasis (LM) occurs when cancer cells spread to the subarachnoid space, representing a serious complication in patients with advanced non-small cell lung cancer (NSCLC). Among the various NSCLC subtypes, adenocarcinoma is most commonly associated with LM (1-3). NSCLC patients with LM generally have a poor prognosis, with median overall survival (OS) ranging from 3.6 to 12 months (1-4), and if left untreated, the median survival can be less than 3 months (5). The clinical manifestations of LM are varied, including headache, nausea, vomiting, seizures, and double vision (6), all of which significantly impair the quality of life and prognosis for these patients.
Treatment options for NSCLC patients with LM depend on factors such as the presence of driver mutations, patient tolerance, and the extent of disease progression. Current treatment strategies include systemic chemotherapy, intrathecal chemotherapy, and whole-brain radiotherapy, though no standard treatment guidelines or consensus have been established for managing NSCLC patients with LM.
In recent years, the rapid advancement of targeted therapies has significantly transformed the treatment landscape of lung cancer, particularly for NSCLC patients harboring driver mutations. The use of tyrosine kinase inhibitors (TKIs) in driver gene-positive NSCLC patients has shown promising anti-tumor activity, notably prolonging progression-free survival (PFS) and OS. As a result, targeted therapy has become a cornerstone of treatment for many lung cancer patients with specific driver gene alterations.
Based on these observations, we hypothesize that molecular targeted therapy could provide therapeutic benefits for NSCLC patients with LM. However, studies evaluating the efficacy of targeted therapies in this patient population are limited, often characterized by small sample sizes and retrospective designs, leading to significant gaps in understanding and inconsistent outcomes. Therefore, further investigation is essential. This study aims to comprehensively evaluate the efficacy and safety of targeted therapy in NSCLC patients with LM, with the goal of providing valuable data to inform treatment strategies for this challenging patient population. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2047/rc).
Methods
Study design and patient inclusion criteria
In this study, 193 NSCLC patients with LM who received targeted therapy at Zhejiang Cancer Hospital between November 2011 and October 2024 were included. Of these, 60 patients received concurrent meningeal-localized therapy, including radiotherapy and chemotherapy, and were classified as the synchronous group. The remaining 133 patients, who did not receive meningeal-targeted therapy, were classified as the asynchronous group. All NSCLC patients were diagnosed with LM by cerebrospinal fluid cytology. This study adhered to the principles outlined in the Declaration of Helsinki and its subsequent amendments and obtained approval to waive individual consent for this retrospective analysis. The study protocol was approved by the Zhejiang Cancer Hospital Ethics Committee (approval number: IRB-2023-377).
Treatment and response assessments
Patient data were collected through a thorough review of medical records and follow-up information. Lesion progression was monitored by computed tomography (CT). Clinical response was evaluated based on RECIST 1.1 criteria, and treatment efficacy was assessed with periodic CT scans, typically every two cycles, or more often if significant disease progression was observed. Responses were categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The objective response rate (ORR) was calculated by adding CR and PR, while the disease control rate (DCR) included CR, PR, and SD. PFS was defined as the time from the start of targeted therapy to the diagnosis of PD. OS was defined as the time from the diagnosis of LM to death or the last follow-up.
Statistical analysis
The last follow-up date was on November 30, 2024, with a complete follow-up rate of 100%. All patients underwent evaluations for PFS and OS. Kaplan-Meier survival analysis was employed to assess patient survival, and the log-rank test was used to compare survival among different prognostic factors. Univariate and multivariable analyses were conducted using Cox regression models. Statistical significance for all tests was set at a two-sided P value of <0.05. The statistical analyses were carried out using SPSS 26.0 (IBM Corporation, Armonk, New York, USA) and GraphPad Prism 10.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Patient characteristics
We conducted a study including 193 NSCLC patients with LM. The cohort consisted of 79 males (40.9%) and 114 females (59.1%). Most patients were under 65 years of age (160 patients, 82.9%), and 47 (24.4%) had a history of smoking. PS scores ranged from 0 to 3, with 154 patients (79.8%) scoring 0 or 1. The most predominant subtype was adenocarcinoma, accounting for 187 cases (96.9%), followed by 4 adenosquamous carcinoma (2.1%), 1 adenocarcinoma with large cell carcinoma (0.5%), and 1 undifferentiated carcinoma (0.5%). The most common driver gene type was EGFR mutation (177, 91.7%), followed by ALK mutation (6, 3.1%), and 10 patients (5.2%) were tested for the remaining rare gene or negative. Thirty-nine patients (20.2%) received first-line treatment, while 154 (79.8%) received posterior-line therapy. Sixty (31.1%) patients received concurrent meningeal therapy with targeted therapy, and 133 (68.9%) did not. Fifty-five (28.5%) patients received antiangiogenic inhibitors in combination with targeted therapy, while 138 (71.5%) did not. A comprehensive overview of the patient characteristics is provided in Table 1. In addition, we also analyzed the patients with combined angiogenesis inhibitors, which is summarized in Table 2.
