
Critical role of spread through air spaces in prognosis of stage IA3 non-small cell lung cancer: insights from a real-world multicenter study
Highlight box
Key findings
• Spread through air spaces (STAS) cell nest subtype was identified as independent prognostic factor in stage IA3 non-small cell lung cancer (NSCLC).
• Tumor-stroma ratio and smoking history strongly impact survival in IA3 NSCLC.
• Novel risk stratification integrates STAS subtypes and histopathology features.
• Predictive tool aids in identifying recurrence-prone patients in early-stage NSCLC.
• This study lays groundwork for tailored adjuvant therapies in early-stage lung cancer.
What is known and what is new?
• Stage IA3 NSCLC is associated with higher recurrence rates and poorer prognosis compared to earlier stages, yet the current tumor-node-metastasis (TNM) staging system does not fully account for critical histopathological factors.
• This study systematically evaluates the prognostic impact of STAS subtypes and related histomorphological factors in stage IA3 NSCLC, identifying the STAS cell nest subtype and tumor stroma ratio as independent prognostic factors for progression-free survival (PFS) and overall survival (OS).
What is the implication, and what should change now?
• The study’s findings suggest that the presence of the STAS cell nest subtype and a tumor-stroma ratio above 50% are significant independent prognostic factors for both PFS and OS in stage IA3 NSCLC, highlighting the need for a more nuanced understanding of histopathological heterogeneity in lung cancer prognosis.
• Current clinical practices and TNM staging guidelines should integrate detailed assessments of STAS subtypes and tumor microenvironment features to better stratify stage IA3 NSCLC patients into risk groups, potentially leading to more personalized and effective treatment strategies, including the consideration of adjuvant therapies for high-risk patients.
Introduction
Lung cancer remains one of the most prevalent and fatal malignancies globally, accounting for 23.6% of new cancer cases and 16.8% of cancer-related deaths in 2022 (1). Among these, non-small cell lung cancer (NSCLC) is the predominant type. Research indicates that pleural invasion, tumor diameter exceeding 4 cm, and vascular tumor emboli significantly heighten recurrence risk in stage IB patients, prompting guidelines to recommend adjuvant therapy for high-risk cases (2). In contrast, the prognostic characteristics and recurrence risks for pathological stage IA3 patients are underexplored, with no guidelines endorsing postoperative adjuvant therapy. Pathological stage IA3 NSCLC is increasingly recognized for its higher postoperative recurrence rates and poorer prognosis compared to IA1/2, with postoperative recurrence rates reaching 10% and 5-year overall survival (OS) rates of approximately 78–80% (3,4). Therefore, effective postoperative adjuvant therapy is critical for improving survival outcomes.
The JCOG 0707 trial compared adjuvant S-1 and Tegafur-Uracil in stage IA NSCLC, showing improved survival in high-risk patients, which supports the potential benefit of adjuvant therapy in selected early-stage cases; notably, these studies only included stage IA patients with tumors larger than 2 cm, meaning the focus of adjuvant therapy research for stage IA was primarily on IA3 patients (5-7). Moreover, these articles underline the necessity for identifying high-risk populations for recurrence. The existing tumor-node-metastasis (TNM) staging system inadequately incorporates essential histopathological factors, which may hinder prognostic assessment in pathological stage IA3 NSCLC patients.
Spread through air spaces (STAS), first described by Kadota et al. in 2015 (8), refers to the spread of lung cancer cells into adjacent air spaces, significantly affecting recurrence rates after the resection of stage I lung adenocarcinoma (9-12). STAS is linked to poor prognosis in NSCLC and other lung cancers (13). Its morphological patterns include single cells, micropapillary clusters, and cell nests, with micropapillary clusters being the most prevalent in lung adenocarcinoma (14). A 2021 study by Xie et al. found that micropapillary clusters and cell nest STAS are independent prognostic indicators; however, including advanced-stage patients limits their applicability to early-stage lung cancer (15). Many existing studies have only performed simplistic evaluations of STAS presence, and while multiple methods are available for assessing STAS, a comprehensive evaluation of its prognostic value in stage IA3 NSCLC is still lacking.
This study is the first to perform a comprehensive assessment of STAS in pathological stage IA3 NSCLC, examining STAS subtypes and various assessment models, including spread distance and the number of affected alveoli. By systematically evaluating the prognostic significance of STAS and other histopathological factors, we provide essential insights into their clinical value. We present this article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2209/rc).
Methods
Patients and clinical data
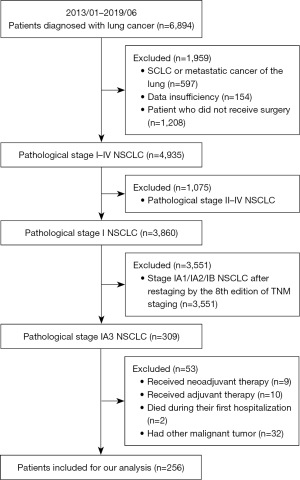
This study included 6,894 patients treated from January 2013 to June 2019 at three hospitals: Fujian Medical University Union Hospital, Ningde Municipal Hospital of Ningde Normal University, and Hui’an County Hospital. After applying strict criteria, 256 patients with pathological stage IA3 NSCLC were identified. They underwent routine examinations as recommended by the guidelines. Most patients had video-assisted thoracoscopic lobectomy; 11 with compromised function underwent segmentectomy.
Inclusion criteria
- Postoperative pathological confirmation of stage IA3 NSCLC according to the 8th edition of the TNM classification.
- Underwent standard radical resection for lung cancer.
- Absence of malignancies involving other organs.
Exclusion criteria
- Pathological confirmation of carcinoma in situ or minimally invasive adenocarcinoma.
- Patients who died within 30 days postoperatively or during the initial hospital admission.
- Patients who received neoadjuvant therapy or adjuvant therapy.
- Postoperative pathology indicating other primary or secondary lung cancers.
- Presence of malignancies involving other organs.
Basic information and clinical data were extracted from electronic medical records, including gender, age, body mass index (BMI), a family history of lung cancer, smoking history, clinical symptoms, tumor location, preoperative carcinoembryonic antigen (CEA) levels, nodule imaging characteristics (ground-glass nodules, mixed-density nodules, solid nodules), surgical approach, tumor volume, pathological type, micropapillary component ratio, and the number of lymph nodes resected. Patient screening flowchart is depicted in Figure 1.
