
Survival outcome after systematic lymphadenectomy in non-small cell lung cancer according to the latest proposed edition of the TNM system
Highlight box
Key findings
• Systematic lymphadenectomy (SLND) does not directly impact survival outcomes in non-small cell lung cancer (NSCLC) patients when assessed according to the latest Tumor, Nodal and Metastasis (TNM) classification.
• Patients with higher N status (e.g., N2) demonstrated a poor survival benefit from SLND.
• The study provides updated evidence supporting the prognostic value of lymph node dissection in stratifying patient outcomes.
What is known and what is new?
• The TNM classification has evolved to improve treatment strategies and diagnostic accuracy, but evidence regarding its impact on survival outcomes in the latest version is still limited.
• This research applies the latest TNM classification to assess survival outcomes, offering new insights into the prognostic role of lymphadenectomy in different nodal statuses.
• Findings suggest that the extent of lymph node dissection remains crucial on the way of optimizing long-term survival under the latest TNM framework.
What is the implication, and what should change now?
• The study reinforces the necessity of SLND in NSCLC surgery but under specific indications (e.g., possible nodal involvement).
• Further studies are needed to refine lymphadenectomy guidelines along with improved staging systems to further improve survival outcomes.
Introduction
In 2024, the American Cancer Society estimated that 340 people were dying daily from lung cancer, remaining the primary cause of cancer-related deaths worldwide (1-5). Surgery in non-small cell lung cancer (NSCLC) with an assessment of mediastinal lymph nodes is a critical component of therapy decisions (6). However, selecting the optimal surgical technique to assess the mediastinal lymph nodes remains a major challenge and subject of debate. Surgical methods include systematic lymph node dissection (SLND) and less invasive methods such as lymph node sampling (LNS) or lobe-specific dissection.
LNS is potentially less invasive, leading to shorter operative durations and possibly reduced postsurgical complications (7). However, since less lymphatic tissue has to be removed, it leads to inadequate tumor staging. During SLND, the lymph nodes are removed together with the adjacent tissue, enabling a more precise staging; however, this is a more invasive procedure that possibly affects patients’ postoperative quality of life.
At the same time, several studies have compared the recurrence and incidence of metastases after each procedure (SLND or LNS) in NSCLC surgery. Previous studies have shown that SLND effectively reduces the incidence of recurrence by eliminating hidden micrometastases (8-11). However, other meta-analyses have shown that SLND and LNS have similar recurrence and distant metastasis rates after the surgery (12), and some other data showed that SLND has even no effect on the incidence of recurrence or distant metastasis (13-15). The absence of clear evidence-based protocols for each technique complicates the decision on the appropriate method for each patient, leading to potential variations in patient outcomes and making it difficult to compare results across different studies. This difficulty has had a negative impact on several levels, starting with the accuracy of the N-staging system, treatment planning, and outcome prediction (16).
The direct influence of surgical procedures, particularly SLND, on long-term survival remains unclear, with conflicting evidence in the literature. The lack of clarity on how specific surgical techniques directly affect patient outcomes highlights the need for further investigation. To address this, we investigate the direct impact of our surgical technique (SLND) on survival rates in operable lung cancer. We believe our findings will provide critical insights and help optimize surgical strategies for patients with NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2086/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Friedrich-Alexander University Erlangen-Nürnberg (194_20 Bc, date of approval 14.07.2020) and individual consent for this retrospective analysis was waived. This retrospective analysis examines data from 367 patients with NSCLC who underwent surgery at the Erlangen University Hospital’s Department of Thoracic Surgery from 2008 to 2019. All patients were followed up based on the 5-year survival rate after tumor resection.
Preoperative assessment and surgical selection criteria
Before surgery, a multidisciplinary tumor board evaluated each case, considering treatment options based on the clinical Tumor, Nodal, Metastasis staging system (cTNM) determined via positron emission tomography-computed tomography (PET-CT) imaging. Surgical candidacy was established for patients with resectable disease, including those with suspected or undetected N1 or N2 involvement. Although diagnostic tools such as transbronchial needle aspiration (TBNA), endobronchial ultrasound (EBUS), and mediastinoscopy were used, some patients with N1 or N2 status remained undetected pre-operatively. Importantly, none of the patients in this cohort received neoadjuvant therapy. In some cases, patients declined neoadjuvant therapy despite eligibility, while others were ineligible due to contraindications or comorbidities. All surgical candidates underwent standardized cardiorespiratory fitness assessments, with patients meeting pulmonary function criteria undergoing anatomical lung resection, while those with insufficient pulmonary reserve received wedge resections.