Table 1
| Characteristics | Non-concurrent therapy (n=133) | Concurrent therapy (n=60) | P value | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Sex | ||||||
| Male | 60 | 45.1 | 19 | 31.7 | ||
| Female | 73 | 54.9 | 41 | 68.3 | 0.08 | |
| Age of leptomeningeal metastasis | ||||||
| ≤65 years | 111 | 83.5 | 49 | 81.7 | ||
| >65 years | 22 | 16.5 | 11 | 18.3 | 0.84 | |
| Smoking history | ||||||
| Never | 97 | 72.9 | 49 | 81.7 | ||
| Former | 36 | 27.1 | 11 | 18.3 | 0.21 | |
| Histology | ||||||
| Adenocarcinoma | 130 | 97.7 | 57 | 95.0 | ||
| Others | 3 | 2.3 | 3 | 5.0 | 0.94 | |
| ECOG PS | ||||||
| ≤1 | 103 | 77.4 | 51 | 85.0 | ||
| ≥2 | 30 | 22.6 | 9 | 15.0 | 0.25 | |
| Driver gene | ||||||
| EGFR | 122 | 91.7 | 55 | 91.7 | ||
| ALK | 2 | 1.5 | 4 | 6.7 | ||
| ROS1 | 2 | 1.5 | 0 | 0.0 | ||
| Others | 7 | 5.3 | 1 | 1.7 | 0.13 | |
| Line of therapy | ||||||
| 1 | 23 | 17.3 | 16 | 26.7 | ||
| ≥2 | 110 | 82.7 | 44 | 73.3 | 0.18 | |
| Combined with angiogenesis inhibitor | ||||||
| Yes | 42 | 31.6 | 13 | 21.7 | ||
| No | 91 | 68.4 | 47 | 78.3 | 0.17 | |
| Other metastasis | ||||||
| Yes | 112 | 84.2 | 56 | 93.3 | ||
| No | 21 | 15.8 | 4 | 6.7 | 0.11 | |
ECOG PS, Eastern Cooperative Oncology Group performance status.
Table 2
| Characteristics | Not combined with angiogenesis inhibitor (n=138) | Combined with angiogenesis inhibitor (n=55) | P value | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Sex | ||||||
| Male | 59 | 42.8 | 20 | 36.4 | ||
| Female | 79 | 57.2 | 35 | 63.6 | 0.52 | |
| Age of leptomeningeal metastasis | ||||||
| ≤65 years | 112 | 81.2 | 48 | 87.3 | ||
| >65 years | 26 | 18.8 | 7 | 12.7 | 0.40 | |
| Smoking history | ||||||
| Never | 104 | 75.4 | 42 | 76.4 | ||
| Former | 34 | 24.6 | 13 | 23.6 | >0.99 | |
| Histology | ||||||
| Adenocarcinoma | 134 | 97.1 | 53 | 96.4 | ||
| Others | 4 | 2.9 | 2 | 3.6 | >0.99 | |
| Driver gene | ||||||
| EGFR | 127 | 92.0 | 50 | 90.9 | ||
| ALK | 3 | 2.2 | 3 | 5.5 | ||
| ROS1 | 1 | 0.7 | 1 | 1.8 | ||
| Others | 7 | 5.1 | 1 | 1.8 | 0.42 | |
| ECOG PS | ||||||
| ≤1 | 111 | 80.4 | 43 | 78.2 | ||
| ≥2 | 27 | 19.6 | 12 | 21.8 | 0.70 | |
| Line of therapy | ||||||
| 1 | 33 | 23.9 | 6 | 10.9 | ||
| ≥2 | 105 | 76.1 | 49 | 89.1 | 0.048 | |
| Local leptomeningeal therapy | ||||||
| Yes | 47 | 34.1 | 13 | 23.6 | ||
| No | 91 | 65.9 | 42 | 76.4 | 0.17 | |
| Other metastasis | ||||||
| Yes | 121 | 87.7 | 47 | 85.5 | ||
| No | 17 | 12.3 | 8 | 14.5 | 0.64 | |
ECOG PS, Eastern Cooperative Oncology Group performance status.