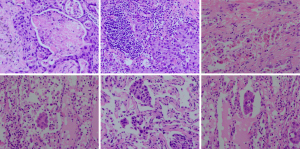
Pathological data were obtained by re-evaluating postoperative pathological slides. STAS was defined as the presence of isolated tumor cells in the air spaces of the lung parenchyma beyond the main tumor edge, assessed by two senior pathologists. Data recorded included tumor location (central/peripheral), laterality (left/right), pathological type, size, volume, tumor-stroma ratio (percentage of stroma in the total tumor area), differentiation degree, micropapillary component ratio, presence of mucin, tumor necrosis percentage (Figure 2A), nerve and vascular invasion, STAS, and Ki67 index. Follow-up strategy involved biannual follow-ups for the first 2 years post-surgery, then annually, through out reassessed according to the 8th edition of the TNM classification. The follow-up visits or telephone interviews. Follow-up evaluations included physical examination, CEA, computed tomography etc. The time of first detection for confirmed tumor recurrence or metastasis was recorded based on imaging or pathology, regardless of tumor marker levels.
Histopathological evaluation
Histological typing of surgical specimens was conducted by two experienced pathologists per the 2015 World Health Organization (WHO) Classification of Tumors of the Lung and the 2011 International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification for lung adenocarcinoma (16,17). An average of 5.8±0.3 slides per case were reviewed. Major NSCLC types included adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and mucoepidermoid carcinoma. Patients with adenocarcinoma in situ and minimally invasive adenocarcinoma were excluded, as these entities are not classified as stage IA3 NSCLC due to their distinct biological behavior and excellent prognosis after complete resection (18).
Tumor budding (Figure 2B), an emerging prognostic biomarker, is defined as isolated single cancer cells or clusters of up to four cells at the invasive tumor front. It is associated with aggressive behavior, local recurrence, distant metastasis, and poor survival outcomes in various cancers (19). Tumor-stroma ratio was visually assessed on hematoxylin and eosin-stained sections using a 4× objective, scoring the most invasive areas in 10% increments. Patients with >50% stroma were classified as stroma-rich, while those with <50% were classified as stroma-poor (20).
STAS was defined as isolated single cells (Figure 2C), micropapillary clusters (Figure 2D), or cell nests (Figure 2E) within the air spaces of the lung parenchyma surrounding the tumor edge (9). Exclusion of false-positive STAS included: (I) random distribution of tumor cells with irregular edges at the tissue section margins or outside the plane; (II) lack of continuous spread process from the main tumor; (III) serrated edges of tumor cell clusters; (IV) linear bands of cells detached from the alveolar walls; (V) artifacts caused by sectioning blades. For slides containing STAS, further subclassification was performed (single cells, cell nests, micropapillary components). All STAS in a 10× objective field were recorded as a single occurrence, with the predominant subtype being the most frequently observed STAS (21). STAS-related metrics included: (I) the distance between the farthest intraluminal cell cluster and the main tumor (1 mm cutoff, Figure 2F); (II) the number of free alveolar spaces (3 alveoli cutoff); (III) total number of invaded alveoli (<3, 3–5, >5); (IV) total number of STAS cells/colonies (5 cells cutoff). Restricted STAS was defined as clusters within ≤3 alveoli from the main tumor mass, while extensive STAS was beyond 3 alveoli (12).
Local recurrence was defined as imaging or pathology-confirmed recurrence in the ipsilateral lobe, bronchial stump, or regional lymph nodes. Distant metastasis was confirmed by imaging or pathology in the contralateral lung, brain, liver, adrenal glands, bones, or other distant organs. Simultaneous local recurrence and distant metastasis were classified as distant metastasis. OS was the duration from diagnosis to death or last follow-up, while progression-free survival (PFS) was the duration from diagnosis to tumor progression or death from any cause.
A total of 256 cases were subjected to follow-up, with the final follow-up date set at December 2022. Successful follow-up was achieved in 228 cases (89.1%), while 28 cases (10.9%) were lost to follow up. For all patients who were lost to follow up, their survival status was verified through the public security household registration system of the People’s Republic of China.
Statistical analysis
Statistical analyses were conducted using SPSS version 27.0. The Shapiro-Wilk test assessed the normality of continuous variables, with non-normally distributed data described as medians and interquartile ranges, and normally distributed data as means and standard deviations. Categorical variables were analyzed using Fisher’s exact test or Chi-squared test, and Pearson correlation tests were applied to quantitative variables. Results were reported with bilateral P values and/or 95% confidence intervals, excluding missing data.
Survival analysis utilized the Kaplan-Meier method to plot curves, with differences assessed by the log-rank test. Univariate Cox regression identified potential risk factors, which were included in multivariate Cox regression if clinically relevant. A P value <0.05 indicated statistical significance.
Risk stratification
Following multivariate analysis, patients were stratified into low-, medium-, and high-risk groups based on the number of independent risk factors identified for PFS. The specific criteria for risk classification are described in detail in the Results section.
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by ethics committee board of Fujian Medical University Union Hospital (No. 2024KY147). All participating institutions were informed and agreed the study. Individual consent for this retrospective analysis was waived.
Results
Patient characteristics
A total of 256 patients were enrolled, with a mean age of 60±0.6 (range, 21–83) years. Gender distribution was nearly equal (50.8% male, 49.2% female). Among them, 157 patients (61.3%) had a BMI between 18 and 24 kg/m2, and 16 (6.3%) had a family history of pulmonary malignancies. Eighty-seven patients (34%) were smokers, and 14 (5.5%) had centrally located tumors. Solid nodules were present in 145 patients (56.6%), and none received preoperative or postoperative adjuvant therapy. The median follow-up period for our cohort was 48 months (range, 6–120 months). Within 5 years, 27 patients experienced recurrence, and 18 died. Adenocarcinoma was the predominant histopathological type, seen in 201 patients (78.6%), with about 25 cases (9.8%) having a combination of two types. STAS was found in 115 patients (44.9%), including 9 with single-cell type, 45 with micropapillary type, and 61 with cell nest type. In 100 patients, tumor cells disseminated via air spaces exceeded five (86.9% of STAS-positive patients). Only two had vascular invasion, and three had neural invasion. Clinical and pathological characteristics are summarized in Table 1.