Preoperative mediastinal staging
Mediastinal staging was conducted preoperatively for all patients as part of the clinical staging process (cTNM). Imaging modalities included contrast-enhanced CT and PET-CT, which were used to assess mediastinal lymph nodes and identify potential metastatic involvement. For patients with radiologically suspicious mediastinal lymph nodes [e.g., nodes with a short-axis diameter ≥10 mm or high fluorodeoxyglucose (FDG) uptake on PET-CT], invasive staging was performed using EBUS-guided TBNA (EBUS-TBNA) or TBNA. In cases where EBUS or TBNA results were inconclusive, mediastinoscopy was employed to provide a definitive histological diagnosis. Despite these efforts, a subset of patients had N1 or N2 disease that was not detectable preoperatively.
Surgical approach
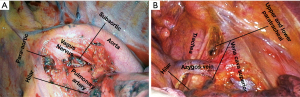
Operative notes and pathology reports were reviewed to assess surgical procedures and lymph node involvement. Utilizing an open surgical approach, systematic lymph node dissection was performed alongside tumor resection. The lymph node map created by the International Association for the Study of Lung Cancer (IASLC) features a proposed classification of lymph node stations into distinct “zones” (17). This comprehensive approach ensures accurate surgical technique. During surgery for right-sided tumors, systematic lymph node dissection included stations 2R and 4R (upper and lower paratracheal). For left-sided tumors, a dissection removed lymph tissue in stations 5 and 6 within the subaortic and paraaortic regions. Independent of tumor laterality, a lymphadenectomy included stations 7, 8, 9, 10, and 11 capturing the subcarinal, paraesophageal, pulmonary ligament, hilar, and interlobar lymph nodes (see Figure 1).
Pathological tumor staging and adjuvant therapy
Following surgery, all patients were re-evaluated in a multidisciplinary tumor conference, where their final pathological staging report was reviewed to establish an individualized adjuvant therapy plan. This approach ensured that treatment decisions were based on the most accurate staging data, optimizing postoperative management and improving patient outcomes. Postoperative nodal staging followed the eighth edition of the IASLC TNM classification, incorporating the ninth edition’s distinction between N2a (single nodal station) and N2b (multiple nodal stations). Additionally, we incorporated the recent adjustments suggested in the ninth edition considering N2a single and N2b multiple nodal involvement (18). Adjuvant treatment was indicated for patients with pathological evidence of nodal involvement (N1 or N2) or other high-risk features, such as large tumor size, or poor tumor differentiation. In particular, patients with N2 disease (both N2a and N2b) were considered for adjuvant chemotherapy, with or without radiotherapy, to address the increased risk of recurrence.
Statistical analysis
The Chi-squared test was utilized to compare the distribution of categorical variables among different groups. Survival was estimated by the Kaplan-Meier method and differences in survival were determined by log rank analysis. The results of the multivariable analysis of independent prognostic factors, which included gender, histologic type, N status, and operative procedure, were assessed by using the Cox proportional hazards regression model. Zero time was the date of pulmonary resection, and the terminal event was death attributable to cancer, a cause other than cancer, or an unknown cause. The resulting P values aided in discerning the impact of various factors on survival rates, with a probability value (P) less than 0.05 considered statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS 28.0).
Results
Patient’s characteristics
The study population consisted of a majority of males (62.1%). Adenocarcinoma was the most common histological tumor type (n=220, 59.9%), representing over half of the cases. Squamous cell carcinoma was the second most frequent (n=126, 34.3%), followed by other tumors (n=21, 5.7%). The most common surgical procedures were lobectomy (n=263, 71.7%), followed by bilobectomy and pneumonectomy. In contrast, wedge resections were performed in fewer cases (n=29, 7.9%). A detailed representation of patient characteristics is provided in Table 1.