Treatment response and survival analysis
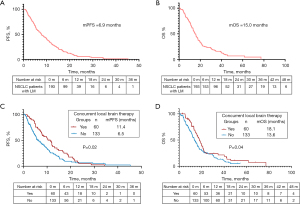
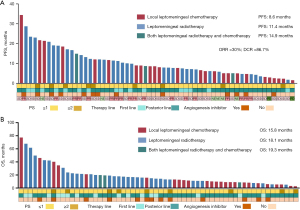
In the entire cohort, the ORR and DCR for targeted therapy were 10.9% and 66.3%, respectively. No patients achieved CR, 31 achieved PR, 107 achieved SD, 19 experienced PD, and 36 were not evaluated. The ORR and DCR in the concurrent group were significantly higher than in the non-concurrent group (ORR: 30.0% vs. 9.8%, P=0.001; DCR: 86.7% vs. 64.7%, P=0.003). Further details are provided in Table 3.
Table 3
| Response | Non-concurrent therapy (n=133) | Concurrent therapy (n=60) | P value |
|---|---|---|---|
| Best response, n (%) | <0.001 | ||
| Partial response | 13 (9.8) | 18 (30.0) | |
| Stable disease | 73 (54.9) | 34 (56.7) | |
| Progressive disease | 17 (12.8) | 2 (3.3) | |
| Not evaluable | 30 (22.6) | 6 (10.0) | |
| Response rate (%) | 9.8 | 30.0 | 0.001 |
| Disease control rate (%) | 64.7 | 86.7 | 0.003 |
| PFS | |||
| Median (months) | 6.5 | 11.4 | 0.02 |
| 95% CI (months) | 4.808–8.259 | 9.362–13.505 | |
| 6-month PFS (%) | 42.1 | 71.7 | <0.001 |
| 12-month PFS (%) | 15.8 | 30.0 | 0.03 |
| OS | |||
| Median (months) | 13.6 | 18.1 | 0.04 |
| 95% CI (months) | 11.221–15.979 | 14.985–21.148 | |
| 12-month OS (%) | 45.9 | 60.0 | 0.09 |
| 24-month OS (%) | 15.8 | 16.7 | 0.88 |
CI, confidence interval; OS, overall survival; PFS, progression-free survival.
In the entire cohort treated with targeted therapy, the mPFS was 6.9 months, and the mOS was 15.0 months. Significant differences in mPFS and mOS were observed between the concurrent and non-concurrent groups (mPFS: 11.4 vs. 6.5 months, P=0.02; mOS: 18.1 vs. 13.6 months, P=0.04).
In the 60 patients who received local meningeal therapy, 25 had received leptomeningeal local chemotherapy (chemo-group), 32 received local meningeal radiotherapy (radio-group), three patients received local meningeal therapy both radiotherapy plus chemotherapy (chemoradio-group). There is no significant difference in the efficacy among the three groups, The PFS between the chemotherapy, radiotherapy, and combination groups was 8.6 vs. 11.4 vs. 14.9 months, P>0.99. OS between the three groups were 15.8 vs. 18.1 vs. 19.3 months, P=0.77. Further information is available in Figures 1,2.
In the subgroup analysis, we divided patients into two groups according to whether they combined with angiogenesis inhibitor. The PFS of the combined group was statistically different from that of the non-combined group (11.4 vs. 6.9 months, P=0.009), but there was no significant difference in OS between the two groups (15.0 vs. 15.5 months, P=0.74). And more details were shown in Figure S1.