Table 1
| Variables | Number | % |
|---|---|---|
| Sex | ||
| Male | 130 | 50.8 |
| Female | 126 | 49.2 |
| Age (years) | ||
| ≥60 | 101 | 39.4 |
| <60 | 155 | 60.6 |
| BMI (kg/m2) | ||
| 18–24 | 157 | 61.3 |
| <18/>24 | 99 | 38.7 |
| Lung cancer family history | ||
| No | 240 | 93.7 |
| Yes | 16 | 6.3 |
| Smoking history | ||
| No | 169 | 66.0 |
| Yes | 87 | 34.0 |
| Symptom | ||
| No | 155 | 60.5 |
| Yes | 101 | 39.5 |
| Tumor location | ||
| Peripheral | 242 | 94.5 |
| Central | 14 | 5.5 |
| Tumor site | ||
| Right | 164 | 64.1 |
| Left | 92 | 35.9 |
| CEA (ng/mL) | ||
| <5 | 217 | 84.8 |
| ≥5 | 39 | 15.2 |
| Solid ingredients | ||
| Solid | 145 | 56.6 |
| GGN/mGGN | 111 | 43.4 |
| Surgical approach | ||
| Lobectomy | 245 | 95.7 |
| Segmentectomy | 11 | 4.3 |
| Tumor diameter (cm) | ||
| >2.5 | 188 | 73.4 |
| ≤2.5 | 68 | 26.6 |
| Pathology type | ||
| Adenocarcinoma | 201 | 78.6 |
| Squamous carcinoma | 15 | 5.8 |
| Large cell carcinoma | 15 | 5.8 |
| Mixed type | 25 | 9.8 |
| Tumor-stroma ratio | ||
| <30% | 80 | 31.2 |
| 30–50% | 152 | 59.4 |
| >50% | 24 | 9.4 |
| Differentiation | ||
| Poorly | 45 | 17.6 |
| Moderately | 160 | 62.5 |
| Well | 51 | 19.9 |
| Vascular invasion | ||
| Yes | 2 | 0.8 |
| No | 254 | 99.2 |
| Nerve invasion | ||
| Yes | 3 | 1.2 |
| No | 253 | 98.8 |
| Mucus composition | ||
| Yes | 242 | 94.5 |
| No | 14 | 5.5 |
| STAS subtypes | ||
| Positive | 115 | 44.9 |
| Single cell invasion | 9 | 3.5 |
| Micropapillary cluster | 45 | 17.6 |
| Cell nest | 61 | 23.8 |
| Distance of STAS from the main tumor (mm) | ||
| <1 | 64 | 25.0 |
| ≥1 | 51 | 19.9 |
| Alveoli count in STAS stroma | ||
| <3 alveoli | 52 | 20.3 |
| ≥3 alveoli | 63 | 24.6 |
| Number of alveoli involved by STAS | ||
| 1–3 | 45 | 17.6 |
| 4–5 | 12 | 4.7 |
| >5 | 58 | 22.6 |
| Tumor budding | ||
| 0 | 158 | 61.7 |
| 1 | 56 | 21.9 |
| 2 | 23 | 9.0 |
| 3 | 19 | 7.4 |
BMI, body mass index; CEA, carcinoembryonic antigen; GGN, ground-glass nodule; mGGN, mixed ground-glass nodule; STAS, spread through air spaces.
Clinicopathological factors affecting STAS dissemination in stage IA3 NSCLC
Before evaluating the prognostic impact of STAS in stage IA3 NSCLC, we analyzed clinicopathological factors linked to extensive STAS. Patients were classified into restricted or extensive STAS groups based on the number of disseminated tumor cells/clusters and their distance from the primary tumor. Pearson correlation showed significant associations between extensive STAS and a family history of pulmonary malignancies (P=0.042), solid tumors (P=0.02), vascular invasion (P=0.002), tumor budding (P=0.009), and STAS distance >1 mm (P<0.001) (Table 2).
Table 2
| Variables | STAS, n | P value | ||
|---|---|---|---|---|
| Absent | ≤3 alveoli | >3 alveoli | ||
| Sex | 0.93 | |||
| Male | 73 | 40 | 17 | |
| Female | 68 | 40 | 18 | |
| Age (years) | 0.86 | |||
| <60 | 56 | 30 | 15 | |
| ≥60 | 85 | 50 | 20 | |
| BMI (kg/m2) | 0.64 | |||
| 18–24 | 90 | 46 | 21 | |
| <18/>24 | 51 | 34 | 14 | |
| Lung cancer family history | 0.042† | |||
| No | 137 | 72 | 31 | |
| Yes | 4 | 8 | 4 | |
| Smoking history | 0.54 | |||
| No | 97 | 51 | 21 | |
| Yes | 44 | 29 | 14 | |
| Symptom | 0.38 | |||
| No | 85 | 52 | 18 | |
| Yes | 56 | 28 | 17 | |
| Tumor location | 0.44 | |||
| Peripheral | 131 | 77 | 34 | |
| Central | 10 | 3 | 1 | |
| Tumor site | 0.61 | |||
| Right | 89 | 50 | 25 | |
| Left | 52 | 30 | 10 | |
| CEA (ng/mL) | 0.46 | |||
| <5 | 123 | 65 | 29 | |
| ≥5 | 18 | 15 | 6 | |
| Solid ingredients | 0.02 | |||
| Solid | 72 | 27 | 12 | |
| GGN/mGGN | 69 | 53 | 23 | |
| Surgical approach | 0.30 | |||
| Lobectomy | 137 | 76 | 32 | |
| Segmentectomy | 4 | 4 | 3 | |
| Tumor diameter (cm) | 0.95 | |||
| ≤2.5 | 104 | 59 | 25 | |
| >2.5 | 37 | 21 | 10 | |
| Pathology type | 0.15 | |||
| Adenocarcinoma/squamous | 136 | 74 | 31 | |
| Large cell/mucus carcinoma | 5 | 6 | 4 | |
| Tumor budding | 0.009 | |||
| No | 100 | 41 | 17 | |
| Yes | 41 | 39 | 18 | |
| Tumor-stroma ratio | 0.61 | |||
| <30% | 46 | 21 | 13 | |
| 30–50% | 83 | 49 | 20 | |
| >50% | 12 | 10 | 2 | |
| Differentiation | 0.39 | |||
| Poorly | 20 | 17 | 8 | |
| Moderately | 88 | 51 | 21 | |
| Well | 33 | 12 | 6 | |
| Vascular invasion | 0.002† | |||
| No | 141 | 80 | 33 | |
| Yes | 0 | 0 | 2 | |
| Ki67 | 0.60 | |||
| <1 | 137 | 78 | 35 | |
| ≥1 | 4 | 2 | 0 | |
| Micropapillary | 0.85 | |||
| No | 113 | 60 | 28 | |
| Yes | 28 | 20 | 7 | |
| Mucus composition | 0.18 | |||
| No | 133 | 75 | 31 | |
| Yes | 5 | 5 | 4 | |
| Tumor necrosis | 0.36 | |||
| No | 122 | 74 | 30 | |
| Yes | 19 | 6 | 5 | |
| Distance of STAS from the main tumor (mm) | <0.001† | |||
| Negative | 141 | 0 | 0 | |
| ≤1 | 0 | 74 | 12 | |
| >1 | 0 | 6 | 23 | |
| Single cell invasion | 0.57 | |||
| Negative | 141 | 73 | 33 | |
| Positive | 0 | 7 | 2 | |
| Cell nest | 0.81 | |||
| Negative | 141 | 37 | 17 | |
| Positive | 0 | 43 | 18 | |
| Micropapillary clusters | 0.58 | |||
| Negative | 141 | 50 | 20 | |
| Positive | 0 | 30 | 15 | |
†, Fisher’s exact test was used. BMI, body mass index; CEA, carcinoembryonic antigen; GGN, ground-glass nodule; mGGN, mixed ground-glass nodule; STAS, spread through air spaces.