Table 1
| Variable | Value |
|---|---|
| Gender | |
| Male | 228 (62.1) |
| Female | 139 (37.9) |
| Tumor location | |
| Right | 229 (62.4) |
| Left | 138 (37.6) |
| Histology | |
| Adenocarcinoma | 220 (59.9) |
| Squamous | 126 (34.3) |
| Other | 21 (5.7) |
| Type of resection | |
| Segmentectomy | 25 (6.8) |
| Lobectomy | 263 (71.7) |
| Bilobectomy | 33 (9.0) |
| Pneumonectomy | 17 (4.6) |
| Wedge resection | 29 (7.9) |
| Tumor stage | |
| I | 156 (42.5) |
| IIA | 62 (16.9) |
| IIB | 80 (21.8) |
| IIIA | 58 (15.8) |
| IIIB | 11 (3.0) |
Data are presented as n (%). Patient demographic and clinical characteristics stratified by gender. Variables include age, histological type, N stage, surgical procedure, and 5-year survival rates. Significant differences were observed between genders (Chi-squared test, P<0.001).
Analysis of the N staging revealed a predominance of patients classified as N0 (n=267, 72.8%), indicating no evidence of lymph node involvement. Within the N1 category (n=61, 16.6%), the majority had only a single lymph node involved. Similarly, the N2 category (n=39, 10.6%) was dominated by cases with single lymph node involvement (n=29, 7.9% of total patients). An analysis of individual intrapulmonary and mediastinal lymph node stations revealed that the interlobar nodes (Gr. 11) emerged as the most frequently metastasized stations. Further details regarding N classification and specific lymph node involvement are listed in Table 2.
Table 2
| Quantitative analysis of lymph nodal status | Value |
|---|---|
| Lymph nodal stage (N) | |
| N0 | 267 (72.8) |
| N1 | 61 (16.6) |
| N1 single level | 52 (14.2) |
| N1 multi-level | 9 (2.5) |
| N2 | 39 (10.6) |
| N2 single level | 29 (7.9) |
| N2 multi-level | 10 (2.7) |
| Positive nodal group | |
| Gr. 2/4 R | 19 (5.2) |
| Gr. 5/6 L | 7 (1.9) |
| Gr. 7 | 12 (3.3) |
| Gr. 8 | 7 (1.9) |
| Gr. 9 | 6 (1.6) |
| Gr. 10 | 19 (5.2) |
| Gr. 11 | 36 (9.8) |
| Number of lymph nodes dissected from each station | |
| Gr. 2/4 R | 1,530 |
| Gr. 5/6 L | 352 |
| Gr. 7 | 1,165 |
| Gr. 8 | 315 |
| Gr. 9 | 491 |
| Gr. 10 | 285 |
| Gr. 11 | 1,246 |
Data are presented as n (%). Summary of lymph node classifications and their corresponding specific nodal involvements, highlighting patterns observed in the study cohort. L, left; Gr., group; R, right.
Five-year survival rate after the surgery
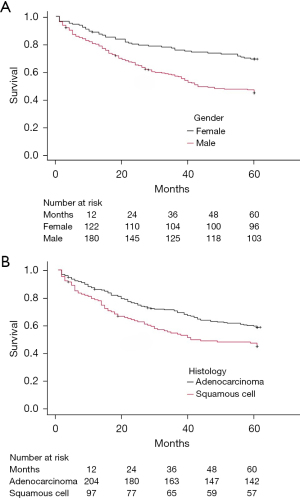
The 5-year survival rate for the patient cohort was 54.2% (n=199). Notably, survival rates differed significantly between genders (P<0.001), with females (n=96, 69.1%) having a higher survival rate compared to males (n=103, 45.2%) (Table 3 and Figure 2). Examining tumor histology, we observed a trend towards slightly better 5-year survival rates for patients with adenocarcinoma (n=126, 57.3%) compared to squamous cell carcinoma (n=57, 45.2%) with a statistical significance between these histological types (P=0.005) (Table 3 and Figure 3). The type of tumor resection did not significantly affect 5-year survival. The patients who underwent tumor anatomical resection (n=180) had a 53.9% survival rate, while those who received wedge resection (n=18) had a 62.1% survival rate. Table 3 comprehensively analyses various factors and their influence on the 5-year survival rate following surgery.
