
Impact of perineural invasion in thoracic esophageal squamous cell carcinoma—a retrospective study with long-term follow-up outcomes
Highlight box
Key findings
• Perineural invasion (PNI) demonstrates a robust correlation with lymphovascular invasion (LVI) and acts as an independent predictor of disease-free survival (DFS) in resectable esophageal squamous cell carcinoma (ESCC) patients. Postoperative adjuvant therapy is not beneficial in ESCC patients.
What is known and what is new?
• The clinical significance of PNI is currently unclear.
• For patients with PNI-negative ESCC who had undergone R0 resection, postoperative adjuvant therapy did not provide a significant survival benefit for patients.
What is the implication, and what should change now?
• Further research is warranted to validate our findings and extend them to a larger and more diverse patient population, thereby enabling the development of more personalized and effective treatment strategies.
Introduction
Esophageal cancer (EC) ranks among the top ten causes of cancer-related mortality in China (1). Globally, there are approximately 604,000 new cases and 544,000 deaths from EC each year, accounting for the sixth-highest number of new deaths from malignant tumors (2). With the widespread adoption of standardized treatments for EC, the overall survival (OS) for advanced EC patients has improved (3). Nevertheless, the five-year OS rate remains below 50% (4,5). Among the factors affecting survival in EC, lymph node metastasis is the most important. However, even in the negative lymph node metastases EC patients, recurrence or metastasis still occurs in more than 40% of the patients (6). Therefore, there is a need to identify more precise biomarkers to guide therapeutic strategy and clinical management, thereby improving patients’ quality of life and OS.
Even among patients with similar pathology tumor-regional lymph node-metastasis (pTNM), there may be variations in OS (7). Lymphovascular invasion (LVI) and perineural invasion (PNI), as prognostic pathological factors, result in poorer prognosis, although they are not part of the pTNM-stage system (8,9). Previous study has indicated that LVI and PNI are risk factors for poor prognosis (10). And the majority of studies demonstrated that LVI is an independent risk factor for tumors (11-14), but whether PNI can act as an independent prognostic factor for EC patients remains controversial (15,16). This controversy may stem from relatively small sample sizes in most studies and differences in diagnostic criteria of PNI, leading to discrepancies in conclusions. Recently, a large sample study has disputed the prognostic role of PNI (17). Therefore, to address this controversial topic, we aimed to clarify the effect of PNI in esophageal squamous cell carcinoma (ESCC) patients.
This study utilized a large-volume EC diagnosis and treatment center database to verify the association between PNI and other clinicopathological characteristics. It evaluated the impact of PNI on the survival of ESCC patients and elucidated the prognostic value of PNI for ESCC patients. Understanding these histological factors not only enhances the accuracy of disease staging but also guides the risk stratification of adjuvant therapy selection. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2043/rc).
Methods
Data collection
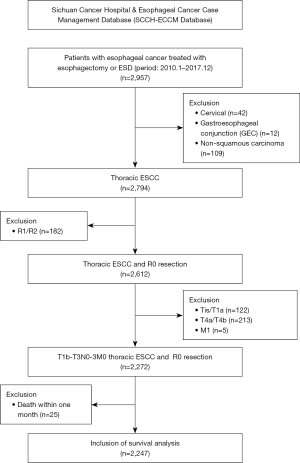
This study retrospectively reviewed the data of 2,957 patients who underwent esophagectomy at the Sichuan Cancer Hospital-Esophageal Cancer Case Management Database (SCCH-ECCM Database) from January 2010 to December 2017. Patients who underwent esophagectomy for ESCC and had postoperative pathologic stage of pT1b–T3N0–3M0 were included in this study. Patients who received preoperative treatment, had incomplete follow-up records and died within one month postoperatively were excluded. There were 2,247 patients included ultimately (Figure 1). Patients’ general information, pathological characteristics, surgery-related details, perioperative treatments, and long-term survival data were collected. The study was approved by the Ethics Committee of Sichuan Cancer Hospital (No. SCCHEC-02-2022-050). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Consent was waived by the Ethics Committee due to the retrospective nature of the study.
Preoperative assessment and treatment
Preoperative assessment encompasses nutritional evaluation, imaging examinations, and evaluation of comorbidities. Patients undergoing surgery and comprehensive treatment should possess a favorable performance status (PS) score. Surgical approaches consist of the McKeown esophagectomy, Ivor-Lewis esophagectomy, and Sweet esophagectomy. Surgical procedures comprise minimally invasive esophagectomy (MIE), open surgery and hybrid surgery. Some patients underwent postoperative adjuvant therapy, comprising chemotherapy, radiotherapy and chemoradiotherapy.
