
Pulmonary ossification: a review
Introduction
Pulmonary ossification (PO) is a very rare respiratory disease that was first described by Luschka. Manifestations include pulmonary calcification and ossification (1,2). This condition is characterized by formation of ectopic bone tissue with or without marrow elements in the lung parenchyma.
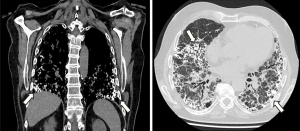
In the past, PO used to be an incidental finding during the autopsy of patients with other respiratory diseases (3,4). However, PO is increasingly found on high-resolution computed tomography (HRCT) scans of the chest. This entity is prevailingly observed in patients with fibrotic lung disease, more specifically in idiopathic pulmonary fibrosis (5,6) (Figure 1).
Clinical characteristics
Nishioka et al. recently carried out a national survey study in Japan to assess the clinical, functional, radiological and histopathological characteristics of patients diagnosed with idiopathic dendriform pulmonary ossification (DPO). Follow-up was performed of 22 cases over a period of 20 years. Most patients were male (82%) with an age at diagnosis of 22 to 56 years [mean 37.9, standard deviation (SD) 9.1]. Around 80% were asymptomatic and disease was discovered during an elective medical visit (7). In a review of 43 patients, the male to female ratio was 6:1 (8). Early symptoms include spontaneous pneumothorax of unclear etiology, although occupation of the visceral pleura by the PO has been suggested as a causative factor (9). Notwithstanding that most patients are asymptomatic at diagnosis, 36% presented with a reduced forced vital capacity (FVC). In addition, over 50% of cases demonstrated a lung function decline as assessed by a reduction in FVC and diffusing capacity for carbon monoxide during follow-up (7,10). Table 1 summarizes the most common clinical features of the disease.
Table 1
| Characteristics | Values |
|---|---|
| Males | 82% |
| Smoking status | |
| Never smokers | 80% |
| Previous lung disease | |
| Pneumothorax | 5% |
| Asthma | 5% |
| Family history | 9% |
| Age at diagnosis (years old), mean | 38 |
| Diagnosis | |
| Medical check-up | 77% |
| Symptomatic case | 18% |
| Incidental in HRCT | 5% |
| KL-6 >500 U/mL | 17% |
| Lung function at diagnosis | |
| FEV1/FVC <70% | 9% |
| FVC <80% | 36% |
| DLCO <80% | 63% |
| Lung function decline | |
| FVC ≥100 mL/year | 24% |
| FVC ≥5%/year | 12% |
| FEV1 ≥100 mL/year | 24% |
| DLCO ≥15%/year | 0% |
| Worsening of symptoms | 14% |
| Follow-up HRCT | |
| Stable | 12% |
| Progression | 88% |
| Overall survival | >90% |
DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; HRCT, high-resolution computed tomography; KL-6, Krebs von den Lungen-6.
Incidence and prevalence
According to the case series available, the incidence of PO ranges from 0.16% to 0.4%. PO is very rarely idiopathic, being most frequently secondary or associated with other diseases. In a series of 1,393 adult autopsies, DPO was identified in 8 cases. Prevalence was 0.5% and incidence was 0.28 cases/year (3). In another series of 10,426 autopsies, a total of 17 cases of DPO were confirmed, thereby accounting for an incidence of 1.63 cases/1,000 autopsies (4). Cases have also been reported within the same family. Such is the case of a 29-year-old male patient and his father, who both developed spontaneous pneumothorax (11). In patients with usual interstitial pneumonia (UIP), the incidence of PO may reach 6.7% following an HRCT or an open pulmonary biopsy (12).
Patterns of PO
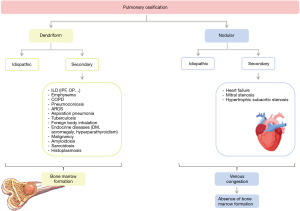
There are two PO patterns: dendriform and nodular. DPO may be idiopathic (4,13) or, most frequently, be associated with primary lung disease. Associated lung diseases include diffuse interstitial lung disease (especially idiopathic pulmonary fibrosis), emphysema; chronic obstructive pulmonary disease; pneumoconiosis; aspiration pneumonia; tuberculosis; inhalation of foreign bodies; sarcoidosis or malignant diseases; among others (3).