Table 3
| Factor | 5-year survival (%) | Log-rank P value | Univariable HR (95% CI) | P value |
|---|---|---|---|---|
| Gender | ||||
| Male | 45.2 | <0.001 | 0.497 (0.350–0.705) | <0.001 |
| Female | 69.1 | Reference | ||
| Type of resection | ||||
| Anatomical resection | 53.9 | 0.98 | 0.900 (0.308–2.635) | 0.84 |
| Wedge resection | 62.1 | Reference | ||
| Histology | ||||
| Adenocarcinoma | 57.3 | 0.005 | 2.387 (0.958–5.947) | 0.06 |
| Squamous cell carcinoma | 45.2 | Reference | ||
| Tumor stage | ||||
| I | 66 | <0.001 | – | – |
| IIA | 33.9 | <0.001 | – | – |
| IIB | 46.3 | 0.13 | – | – |
| IIIA | 53.4 | 0.97 | – | – |
| IIIB | 63.6 | 0.44 | – | – |
HR and 95% CI for variables influencing 5-year survival. Factors include gender, N factor, histological type, and type of surgical procedure. Female gender and early-stage disease (N0) were independently associated with improved survival (P<0.05). CI, confidence interval; HR, hazard ratio.
Impact of N stage and metastasized lymph node stations
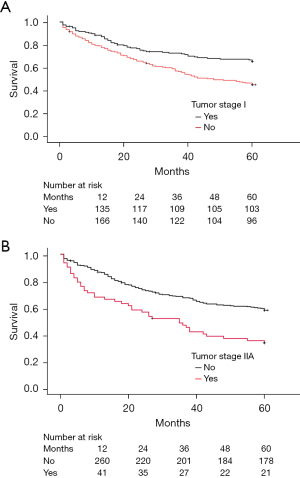
The 5-year survival rates did not differ significantly among the N stages. Patients with the N0 stage had a survival rate of 56.6% (n=151), followed by N1 at 45.9% (n=28), and N2 at 51.3% (n=20) (Table 4). Additionally, single-level lymph node involvement did not show a statistically significant difference in 5-year survival, with N1 single at 46.2% (n=24) and N2 single at 65.5% (n=19) (Table 4). However, multiple lymph node involvement in N1 did not significantly affect survival. N2 multiple-level involvement was associated with a much lower survival rate of 10.0% (n=1. P<0.001) (Table 4 and Figure 4).
Table 4
| Lymph node status | 5-year survival | Log-rank P value |
|---|---|---|
| N status | ||
| N0 | 151/267 (56.6) | 0.12 |
| N1 | 28/61 (45.9) | 0.23 |
| N1 single level | 24/52 (46.2) | 0.21 |
| N1 multi-level | 4/9 (44.4) | 0.66 |
| N2 | 20/39 (51.3) | 0.43 |
| N2 single level | 19/29 (65.5) | 0.45 |
| N2 multi-level | 1/10 (10.0) | <0.001 |
| N station | ||
| Gr. 2/4 R | 9 (47.4) | 0.43 |
| Gr. 5/6 L | 4 (57.1) | 0.95 |
| Gr. 7 | 6 (50.0) | 0.49 |
| Gr. 8 | 1 (14.3) | <0.001 |
| Gr. 9 | 2 (33.3) | 0.45 |
| Gr. 10 | 7 (36.8) | 0.16 |
| Gr. 11 | 15 (41.7) | 0.04 |
Comparison of 5-year survival rates for patients with different nodal group involvements. The paraesophageal lymph node group (Group 8) was associated with the lowest survival (14.3%, P<0.001). L, left; Gr., group; R, right.
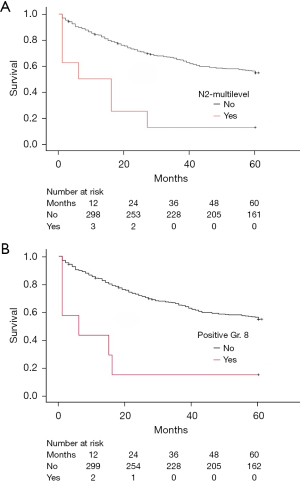
We also examined the influence of each lymph node station on 5-year survival rates. The differences in 5-year survival rates varied significantly between N stages and individual lymph node stations. For example, there were notable differences in survival rates between patients with the N2 stage and positive involvement in Group 8 or Group 7 lymph nodes (Tables 3,4). Similarly, the mediastinal and pulmonary groups showed a consistent trend. Table 4 presents a detailed analysis of each N stage and each lymph node group and their impact on the 5-year survival rate following surgery.