Correlation between clinicopathological data and OS
Cox regression identified independent risk factors for OS. Univariate analysis showed that smoking history (P<0.001), CEA ≥5 ng/mL (P=0.008), solid components (P=0.02), tumor-stroma ratio (P<0.001), STAS cell nests (P=0.005), and STAS in ≥5 alveoli (P=0.006) affected prognosis in stage IA3 lung adenocarcinoma. Multivariate analysis confirmed smoking history (P=0.004), CEA ≥5 ng/mL (P=0.01), tumor-stroma ratio (P=0.001), and STAS cell nests (P=0.005) as independent risk factors for OS (Table 3).
Table 3
| Variables | Univariate analysis | Multiplicity | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex (vs. male) | 0.09 | – | |||
| Female | 2.32 (0.87–6.20) | – | |||
| Age (years) (vs. <60) | 0.08 | – | |||
| ≥60 | 3.07 (0.89–10.62) | – | |||
| BMI (kg/m2) (vs. 18–24) | 0.35 | – | |||
| <18/>24 | 0.92 (0.77–1.10) | – | |||
| Lung cancer family history (vs. no) | 0.79 | – | |||
| Yes | 1.32 (0.18–9.99) | – | |||
| Smoking history (vs. no) | <0.001 | 0.004 | |||
| Yes | 5.07 (1.90–13.54) | 4.51 (1.62–12.55) | |||
| Symptom (vs. no) | 0.84 | – | |||
| Yes | 0.91 (0.35–2.34) | – | |||
| Tumor location (vs. peripheral) | 0.72 | – | |||
| Central | 0.69 (0.09–5.18) | – | |||
| Tumor site (vs. right) | 0.42 | – | |||
| Left | 0.65 (0.23–1.83) | – | |||
| CEA (ng/mL) (vs. <5) | 0.008 | 0.01 | |||
| ≥5 | 3.51 (1.39–8.85) | 3.41 (1.34–8.69) | |||
| Solid ingredients (vs. solid) | 0.02 | – | |||
| GGN/mGGN | 5.14 (1.18–22.37) | – | |||
| Surgical approach (vs. lobectomy) | 0.53 | – | |||
| Segmentectomy | 1.93 (0.25–14.68) | – | |||
| Tumor diameter (cm) (vs. ≤2.5) | 0.32 | – | |||
| >2.5 | 1.62 (0.63–4.19) | – | |||
| Pathology type (vs. adenocarcinoma/squamous carcinoma) | 0.15 | – | |||
| Large cell/mucus carcinoma | 2.51 (0.73–8.67) | – | |||
| Tumor budding (vs. no) | 0.45 | – | |||
| Yes | 1.42 (0.56–3.61) | – | |||
| Tumor-stroma ratio (vs. <30%) | <0.001 | 0.001 | |||
| 30–50% | 2.89 (0.64–13.06) | 1.94 (0.41–9.04) | |||
| >50% | 13.01 (2.51–67.29) | 5.57 (1.93–16.10) | |||
| Differentiation (vs. poorly) | 0.058 | – | |||
| Moderately | 0.49 (0.18–1.34) | – | |||
| Well | 0.46 (0.21–1.03) | – | |||
| Micropapillary (vs. no) | 0.32 | – | |||
| Yes | 1.64 (0.62–4.38) | – | |||
| Vascular invasion (vs. no) | 0.81 | – | |||
| Yes | 0.049 (0.000–3,289) | – | |||
| Ki67 (vs. <1) | 0.41 | – | |||
| ≥1 | 2.33 (0.31–17.49) | – | |||
| STAS (vs. negative) | 0.39 | – | |||
| Positive | 1.51 (0.60–3.82) | – | |||
| Mucus composition (vs. no) | 0.77 | – | |||
| Yes | 0.74 (0.10–5.56) | – | |||
| Single cell invasion (vs. negative) | 0.25 | – | |||
| Positive | 2.39 (0.55–10.41) | – | |||
| Cell nest (vs. negative) | 0.005 | 0.005 | |||
| Positive | 3.85 (1.52–9.75) | 3.92 (1.52–10.12) | |||
| Micropapillary clusters (vs. negative) | 0.23 | – | |||
| Positive | 0.03 (0–8.10) | – | |||
| Distance of STAS from the main tumor (mm) (vs. <1) | 0.52 | – | |||
| ≥1 | 1.49 (0.43–5.16) | – | |||
| Alveoli count in STAS stroma (vs. <3) | 0.14 | – | |||
| ≥3 | 2.03 (0.79–5.25) | – | |||
| Number of alveoli involved by STAS (vs. <5) | 0.006 | – | |||
| ≥5 | 3.69 (1.45–9.34) | – | |||
| Tumor necrosis (vs. <1%) | 0.62 | – | |||
| ≥1% | 0.69 (0.16–3.00) | – | |||
BMI, body mass index; CEA, carcinoembryonic antigen; CI, confidence interval; GGN, ground-glass nodule; HR, hazard ratio; mGGN, mixed ground-glass nodule; STAS, spread through air spaces.
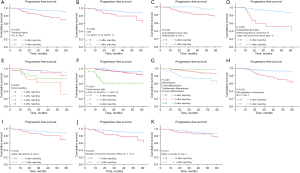
Kaplan-Meier curves revealed significant OS differences for smoking history (Figure 3A), CEA ≥5 ng/mL (Figure 3B), solid components (Figure 3C), tumor-stroma ratio (Figure 3D), STAS cell nests (Figure 3E), and STAS ≥5 alveoli (Figure 3F). STAS presence was not significant for OS (P=0.38, Figure 3G).