Likewise, the nodular pulmonary ossification (NPO) form, the most frequent type of PO, can be idiopathic or secondary. Unlike DPO, secondary NPO forms have been associated with passive venous congestion caused by chronic heart failure, mitral stenosis and hypertrophic subaortic stenosis, to name a few (14). Figure 2 shows the most frequent causes of PO described in literature.
Pathogeny
The pathogenesis of PO is not fully understood, and several hypotheses have been suggested to account for its etiology. The most widely studied hypothesis is that PO develops by organization of an alveolar exudate in response to chronic insults, which leads to the development of fibro-osseus metaplasia. Another theory is that angiogenesis and subsequent anoxia and fibrotic lung repair generate an acidic ambient that ultimately stimulates the proliferation of fibroblasts and bone formation (5,15). One of the main differences in the pathogenesis of DPO and NPO is that the ectopic bone tissue of the former contains a marrow component. Thus, the formation of bone marrow tissue is not secondary to the precipitation of calcium and phosphate in lung tissue or hypercalcemia, but it is a metaplastic bone formation in the lung interstitium (13).
Likewise, DPO is associated with a dendriform pattern characterized by a branched form resembling a tree that expands throughout the interstitium and alveolar septa. Bone spicules show a regular dichotomous pattern that branches at angles of 60° to 70° roughly every 2.0 cm (3). Hence, DPO may progress to interstitial fibrosis and has been considered a radiological and histological marker included in the phenotype of progressive pulmonary fibrosis (16).
Other predisposing agents have been associated with the pathogenesis of PO, including the transforming growth factor beta (TGF-β). This post-inflammatory mediator stimulates the proliferation of osteoblasts and chrondrocytes (17). Serum levels of Krebs von den Lungen-6 (KL-6), a glycoprotein produced by type II pneumocytes in response to cell insults, have been suggested as a predictive factor of progression. In the light that disease progression in PO is associated with an increase in the number of ossified lesions and surrounding reactive fibrosis, it could cause an increase in the levels of KL-6 in serum, a known marker of interstitial lung disease. Hence, KL-6 emerges as a potential predictor of progression (18). On another note, cases have been reported of ectopic bone formation in vitro induced by other mediators, including bone morphogenic protein or interleukin-1 and interleukin-4 (19).
Genetic aspects and their link to PO
Azuma et al. have first described a familial clustering of DPO (11). There are many molecular biological hypotheses that lead to the formation of ectopic bone in the lungs. The bone morphogenetic protein (BMP) genes and TGF-β gene families have been hypothesized as a conceivable pathogenesis of DPO, because they regulate ectopic bone formation (20). These genes are associated with the embryonic molecular regulation of limb growth. BMP signaling molecules regulate the morphogenesis of the limbs together with other signaling molecules, including fibroblast growth factors (21). There might be a relationship between ectopic bone formation in the lung and bone hypoplasia but further studies are needed to better elucidate these mechanisms.
Histological diagnostic approach
Diffuse PO is a rare disease increasingly diagnosed in vivo, although it is most frequently found post-mortem. Although PO is commonly an incidental finding on an HRCT performed for other cause, it is also found by surgical biopsy, a lung tissue sample obtained for other purposes. Other types of less-invasive lung biopsies, including transbronchial lung biopsy (TBLB) are not suitable for correct diagnosis as the sample of pulmonary interstitium obtained is too small. A valid and acceptable alternative is transbronchial lung cryobiopsy. This new procedure has been proven to have a superior diagnostic performance for interstitial lung disease, as compared to TBLB. However, this procedure is associated with a slightly higher risk for secondary pneumothorax and lung hemorrhages, as compared to TBLB. Conversely, the risk for these events is lower in cryobiopsy than in surgical lung biopsy (22,23).
Dendriform lung ossification as a phenotype of interstitial lung abnormality (ILA)
ILAs are defined as incidental radiological findings on HRCT, including ground-glass opacities; reticular abnormalities; lung architectural distortion; bronchiectasis; and honeycomb pattern or cysts involving over 5% of the lung surface (24). ILA is considered an early stage of radiological involvement that may progress to defined interstitial lung disease over time. However, the pathogenesis and course of ILA are still poorly understood (25).