In addition, we further explored the effect of patients with EGFR driver gene mutation regarding three-generation TKI drug therapy. One hundred and seventy-seven patients with EGFR gene mutation had a PFS of 7.8 months and an OS of 15.0 months (PFS: first vs. second vs. third: 11.4 vs. 7.4 vs. 7.8 months, OS: first vs. second vs. third: 19.9 vs. 21.9 vs. 14.1 months). Further information is available in Figure S2.
Multivariate analyses of the clinical features and prognoses
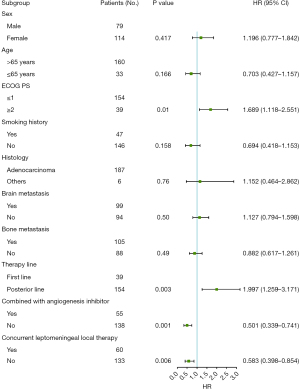
Through multivariate analysis of mPFS, we evaluated the following criteria: sex, age, Eastern Cooperative Oncology Group performance status (ECOG PS), smoking history, histology, brain metastases, bone metastases, lines of therapy, concurrent local leptomeningeal therapy and angiogenesis inhibitor therapy. Among them, ECOG PS score [P=0.01, 95% confidence interval (CI): 1.118–2.551], therapy lines (P=0.003, 95% CI: 1.259–3.171), combination with angiogenesis inhibitors (P=0.001, 95% CI: 0.339–0.741), and receipt of local leptomeningeal therapy (P=0.006, 95% CI: 0.398–0.854) as independent factors influencing PFS. The details are shown in Figure 3.
In addition, we also performed a further multivariate analysis of 177 patients with EGFR mutations, as shown in Figure S3.
Discussion
Our study systematically evaluated the efficacy of targeted therapy in NSCLC patients with LM, and represents the largest cohort to date examining the impact of targeted therapy in combination with local chemotherapy, radiotherapy, and angiogenesis inhibitors. The concurrent administration of local radiotherapy and chemotherapy significantly prolonged both PFS and OS in patients with LM, with PFS reaching 11.4 months and OS extending to 18.1 months. Regarding treatment regimens, the combination of targeted therapy and anti-angiogenic agents also demonstrated notable clinical benefit, with a PFS of 11.4 months.
The treatment of LM in lung cancer remains a complex challenge, as the poor permeability of the blood-brain barrier to traditional chemotherapeutic drugs often renders conventional cytotoxic chemotherapy ineffective in NSCLC patients with LM (7), and the disease progresses rapidly, significantly impacting the patient’s quality of life. In recent years, targeted therapies for NSCLC patients have demonstrated promising therapeutic outcomes, and it also had good therapeutic effects for leptomeningeal lesions that were difficult to achieve with traditional chemotherapy (8). However, there have been limited studies on the efficacy of targeted therapy in patients with LM, and there is also a large discrepancy between existing studies (9-20). Despite these disparities, a consistent finding is that TKIs exhibit favorable efficacies in patients with LM, with some studies reporting survival periods of up to 18.0 months (14).
Local treatment of leptomeningeal lesions, including intrathecal chemotherapy and radiotherapy, is an important therapeutic modality. NSCLC patients with LM often present with significant symptoms such as headache and vomiting, and typically have a worse baseline performance status, which makes them less tolerant to anti-tumor therapies compared to other lung cancer patients. Our study also highlighted that the ECOG PS score was an independent factor influencing PFS (P=0.01, 95% CI: 1.118–2.551). This suggests that improving the physical status of patients with LM could potentially enhance survival outcomes. One direct approach for treating leptomeningeal lesions is intrathecal chemotherapy, which delivers an optimal drug concentration directly to the cerebrospinal fluid. Morris et al. (21) reported that seven patients who received intrathecal chemotherapy survived for 18 months, significantly longer than the 83 patients who did not receive this treatment (P=0.001). Radiotherapy, particularly whole-brain radiation therapy (WBRT), is another modality used to manage meningeal lesions, though its role in prolonging survival remains debated. Liao et al. showed that WBRT improved OS (WBRT vs. non-WBRT: 10.9 vs. 2.4 months, P=0.002), and WBRT was identified as an independent predictor of prolonged survival (2). Similarly, our study demonstrated significantly longer PFS and OS in patients receiving concurrent treatment, including intrathecal chemotherapy and WBRT (mPFS: 11.4 vs. 6.5 months, P=0.02; mOS: 18.1 vs. 13.0 months, P=0.04). These findings provide further real-world evidence supporting the use of targeted therapy in patients with leptomeningeal lesions.