Correlation between clinicopathological data and PFS
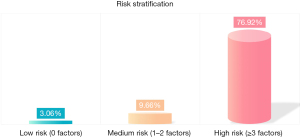
Univariate analysis identified smoking history (P=0.001), CEA ≥5 ng/mL (P=0.006), solid components (P=0.003), histopathological type (P<0.001), tumor budding (P=0.004), tumor-stroma ratio (P<0.001), tumor differentiation (P<0.001), micropapillary components (P<0.001), STAS cell nests (P=0.02), and STAS in ≥5 alveoli (P=0.001) as risk factors for PFS in stage IA3 lung adenocarcinoma. Multivariate analysis confirmed smoking history (P=0.001), STAS cell nests (P=0.01), histopathological type (P=0.001), tumor-stroma ratio (P=0.004), and micropapillary components (P<0.001) as independent risk factors (Table 4). The 256 patients were divided into low-, medium-, and high-risk groups based on independent risk factors. The low-risk group (0 factors) had a 3.06% positive rate (3/98), the medium-risk group (1–2 factors) had 9.66% (14/145), and the high-risk group (3+ factors) had 76.92% (10/13) (Figure 4).
Table 4
| Variables | Univariate analysis | Multiplicity | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex (vs. male) | 0.02 | – | |||
| Female | 0.38 (0.17–0.86) | – | |||
| Age (years) (vs. <60) | 0.58 | – | |||
| ≥60 | 1.25 (0.56–2.79) | – | |||
| BMI (kg/m2) (vs. 18–24) | 0.21 | – | |||
| <18/>24 | 0.58 (0.24–1.37) | – | |||
| Lung cancer family history (vs. no) | 0.41 | – | |||
| Yes | 0.046 (0.000–71.33) | – | |||
| Smoking history (vs. no) | 0.001 | 0.001 | |||
| Yes | 3.58 (1.66–7.74) | 4.56 (1.92–10.82) | |||
| Symptom (vs. no) | 0.41 | – | |||
| Yes | 0.71 (0.32–1.59) | – | |||
| Tumor location (vs. peripheral) | 0.99 | – | |||
| Central | 0.99 (0.23–4.20) | – | |||
| Tumor site (vs. right) | 0.17 | – | |||
| Left | 1.71 (0.80–3.63) | – | |||
| CEA (ng/mL) (vs. <5) | 0.006 | – | |||
| ≥5 | 2.90 (1.36–6.20) | – | |||
| Solid ingredients (vs. solid) | 0.003 | – | |||
| GGN/mGGN | 8.92 (2.11–37.68) | – | |||
| Surgical approach (vs. lobectomy) | 0.92 | – | |||
| Segmentectomy | 1.12 (0.15–8.31) | – | |||
| Tumor diameter (cm) (vs. ≤2.5) | 0.12 | – | |||
| >2.5 | 1.83 (0.85–3.95) | – | |||
| Pathology type (vs. adenocarcinoma/squamous carcinoma) | <0.001 | 0.001 | |||
| Large cell/mucus carcinoma | 6.81 (2.97–15.59) | 5.31 (2.02–13.92) | |||
| Tumor budding (vs. no) | 0.004 | – | |||
| Yes | 3.17 (1.45–6.95) | – | |||
| Tumor-stroma ratio (vs. <30%) | <0.001 | 0.004 | |||
| 30–50% | 1.51 (0.54–4.19) | 1.94 (0.64–5.92) | |||
| >50% | 3.40 (1.72–6.70) | 2.66 (1.35–5.21) | |||
| Differentiation (vs. poorly) | <0.001 | – | |||
| Moderately | 0.29 (0.13–0.63) | – | |||
| Well | 0.286 (0.15–0.56) | – | |||
| Micropapillary (vs. no) | <0.001 | <0.001 | |||
| Yes | 3.94 (1.85–8.39) | 4.84 (2.10–11.13) | |||
| Vascular invasion (vs. no) | 0.77 | – | |||
| Yes | 0.05 (0.00–1,388,023) | – | |||
| Ki67 (vs. <1) | 0.13 | – | |||
| ≥1 | 3.02 (0.71–12.82) | – | |||
| STAS (vs. negative) | 0.11 | – | |||
| Positive | 1.88 (0.87–4.06) | – | |||
| Mucus composition (vs. no) | 0.08 | – | |||
| Yes | 2.63 (0.91–7.63) | – | |||
| Single cell invasion (vs. negative) | 0.59 | – | |||
| Positive | 1.49 (0.35–6.33) | – | |||
| Cell nest (vs. negative) | 0.02 | 0.01 | |||
| Positive | 2.36 (1.09–5.10) | 2.88 (1.28–6.48) | |||
| Micropapillary clusters (vs. negative) | 0.48 | – | |||
| Positive | 1.35 (0.59–3.09) | – | |||
| Distance of STAS from the main tumor (mm) (vs. <1) | 0.42 | – | |||
| ≥1 | 1.23 (0.74–2.06) | – | |||
| Alveoli count in STAS stroma (vs. <3) | 0.82 | – | |||
| ≥3 | 1.10 (0.47–2.61) | – | |||
| Number of alveoli involved by STAS (vs. <5) | 0.001 | – | |||
| ≥5 | 3.45 (1.62–7.35) | – | |||
| Tumor necrosis (vs. <1%) | 0.55 | – | |||
| ≥1% | 1.34 (0.51–3.56) | – | |||
BMI, body mass index; CEA, carcinoembryonic antigen; CI, confidence interval; GGN, ground-glass nodule; HR, hazard ratio; mGGN, mixed ground-glass nodule; STAS, spread through air spaces.
Kaplan-Meier survival curves revealed significant differences in PFS based on smoking history (Figure 5A), CEA ≥5 ng/mL (Figure 5B), solid components (Figure 5C), histopathological type (Figure 5D), tumor budding (Figure 5E), tumor-stroma ratio (Figure 5F), tumor differentiation (Figure 5G), micropapillary components (Figure 5H), STAS cell nests (Figure 5I), and STAS involving ≥5 alveoli (Figure 5J). STAS presence did not show a statistically significant difference in PFS (P=0.10, Figure 5K).
Recurrence patterns
Among the 256 patients with stage IA3 NSCLC, 27 patients (10.5%) experienced recurrence within the 5-year follow-up period. The recurrence patterns were as follows:
- Local recurrence: 8 patients (3.1%) had recurrence in the ipsilateral lobe, bronchial stump, or regional lymph nodes.