The typical radiological findings of DPO on HRCT are calcified branching structures predominantly distributed in the lower lobes, without any other remarkable finding (7,8). However, recent studies suggest that the idiopathic form could be included in the group of disorders associated with ILA (25,26). A retrospective cohort study of 16 patients conducted by Ueno et al. revealed that all radiological lesions of patients with DPO were associated with a histological finding of dendriform ossification, cicatricial PO and/or fibrosis. In these cases, lesions had a lower lobe distribution and a linear branching pattern, which is consistent with the distribution described in ILAs. It is worth noting that some of the POs in these series did not exhibit high attenuation in the HRCT bone window level. This could be explained by the small size of abnormalities and the reduced fat fraction of bone marrow (26).
Considering defined interstitial lung disease, the UIP pattern also shows radiological and histological PO, which is one of the most common forms of ILA. Therefore, bone formation in ILA may resemble that associated with the UIP pattern. In the light of their shared features, DPO, as an ILA phenotype, has been suggested to be an early stage of disease that may ultimately progress to interstitial lung disease (12).
On this line, in their study of patients with interstitial lung disease, Kim et al. found that DPO was found in 5 of 75 patients with pathologically proven UIP (12). The presence of DPO can help the diagnose of UIP pattern and it can be considered as an ancillary finding of fibrosis. In the study published by Egashira et al., DPO was found to have a much higher prevalence in UIP patients than in other non-idiopathic pulmonary fibrosis fibrotic interstitial lung diseases (5). Many studies have shown the relationship between IPF and DPO, helping the differentiation of idiopathic pulmonary fibrosis from other interstitial lung diseases; they have also demonstrated that DPO is highly associated with a higher grade of fibrosis and to poor survival. Thus, DPO could reflect the severity of fibrosis (27,28).
Conclusions
PO is a low-prevalence entity more frequent in men than in women that occurs in early stages of life. This disease has an indolent course that may progressively cause lung function impairment. Although it is generally idiopathic, it may be associated with other diseases, prevailingly lung interstitial diseases. An association has been observed with the UIP radiological pattern. HRCT is the technology of choice for diagnosis, as it allows for differential diagnosis with other diseases and spares the use of more aggressive diagnostic techniques. As a specific treatment is not currently available, treatment is aimed at preventing progression to fibrosing interstitial lung disease. For such reason, early diagnosis and appropriate management of associated comorbidities are crucial. Further research and long-term follow-up are necessary for a better understanding of the pathogenesis and prognosis of this disease.
Acknowledgments
None.
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2083/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2083/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Luschka H. Ramified ossification of the pulmonary parenchyma. Wirchows Arch 1856;10:500-5. [Crossref]
- Chan ED, Morales DV, Welsh CH, et al. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 2002;165:1654-69. [Crossref] [PubMed]
- Lara JF, Catroppo JF, Kim DU, et al. Dendriform pulmonary ossification, a form of diffuse pulmonary ossification: report of a 26-year autopsy experience. Arch Pathol Lab Med 2005;129:348-53. [Crossref] [PubMed]
- Tseung J, Duflou J. Diffuse pulmonary ossification: an uncommon incidental autopsy finding. Pathology 2006;38:45-8. [Crossref] [PubMed]
- Egashira R, Jacob J, Kokosi MA, et al. Diffuse Pulmonary Ossification in Fibrosing Interstitial Lung Diseases: Prevalence and Associations. Radiology 2017;284:255-63. [Crossref] [PubMed]