In addition, vascular endothelial growth factor (VEGF) plays a key role in angiogenesis and is an important therapeutic target in NSCLC patients. Elevated levels of VEGF in the cerebrospinal fluid are associated with poor prognosis (22). It is believed that angiogenesis inhibitors exert their effects on LM by reducing the distribution of interstitial and cerebrospinal fluid, thereby alleviating cerebral edema and enhancing drug penetration into the cerebrospinal fluid. A study by Reijneveld et al. (23) reported that mice treated with angiostatin showed prolonged survival, with nearly one-fifth achieving long-term survival. Additionally, preclinical studies have demonstrated synergistic antitumor effects when EGFR inhibitors are combined with VEGF/VEGFR pathway blockade (24,25). This combination may provide a dual therapeutic benefit for patients with LM. In a retrospective analysis of 27 EGFR-mutant lung cancer patients with LM, those treated with osimertinib with or without bevacizumab showed that the group receiving the angiogenesis inhibitor had a significantly longer mPFS compared to the group without (10.6 vs. 5.5 months, P=0.04). Furthermore, the OS was also prolonged in the angiogenesis inhibitor-treated group (18.0 vs. 13.7 months, P=0.046). The study also found that angiogenesis inhibitor treatment significantly increased the concentration of osimertinib in mouse brain tissue (26). Similarly, our study found comparable results, showing that the combination of targeted therapy with an angiogenesis inhibitor significantly improved PFS compared to the group not receiving the inhibitor (11.4 vs. 6.9 months, P=0.009). Thus, combining targeted therapy with angiogenesis inhibitors may provide long-term benefits to patients during the treatment process. Nevertheless, additional prospective studies are required to substantiate this conclusion.
Our study had certain limitations. First, the lack of a standardized treatment plan and consensus on targeting meningeal metastasis, along with variations in clinicians’ habits and preferences, may have introduced bias to the study. Second, being a retrospective study, there is a potential for loss-to-follow-up during data collection. In addition, due to the large time span of medical record collection, some patients have not yet adopted the standard Response Assessment in Neuro-Oncology for Leptomeningeal Metastasis (RANO-LM) criteria. In order to ensure the unification of evaluation criteria, RECIST 1.1 was used in this study.
Conclusions
Overall, targeted therapy has shown promising efficacy in NSCLC patients with LM, particularly in those with concurrent leptomeningeal lesions undergoing radiotherapy or chemotherapy. Additionally, the combination of angiogenesis inhibitors and targeted therapy has been demonstrated to effectively prolong PFS in these patients. We hope that the findings of this study will provide valuable insights for optimizing treatment strategies for this patient population.