- Distant metastasis: 19 patients (7.4%) developed metastasis to distant organs, including the contralateral lung (6 patients, 2.3%), brain (5 patients, 2.0%), bones (4 patients, 1.6%), liver (3 patients, 1.2%), and adrenal glands (1 patient, 0.4%).
Discussion
This study offers a novel and comprehensive assessment of STAS in pathological stage IA3 NSCLC, being one of the first to systematically analyze its clinicopathological implications. Our results show that specific STAS subtypes, especially cell nest clusters, significantly impact OS and PFS, independent of STAS presence. The STAS positivity rate in stage IA3 NSCLC patients was 44.9%. By identifying key risk factors like a high tumor-stroma ratio, smoking history, and micropapillary components, this research underscores the need for a multidimensional analysis of STAS to improve prognosis and personalize management for high-risk patients. This approach addresses a gap in the TNM staging system, which does not adequately consider these histopathological features.
Our study also investigated factors influencing STAS diffusion distance. Variations in fixation protocols between laboratories may lead to variable alveolar expansion, potentially introducing errors during section preparation. However, observing the number of alveoli affected by airspace spread offers a more objective assessment. Following the classification scheme by Warth et al., we categorized STAS diffusion distances as restrictive or extensive (12). Our correlation analysis revealed that a family history of lung cancer affects STAS diffusion distance, possibly related to specific lung cancer susceptibility genes (22-24). Additionally, we observed an association between tumor solid component proportion and extensive STAS, consistent with prior findings by Yagi et al. (25). Our study also indicates that STAS greater than 1 millimeter is related to extensive STAS, suggesting a correlation between the two STAS assessment methods. Evaluating alveolar numbers may exclude some subjective factors, making the measurement method more objective.
Moreover, we discovered correlations between vascular tumor emboli and STAS diffusion distance, with two samples containing vascular tumor emboli having STAS diffusion involving more than three alveoli. Tumor budding was also associated with extensive STAS, indicating that STAS, tumor budding, and vascular tumor emboli may represent morphological translations of the same epithelial-mesenchymal transition phenomenon. Previous studies have linked the presence of STAS to reduced E-cadherin expression in adenocarcinomas (26). Tumor emboli may be associated with epithelial-mesenchymal transition processes (27). During tumor budding, E-cadherin and other proteins are involved in the epithelial-mesenchymal transition process (28). However, our study primarily included stage IA3 NSCLC patients, and vascular tumor emboli are not commonly observed in early-stage patients. Thus, we cannot draw definitive conclusions about the relationship between vascular tumor emboli and distant alveolar spread, necessitating larger, multicenter studies for confirmation.
Contrary to previous research, we found that STAS positivity and diffusion distance are not risk factors affecting OS and PFS in stage IA3 NSCLC patients. We propose that the impact of STAS on early-stage patients’ prognosis may be related to specific STAS subtypes. Further analysis of the three STAS subtypes corroborated this hypothesis. We found that among the three subtypes, cell nest STAS is an independent risk factor for OS and PFS in stage IA3 NSCLC patients. In 2021, Xie et al. demonstrated that micropapillary STAS and cell nest STAS are independent prognostic indicators in NSCLC patients (15). Cell nest STAS is more common in solid-type NSCLC patients (33.6% solid vs. 10.9% ground-glass component), consistent with prior research (15). In 2017, Lu et al. found that all STAS lesions in squamous cell carcinoma were composed of cell nests and were independent prognostic indicators (29). Combining literature with our findings, cell nest STAS is an independent risk factor for poor prognosis in stage IA3 NSCLC patients and warrants particular attention when assessing prognosis and considering early intervention.
Survival analysis revealed that the number of alveoli involved in airspace spread also affects OS and PFS. Univariate analysis showed that involvement of more than five alveoli has a statistically significant impact on OS and PFS, though the difference was not apparent in multivariate analysis. Warth et al. classified STAS into restrictive and extensive types based on measured distances or the number of intervening alveoli (12). Uruga et al. categorized STAS into no STAS, low STAS, and high STAS types by counting cellular colonies under microscopy (30). The former indicates that different pathological types of STAS exhibit varying diffusion patterns, but the impact on survival prognosis lacks statistical significance, a finding consistent with subsequent research (29). The latter study indicates that high STAS is a significant factor for poor survival prognosis, commonly seen in solid invasive adenocarcinomas with pleural and lymphovascular invasion, and tumors ≥10 mm. High STAS is positively correlated with recurrence rates, aligning with our univariate analysis results. Therefore, in early-stage NSCLC, particularly stage IA3, focusing solely on STAS is insufficient; emphasis should be placed on the STAS cell nest subtype and the number of affected alveoli.
Furthermore, we found that the tumor-stroma ratio is also an independent risk factor for OS and PFS in stage IA3 NSCLC patients. Previous studies have shown that a tumor-stroma ratio greater than 50% correlates with reduced OS in NSCLC patients (20,31,32). Our findings are consistent with these conclusions. A high tumor-stroma ratio in lung cancer patients typically indicates poorer prognosis, potentially due to the influence on tumor cell growth and dissemination, and deterioration of the tumor microenvironment affecting treatment efficacy. Thus, reducing the tumor-stroma ratio may be a crucial strategy for improving lung cancer patient outcomes. Related research suggests that cancer-associated fibroblasts, as a major component of the tumor stroma, promote tumor proliferation, invasion, and metastasis by producing and secreting various cytokines and growth factors, as well as inducing angiogenesis. Additionally, extracellular matrix remodeling caused by proteolysis also contributes to NSCLC metastasis, with over 70% of patient deaths attributed to extracellular matrix remodeling (33-36).
Univariate and multivariate analyses revealed a strong link between smoking history and poorer prognosis in early-stage NSCLC. Smokers had significantly lower OS and PFS than non-smokers, consistent with prior research (37,38). Smoking adversely affects lung cancer prognosis by increasing tumor size, differentiation, inflammatory response, and impairing immune function, promoting tumor progression and metastasis. While smoking has limited impact on long-term survival in squamous cell carcinoma, differences in epidermal growth factor receptor (EGFR) levels may influence the prognosis of adenocarcinoma patients (39-41).