Discussion
According to the proposed revision of the forthcoming ninth edition of the TNM classification for lung cancer, the 5-year survival rate varies from 80% in patients with the N0 stage to 58% in patients with N1 stage and in N2 disease the survival rate was around 50–40% (18). We conducted our analysis using our standardized surgical approach SLND. Accordingly, we have analyzed our database according to the proposed changes to the 9th edition of the N staging classification, which includes single and multi-level involvement of N2 disease (N2a and N2b) (18).
Our analysis revealed that N0 had a 5-year survival rate of 56.6% (P=0.12). Depending on the data of the 9th edition of the N staging classification, the survival rate is geographically dependent; in Europe, it is 60% (cN0), which is more or less in line with our results (18). However, the 5-year survival rate after tumor resection in the entire proposed cohort is 80%, and the proposed data offer no explanation as to why there was a dramatic change in survival in this patient group (17,19). We also found that these results were similar to the earlier data from the 7th edition (16). This patient group accounts for the largest proportion of operable NSCLC, and the aggressive approach of SLND contributes to the moderate 5-year survival rate by reducing the quality of life of these patients. Therefore, we advocate for a more targeted approach to lymph node dissection, which should also be considered in early-stage NSCLC (cN0).
For N1 stage, we also classified these according to single- and multiple-level involvement. The 5-year survival rate for N1 was 45.9% (P=0.23), for single-level involvement 46.2% (P=0.21) and for multi-level involvement 44.4% (P=0.66). These findings align with the proposed TNM revision, which reports a 59% proportion of N1 disease without differentiation among individual stages of N1 involvement. Our analysis of specific anatomical stations within the N1 intrapulmonary lymph nodes shows that stations 9, 10, and 11 are linked to slightly poorer survival outcomes. Nevertheless, a reclassification has been proposed, suggesting the downstaging of N1 disease from stage IIB (T1N1) to stage IIA (T1N1) (18).
For N2 disease, the 5-year survival rate was approximately 51.3% (P=0.43), with significant variation depending on the extent of involvement: survival was around 65.5% (P=0.45) for single-level N2 involvement but dropped to 10.0% (P<0.001) for more extensive disease (multi-level involvement). However, this represents a relatively small patient cohort. The survival analysis of mediastinal lymph nodes (stations 2/4, 7, and 8) showed varied outcomes, with station 8 standing out as significant, demonstrating a survival rate of 14.3% (P<0.001). While our technique (SLND) consistently offers a survival advantage across various N stages, the prognosis significantly worsens with the involvement of multiple lymph nodes, particularly in N2 stages. A closer examination of individual mediastinal and pulmonary lymph node stations has provided clearer insights into their direct impact on survival.
While LNS and systematic dissection appear to be equally accurate in the early stages of disease, a thorough assessment by systematic dissection is essential in advanced cases (N2) (15,20,21). However, it is considerable that approximately two-thirds of N2 patients show a distant metastasis as initial disease relapse not locoregional (8,22). Furthermore, the increasing utilization of advanced imaging and biopsy techniques, such as PET scans and EBUS, in pre-operative planning shows promise (23). These technologies enable a more precise, non-invasive assessment of lymph node involvement, potentially reducing the necessity for extensive surgical dissection in selected cases. Consequently, a more selective application of SLND or other surgical procedures could be considered, limiting their use to patients with specific indications rather than applying them universally across all tumor stages. Such a strategy would help mitigate the potential drawbacks of SLND and other surgical approaches, including longer operative times, higher complication rates, and extended recovery periods.
Our analysis highlighted also limitations in the N staging system’s capacity to distinguish 5-year survival rates across all NSCLC stages. Although it showed some improvement in predicting prognosis for advanced tumors (such as N2) when considering the extent of lymph node involvement within each N stage, the overall differentiation between stages remained limited. Integrating information on specific lymph node stations into the N staging system could enhance prognostic accuracy. For example, documenting the precise anatomical location of affected lymph nodes may provide more nuanced prognostic insights. However, any modifications to N classification must balance complexity with clinical practicality to ensure widespread applicability.