Acknowledgments
The authors would like to appreciate all patients and their families for their cooperation and participation. Additionally, we are thankful to all research staff and co-investigators involved in this research.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2047/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2047/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2047/prf
Funding: The study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2047/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Approval of the study protocol was obtained from Zhejiang Cancer Hospital Institutional Review Board (approval number: IRB-2023-377). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal Metastases in Patients with NSCLC with EGFR Mutations. J Thorac Oncol 2016;11:1962-9. [Crossref] [PubMed]
- Liao BC, Lee JH, Lin CC, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for Non-Small-Cell Lung Cancer Patients with Leptomeningeal Carcinomatosis. J Thorac Oncol 2015;10:1754-61. [Crossref] [PubMed]
- Umemura S, Tsubouchi K, Yoshioka H, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer 2012;77:134-9. [Crossref] [PubMed]
- Riess JW, Nagpal S, Iv M, et al. Prolonged survival of patients with non-small-cell lung cancer with leptomeningeal carcinomatosis in the modern treatment era. Clin Lung Cancer 2014;15:202-6. [Crossref] [PubMed]
- Park JH, Kim YJ, Lee JO, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer 2012;76:387-92. [Crossref] [PubMed]
- Le Rhun E, Weller M, Brandsma D, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol 2017;28:iv84-99. [Crossref] [PubMed]
- Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev 2017;53:128-37. [Crossref] [PubMed]
- Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol 2018;19:e43-55. [Crossref] [PubMed]
- Yang JCH, Kim SW, Kim DW, et al. Osimertinib in Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J Clin Oncol 2020;38:538-47. [Crossref] [PubMed]
- Wu YL, Zhao Q, Deng L, et al. Leptomeningeal metastasis after effective first-generation EGFR TKI treatment of advanced non-small cell lung cancer. Lung Cancer 2019;127:1-5. [Crossref] [PubMed]
- Nanjo S, Hata A, Okuda C, et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer 2018;118:32-7. [Crossref] [PubMed]
- Kuiper JL, Hendriks LE, van der Wekken AJ, et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: A retrospective cohort analysis. Lung Cancer 2015;89:255-61. [Crossref] [PubMed]
- Jackman DM, Cioffredi LA, Jacobs L, et al. A phase I trial of high dose gefitinib for patients with leptomeningeal metastases from non-small cell lung cancer. Oncotarget 2015;6:4527-36. [Crossref] [PubMed]
- Saboundji K, Auliac JB, Pérol M, et al. Efficacy of Osimertinib in EGFR-Mutated Non-Small Cell Lung Cancer with Leptomeningeal Metastases Pretreated with EGFR-Tyrosine Kinase Inhibitors. Target Oncol 2018;13:501-7. [Crossref] [PubMed]
- Xu Z, Hao X, Wang Q, et al. Intracranial efficacy and safety of furmonertinib 160 mg with or without anti-angiogenic agent in advanced NSCLC patients with BM/LM as salvage therapy. BMC Cancer 2023;23:206. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal Metastases in Patients with NSCLC with EGFR Mutations. J Thorac Oncol 2016;11:1962-9. [Crossref] [PubMed]
- Nosaki K, Yamanaka T, Hamada A, et al. Erlotinib for Non-Small Cell Lung Cancer with Leptomeningeal Metastases: A Phase II Study (LOGIK1101). Oncologist 2020;25:e1869-78. [Crossref] [PubMed]
- Ahn MJ, Chiu CH, Cheng Y, et al. Osimertinib for Patients With Leptomeningeal Metastases Associated With EGFR T790M-Positive Advanced NSCLC: The AURA Leptomeningeal Metastases Analysis. J Thorac Oncol 2020;15:637-48. [Crossref] [PubMed]
- Park S, Lee MH, Seong M, et al. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol 2020;31:1397-404. [Crossref] [PubMed]
- Lu ZQ, Cai J, Wang X, et al. Osimertinib combined with bevacizumab for leptomeningeal metastasis from EGFR-mutation non-small cell lung cancer: A phase II single-arm prospective clinical trial. Thorac Cancer 2021;12:172-80. [Crossref] [PubMed]
- Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol 2012;7:382-5. [Crossref] [PubMed]
- Herrlinger U, Wiendl H, Renninger M, et al. Vascular endothelial growth factor (VEGF) in leptomeningeal metastasis: diagnostic and prognostic value. Br J Cancer 2004;91:219-24. [Crossref] [PubMed]
- Reijneveld JC, Taphoorn MJ, Kerckhaert OA, et al. Angiostatin prolongs the survival of mice with leptomeningeal metastases. Eur J Clin Invest 2003;33:76-81. [Crossref] [PubMed]
- Byers LA, Heymach JV. Dual targeting of the vascular endothelial growth factor and epidermal growth factor receptor pathways: rationale and clinical applications for non-small-cell lung cancer. Clin Lung Cancer 2007;8:S79-85. [Crossref] [PubMed]
- Swinson DE, O’Byrne KJ. Interactions between hypoxia and epidermal growth factor receptor in non-small-cell lung cancer. Clin Lung Cancer 2006;7:250-6. [Crossref] [PubMed]
- Yi Y, Cai J, Xu P, et al. Potential benefit of osismertinib plus bevacizumab in leptomeningeal metastasis with EGFR mutant non-small-cell lung cancer. J Transl Med 2022;20:122. Erratum in: J Transl Med 2022;20:292. [Crossref] [PubMed]