Our study also revealed that tumor budding is significantly associated with extensive STAS, affecting OS and PFS. Tumor budding is a manifestation of epithelial-mesenchymal transition and is confirmed as a poor prognostic factor in various tumors, related to invasive behavior, local recurrence, distant metastasis, and lower survival rates (19). Poor tumor differentiation correlates negatively with PFS but has a limited impact on OS, suggesting that differentiation affects recurrence but not survival. Elevated CEA levels are associated with poorer OS and are a risk factor for brain metastases (42,43). Our analysis of CEA ≥5 ng/mL also affects PFS in univariate analysis, and multivariate analysis confirms CEA as an effective prognostic marker for OS in NSCLC patients, aligning with guidelines recommending regular CEA monitoring. Research by Asamura et al. indicated that a consolidation-to-tumor ratio (CTR) value >0.5 suggests high invasiveness, while CTR values <0.5 in ground-glass nodules are associated with better prognosis (44,45). Our analysis also showed that stage IA3 NSCLC patients with a predominantly solid component had poorer OS and PFS, and solid tumors had higher cell nest STAS proportions. The presence of micropapillary components in solid tumors is a risk factor for recurrence, even in early postoperative patients, and is associated with poor prognosis (46-48).
The need for postoperative adjuvant therapy in stage IA3 NSCLC remains debated. Hamada et al. suggested that adjuvant chemotherapy with tegafur-uracil might improve survival in stage IA NSCLC, particularly in high-risk groups (7). Kunitoh’s JCOG 0707 phase III study also highlighted the benefits of adjuvant chemotherapy in patients with completely resected, node-negative NSCLC, further emphasizing its value in selecting high-risk patients for treatment (6). Yamamoto et al. explored adjuvant therapy in stage IA (tumor >2 cm) to III NSCLC, indicating its potential role in specific high-risk populations, including stage IA3 cases (5). These findings suggest that some stage IA3 NSCLC patients could benefit from adjuvant therapy, making it essential to identify those at high risk of recurrence and poor prognosis. Smokers (P=0.001), STAS cell nests (P=0.01), histopathological type (P=0.001), tumor-stroma ratio (P=0.004), and micropapillary components (P<0.001) were independent risk factors for postoperative PFS in stage IA3 NSCLC. The recurrence rate in the high-risk group, defined as having three or more positive factors, reached 76.92%. High recurrence rates in high-risk groups necessitate enhanced monitoring and individualized treatment strategies to improve outcomes for these patients.
Despite the unavoidable selection bias inherent in retrospective designs, we enhanced the reliability of the study through dual reading, consensus meetings, and comprehensive assessment of each case using at least five pathological slides. Additionally, the inclusion of a multi-center study improves the applicability of our research. Most findings align with existing literature. A few years ago, limited economic resources hindered the implementation of routine next-generation sequencing (NGS), resulting in incomplete EGFR data. Future research will further explore these results through genomics and proteomics.
Conclusions
In summary, this study delineated the cell nest STAS subtype, tumor-stroma ratio, and smoking history as independent prognostic factors significantly influencing PFS and OS in stage IA3 NSCLC patients. It represented the first comprehensive exploration of the prognostic ramifications of diverse STAS evaluation models within this population, yielding novel insights for risk stratification. A meticulous examination of STAS subtypes and histopathological heterogeneity, coupled with the identification of high-risk patients based on prognostic indicators, is imperative for refining prognostic evaluations and optimizing clinical decision-making in pathological stage IA3 NSCLC. Future studies should validate STAS subtypes’ prognostic significance, explore molecular pathways (e.g., epithelial-mesenchymal transition, tumor microenvironment), and evaluate adjuvant therapy in high-risk stage IA3 NSCLC to refine risk stratification and improve outcomes.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2209/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2209/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2209/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2209/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by ethics committee board of Fujian Medical University Union Hospital (No. 2024KY147). All participating institutions were informed and agreed the study. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Expert Consensus Panel. The American Association for Thoracic Surgery (AATS) 2023 Expert Consensus Document: Staging and multidisciplinary management of patients with early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg 2023;166:637-54. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Yamamoto H, Soh J, Okumura N, et al. Randomized phase II study of daily versus alternate-day administrations of S-1 for the elderly patients with completely resected pathological stage IA (tumor diameter > 2 cm)-IIIA of non-small cell lung cancer: Setouchi Lung Cancer Group Study 1201. PLoS One 2023;18:e0285273. [Crossref] [PubMed]
- Kunitoh H, Tsuboi M, Wakabayashi M, et al. A phase III study of adjuvant chemotherapy in patients with completely resected, node-negative non-small cell lung cancer (JCOG 0707). JTCVS Open 2020;4:90-102. [Crossref] [PubMed]
- Hamada C, Tsuboi M, Ohta M, et al. Effect of postoperative adjuvant chemotherapy with tegafur-uracil on survival in patients with stage IA non-small cell lung cancer: an exploratory analysis from a meta-analysis of six randomized controlled trials. J Thorac Oncol 2009;4:1511-6. [Crossref] [PubMed]
- Kadota K, Nitadori JI, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Dagher S, Sulaiman A, Bayle-Bleuez S, et al. Spread Through Air Spaces (STAS) Is an Independent Prognostic Factor in Resected Lung Squamous Cell Carcinoma. Cancers (Basel) 2022;14:2281. [Crossref] [PubMed]
- Yokoyama S, Murakami T, Tao H, et al. Tumor Spread Through Air Spaces Identifies a Distinct Subgroup With Poor Prognosis in Surgically Resected Lung Pleomorphic Carcinoma. Chest 2018;154:838-47. [Crossref] [PubMed]