A key limitation of my study is the relatively small sample size, as it was conducted within a single center. This could introduce potential biases, particularly in terms of patient selection and institutional treatment practices. The findings may not be fully generalizable to a broader population, and some of the observed trends could be influenced by local clinical protocols or demographic factors specific to the study group. Future research involving larger, multi-center cohorts will be necessary to validate these findings and confirm their broader applicability. During the study period, most surgeries were conducted via open thoracotomy, as video-assisted thoracoscopic surgery (VATS) had not yet been widely adopted at our department (Department of Thoracic Surgery, Erlangen University Hospital). Our aim was to evaluate each technique independently, without intending to favor one over the other.
Conclusions
Our findings show no evidence of a direct effect of lymph node dissection on survival rates across different tumor stages. While comprehensive lymph node examination is vital for accurate staging, our findings demonstrate that the current N-staging system falls short in differentiating survival outcomes across all stages. Therefore, we advocate for more focused strategy treating each clinical tumor stage as a unique condition that requires a specific treatment plan.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2086/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2086/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2086/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2086/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Friedrich-Alexander University Erlangen-Nürnberg (194_20 Bc, date of approval 14.07.2020) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med 2020;41:1-24. [Crossref] [PubMed]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. [Crossref] [PubMed]
- Gridelli C, Rossi A, Maione P, et al. Vaccines for the treatment of non-small cell lung cancer: a renewed anticancer strategy. Oncologist 2009;14:909-20. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Chiappetta M. The lymphadenectomy in non-small cell lung cancer. Video-assist Thorac Surg 2022;7:2. [Crossref]
- Dezube AR, Mazzola E, Bravo-Iñiguez CE, et al. Analysis of Lymph Node Sampling Minimums in Early Stage Non-Small-Cell Lung Cancer. Semin Thorac Cardiovasc Surg 2021;33:834-45. [Crossref] [PubMed]
- Wu Yl, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [Crossref] [PubMed]
- Su X, Wang X, Long H, et al. Mediastinal lymph node dissection affects survival in patients with stage I non-small cell lung cancer. Thorac Cardiovasc Surg 2008;56:226-30. [Crossref] [PubMed]
- Lardinois D, Suter H, Hakki H, et al. Morbidity, survival, and site of recurrence after mediastinal lymph-node dissection versus systematic sampling after complete resection for non-small cell lung cancer. Ann Thorac Surg 2005;80:268-74; discussion 274-5. [Crossref] [PubMed]
- Meng D, Zhou Z, Wang Y, et al. Lymphadenectomy for clinical early-stage non-small-cell lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2016;50:597-604. [Crossref] [PubMed]
- Huang X, Wang J, Chen Q, et al. Mediastinal lymph node dissection versus mediastinal lymph node sampling for early stage non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2014;9:e109979. [Crossref] [PubMed]
- Mokhles S, Macbeth F, Treasure T, et al. Systematic lymphadenectomy versus sampling of ipsilateral mediastinal lymph-nodes during lobectomy for non-small-cell lung cancer: a systematic review of randomized trials and a meta-analysis. Eur J Cardiothorac Surg 2017;51:1149-56. [Crossref] [PubMed]
- Luo J, Yang S, Dong S. Selective Mediastinal Lymphadenectomy or Complete Mediastinal Lymphadenectomy for Clinical Stage I Non-Small Cell Lung Cancer: A Meta-Analysis. Adv Ther 2021;38:5671-83. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Osarogiagbon RU, Van Schil P, Giroux DJ, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Overview of Challenges and Opportunities in Revising the Nodal Classification of Lung Cancer. J Thorac Oncol 2023;18:410-8. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Huang J, Osarogiagbon RU, Giroux DJ, et al. The International Association for the Study of Lung Cancer Staging Project for Lung Cancer: Proposals for the Revision of the N Descriptors in the Forthcoming Ninth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2024;19:766-85.
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg 2000;70:358-65; discussion 365-6. [Crossref] [PubMed]
- Izbicki JR, Thetter O, Habekost M, et al. Radical systematic mediastinal lymphadenectomy in non-small cell lung cancer: a randomized controlled trial. Br J Surg 1994;81:229-35. [Crossref] [PubMed]
- Caglar HB, Baldini EH, Othus M, et al. Outcomes of patients with stage III nonsmall cell lung cancer treated with chemotherapy and radiation with and without surgery. Cancer 2009;115:4156-66. [Crossref] [PubMed]
- Schmidt-Hansen M, Baldwin DR, Hasler E, et al. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev 2014;2014:CD009519. [Crossref] [PubMed]