- Slabbynck H, de Beukelaar T, De Surgeloose D, et al. Predominant dendriform pulmonary ossification in a usual interstitial pneumonia-like distribution: to be distinguished from idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2017;34:251-6. [PubMed]
- Nishioka Y, Toyoda Y, Egashira R, et al. Nationwide retrospective observational study of idiopathic dendriform pulmonary ossification: clinical features with a progressive phenotype. BMJ Open Respir Res 2022;9:e001337. [Crossref] [PubMed]
- Fernández-Bussy S, Labarca G, Pires Y, et al. Dendriform pulmonary ossification. Respir Care 2015;60:e64-7. [Crossref] [PubMed]
- Kato T, Ishikawa K, Kadoya M, et al. Spontaneous pneumothorax in a patient with dendriform pulmonary ossification: report of a case. Surg Today 2012;42:903-8. [Crossref] [PubMed]
- Matsuo H, Handa T, Tsuchiya M, et al. Progressive Restrictive Ventilatory Impairment in Idiopathic Diffuse Pulmonary Ossification. Intern Med 2018;57:1631-6. [Crossref] [PubMed]
- Azuma A, Miyamoto H, Enomoto T, et al. Familial clustering of dendriform pulmonary ossification. Sarcoidosis Vasc Diffuse Lung Dis 2003;20:152-4. [PubMed]
- Kim TS, Han J, Chung MP, et al. Disseminated dendriform pulmonary ossification associated with usual interstitial pneumonia: incidence and thin-section CT-pathologic correlation. Eur Radiol 2005;15:1581-5. [Crossref] [PubMed]
- Jaderborg JM, Dunton RF. Rare clinical diagnosis of dendriform pulmonary ossification. Ann Thorac Surg 2001;71:2009-11. [Crossref] [PubMed]
- Ahari JE, Delaney M. Dendriform pulmonary ossification: a clinical diagnosis with 14-year follow-up. Chest 2007;132:701A. [Crossref]
- Gielis J, Torfs M, Luijks M, et al. Nodular pulmonary ossifications in differential diagnosis of solitary pulmonary nodules. Eur Respir J 2011;37:966-8. [Crossref] [PubMed]
- Palermo M, Tiralongo F, Distefano G, et al. Quantitative Evaluation of Fibrosis in IPF Patients: Meaning of Diffuse Pulmonary Ossification. Diagnostics (Basel) 2021;11:113. [Crossref] [PubMed]
- Peros-Golubicić T, Tekavec-Trkanjec J. Diffuse pulmonary ossification: an unusual interstitial lung disease. Curr Opin Pulm Med 2008;14:488-92. [Crossref] [PubMed]
- Ohnishi H, Yokoyama A, Kondo K, et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med 2002;165:378-81. [Crossref] [PubMed]
- Poletti V, Costabel U, Casoni GL, et al. Rare infiltrative lung diseases: a challenge for clinicians. Respiration 2004;71:431-43. [Crossref] [PubMed]
- Fried ED, Godwin TA. Extensive diffuse pulmonary ossification. Chest 1992;102:1614-5. [Crossref] [PubMed]
- Lyons K, Ezaki M. Molecular regulation of limb growth. J Bone Joint Surg Am 2009;91:47-52. [Crossref] [PubMed]
- Tsai AP, English JC, Murphy D, et al. Recurrent pneumothorax related to diffuse dendriform pulmonary ossification in genetically predisposed individual. Respirol Case Rep 2017;5:e00211. [Crossref] [PubMed]
- Martinez JB, Ramos SG. Dendriform pulmonary ossification. Lancet 2013;382:e22. [Crossref] [PubMed]
- Hatabu H, Hunninghake GM, Richeldi L, et al. Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Med 2020;8:726-37. [Crossref] [PubMed]
- Chae KJ, Chung MJ, Jin GY, et al. Radiologic-pathologic correlation of interstitial lung abnormalities and predictors for progression and survival. Eur Radiol 2022;32:2713-23. [Crossref] [PubMed]
- Ueno M, Egashira R, Hashisako M, et al. Idiopathic dendriform pulmonary ossification as the phenotype of interstitial lung abnormalities: CT-pathologic correlation and prevalence. Jpn J Radiol 2024;42:993-1002. [Crossref] [PubMed]
- Travis WD, Matsui K, Moss J, et al. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol 2000;24:19-33. [Crossref] [PubMed]
- Lloyd CR, Walsh SL, Hansell DM. High-resolution CT of complications of idiopathic fibrotic lung disease. Br J Radiol 2011;84:581-92. [Crossref] [PubMed]