- Xu X, Shen W, Wang D, et al. Clinical features and prognosis of resectable pulmonary primary invasive mucinous adenocarcinoma. Transl Lung Cancer Res 2022;11:420-31. [Crossref] [PubMed]
- Warth A, Muley T, Kossakowski CA, et al. Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. Am J Surg Pathol 2015;39:793-801. [Crossref] [PubMed]
- Jia M, Yu S, Gao H, et al. Spread Through Air Spaces (STAS) in Lung Cancer: A Multiple-Perspective and Update Review. Cancer Manag Res 2020;12:2743-52. [Crossref] [PubMed]
- Dai C, Xie H, Su H, et al. Tumor Spread through Air Spaces Affects the Recurrence and Overall Survival in Patients with Lung Adenocarcinoma >2 to 3 cm. J Thorac Oncol 2017;12:1052-60. [Crossref] [PubMed]
- Xie H, Su H, Zhu E, et al. Morphological Subtypes of Tumor Spread Through Air Spaces in Non-Small Cell Lung Cancer: Prognostic Heterogeneity and Its Underlying Mechanism. Front Oncol 2021;11:608353. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Sorensen AG, Patel S, Harmath C, et al. Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol 2001;19:551-7. [Crossref] [PubMed]
- Lugli A, Zlobec I, Berger MD, et al. Tumour budding in solid cancers. Nat Rev Clin Oncol 2021;18:101-15. [Crossref] [PubMed]
- Xi KX, Wen YS, Zhu CM, et al. Tumor-stroma ratio (TSR) in non-small cell lung cancer (NSCLC) patients after lung resection is a prognostic factor for survival. J Thorac Dis 2017;9:4017-26. [Crossref] [PubMed]
- Blaauwgeers H, Flieder D, Warth A, et al. A Prospective Study of Loose Tissue Fragments in Non-Small Cell Lung Cancer Resection Specimens: An Alternative View to "Spread Through Air Spaces". Am J Surg Pathol 2017;41:1226-30. [Crossref] [PubMed]
- Matakidou A, Eisen T, Houlston RS. Systematic review of the relationship between family history and lung cancer risk. Br J Cancer 2005;93:825-33. [Crossref] [PubMed]
- LoPiccolo J, Gusev A, Christiani DC, et al. Lung cancer in patients who have never smoked - an emerging disease. Nat Rev Clin Oncol 2024;21:121-46. [Crossref] [PubMed]
- Cannon-Albright LA, Carr SR, Akerley W. Population-Based Relative Risks for Lung Cancer Based on Complete Family History of Lung Cancer. J Thorac Oncol 2019;14:1184-91. [Crossref] [PubMed]
- Yagi Y, Aly RG, Tabata K, et al. Three-Dimensional Histologic, Immunohistochemical, and Multiplex Immunofluorescence Analyses of Dynamic Vessel Co-Option of Spread Through Air Spaces in Lung Adenocarcinoma. J Thorac Oncol 2020;15:589-600. [Crossref] [PubMed]
- Ikeda T, Kadota K, Yoshida C, et al. The epithelial-mesenchymal transition phenotype is associated with the frequency of tumor spread through air spaces (STAS) and a High risk of recurrence after resection of lung carcinoma. Lung Cancer 2021;153:49-55. [Crossref] [PubMed]
- Takemoto A, Okitaka M, Takagi S, et al. A critical role of platelet TGF-β release in podoplanin-mediated tumour invasion and metastasis. Sci Rep 2017;7:42186. [Crossref] [PubMed]
- Taira T, Ishii G, Nagai K, et al. Characterization of the immunophenotype of the tumor budding and its prognostic implications in squamous cell carcinoma of the lung. Lung Cancer 2012;76:423-30. [Crossref] [PubMed]
- Lu S, Tan KS, Kadota K, et al. Spread through Air Spaces (STAS) Is an Independent Predictor of Recurrence and Lung Cancer-Specific Death in Squamous Cell Carcinoma. J Thorac Oncol 2017;12:223-34. [Crossref] [PubMed]
- Uruga H, Fujii T, Fujimori S, et al. Semiquantitative Assessment of Tumor Spread through Air Spaces (STAS) in Early-Stage Lung Adenocarcinomas. J Thorac Oncol 2017;12:1046-51. [Crossref] [PubMed]
- Wang Z, Liu H, Zhao R, et al. Tumor-stroma ratio is an independent prognostic factor of non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2013;16:191-6. [Crossref] [PubMed]
- Zhang T, Xu J, Shen H, et al. Tumor-stroma ratio is an independent predictor for survival in NSCLC. Int J Clin Exp Pathol 2015;8:11348-55.
- Lee SS, Cheah YK. The Interplay between MicroRNAs and Cellular Components of Tumour Microenvironment (TME) on Non-Small-Cell Lung Cancer (NSCLC) Progression. J Immunol Res 2019;2019:3046379. [Crossref] [PubMed]
- Sung PJ, Rama N, Imbach J, et al. Cancer-Associated Fibroblasts Produce Netrin-1 to Control Cancer Cell Plasticity. Cancer Res 2019;79:3651-61. [Crossref] [PubMed]
- Wood SL, Pernemalm M, Crosbie PA, et al. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev 2014;40:558-66. [Crossref] [PubMed]
- Zhang X, Ma H, Zhang L, et al. Predictive Role of Tumor-Stroma Ratio for Survival of Patients With Non-Small Cell Lung Cancer: A Meta-Analysis. Pathol Oncol Res 2021;27:1610021. [Crossref] [PubMed]
- Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 2010;340:b5569. [Crossref] [PubMed]
- Hung JJ, Jeng WJ, Hsu WH, et al. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol 2012;7:1115-23. [Crossref] [PubMed]
- Kawai H, Tada A, Kawahara M, et al. Smoking history before surgery and prognosis in patients with stage IA non-small-cell lung cancer--a multicenter study. Lung Cancer 2005;49:63-70. [Crossref] [PubMed]
- Takamori S, Shimokawa M, Matsubara T, et al. Prognostic Impact of Smoking Period in Patients with Surgically Resected Non-small Cell Lung Cancer. Ann Surg Oncol 2021;28:685-94. [Crossref] [PubMed]
- Poullis M, McShane J, Shaw M, et al. Smoking status at diagnosis and histology type as determinants of long-term outcomes of lung cancer patients. Eur J Cardiothorac Surg 2013;43:919-24. [Crossref] [PubMed]
- Jiang ZF, Wang M, Xu JL. Thymidine kinase 1 combined with CEA, CYFRA21-1 and NSE improved its diagnostic value for lung cancer. Life Sci 2018;194:1-6. [Crossref] [PubMed]
- Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012;76:138-43. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Nakao M, Oikado K, Sato Y, et al. Prognostic Stratification According to Size and Dominance of Radiologic Solid Component in Clinical Stage IA Lung Adenocarcinoma. JTO Clin Res Rep 2022;3:100279. [Crossref] [PubMed]
- Xu SJ, Tu JH, Chen H, et al. A Multi-institutional Analysis of the Combined Effect of Micropapillary Component and Consolidation-to-Tumor Ratio >0.5 on the Prognosis of Pathological, Stage IA3, Lung Adenocarcinoma. Ann Surg Oncol 2023;30:5843-53. [Crossref] [PubMed]
- Watanabe K, Sakamaki K, Ito H, et al. Impact of the micropapillary component on the timing of recurrence in patients with resected lung adenocarcinoma. Eur J Cardiothorac Surg 2020;58:1010-8. [Crossref] [PubMed]
- Zheng Y, Ju S, Huang R, et al. Lymph node metastasis risk factors in clinical stage IA3 lung adenocarcinoma. J Cancer Res Ther 2023;19:34-8. [Crossref] [PubMed]