Pathological diagnosis
All pathological specimens underwent staining using the standard hematoxylin and eosin (H&E) staining protocol. Sections were analyzed under a microscope and reviewed by two senior pathologists. PNI was characterized by the infiltration of cancer cells into any layer of the perineurium (including the epineurium, perineurium, and endoneurium) (18). Postoperative pathological staging is adjusted according to the 8th edition of the American Joint Committee on Cancer (AJCC 8th) TNM-stage for ESCC.
Follow-up
After discharge, patients received regular outpatient follow-up or telephone follow-up. During the initial two years post-surgery, outpatient follow-up occurred every three months, incorporating chest and abdominal computed tomography (CT) scan, with contrast-enhanced CT utilized as necessary to detect local recurrence or metastasis. Between the 2nd and 5th years post-surgery, outpatient follow-up took place biannually, transitioning to yearly telephone follow-up thereafter. OS was defined as the duration from the date of surgery until the last follow-up or occurrence of death (whichever transpired first). DFS was defined as the period from the date of surgery until the occurrence of disease recurrence or death from any cause (whichever came first). Recurrence types comprised local and regional lymph node recurrence, while metastasis encompassed distant lymph node and organ metastasis. Tumor recurrence and metastasis were assessed via CT/enhanced CT, magnetic resonance imaging (MRI), ultrasonography, and endoscopic examination.
Statistical analysis
Data analysis was conducted utilizing SPSS 27.0 (SPSS Inc., Chicago, IL, USA) statistical software. Normally distributed continuous variables were expressed as mean ± standard deviation, while skewed distributed continuous variables were represented by median. Count data or categorical variables were displayed as absolute numbers or percentages. Continuous variables were categorized into subgroups based on their median or clinical significance. The t-test were used to compare groups. To compare categorical variables, the Chi-squared test was used. A linear model was used in the multifactorial analysis associated with PNI. Survival analysis was performed utilizing Kaplan-Meier curves, with significance evaluated via the log-rank test. Univariate and multivariate survival analyses were carried out utilizing Cox proportional hazards models. It was statistically significant if two-tailed P values less than 0.05.
Results
Patient characteristics
This study included 2,247 patients with thoracic ESCC, of whom 1,828 (81.4%) were male. The mean age of the patients was 62±8 years. MIE was conducted in 905 patients (40.3%). McKeown esophagectomy was undertaken in 1,580 cases (70.3%), Ivor-Lewis esophagectomy in 632 cases (28.1%). The distribution of tumor location was predominantly in the middle thoracic portion with 1,220 cases (54.3%). The median length of the tumor specimen was 4 cm. Postoperative pathology showed that most patients were moderate or poorly differentiated (82.5%). Pathological results revealed that there were 1,547 cases (68.8%) classified as pT3-stage. Approximately 55.5% of patients (1,247/2,247) exhibited postoperative lymph node-positive metastasis. The detection rates of LVI and PNI were 16.4% and 18.0%, respectively. Postoperatively, 1,143 patients underwent adjuvant therapy (Table 1). Further subgroup analysis indicated that 190 patients with PNI-positive did not receive postoperative adjuvant therapy, the other 214 patients with PNI-positive received postoperative adjuvant therapy. Among the patients with PNI-negative, 914 did not receive postoperative adjuvant therapy, while 929 received postoperative adjuvant therapy.
Table 1
| Variables | Value |
|---|---|
| Total | 2,247 |
| Sex | |
| Male | 1,828 (81.4) |
| Female | 419 (18.6) |
| Age (years) | 62±8 [34–90] |
| Tumor site | |
| Upper thoracic portion | 520 (23.1) |
| Middle thoracic portion | 1,220 (54.3) |
| Lower thoracic portion | 507 (22.6) |
| Differentiation | |
| High | 393 (17.5) |
| Middle | 937 (41.7) |
| Low | 917 (40.8) |
| Tumor lengths (cm) | 4 [0–15] |
| Minimal invasive approach | |
| MIE | 905 (40.3) |
| Hybrid | 220 (9.8) |
| Open | 1,122 (49.9) |
| Surgical approach | |
| McKeown | 1,580 (70.3) |
| Ivor-Lewis | 632 (28.1) |
| Sweet | 35 (1.6) |
| pT-stage | |
| T1 | 201 (8.9) |
| T2 | 499 (22.2) |
| T3 | 1,547 (68.8) |
| pN-stage | |
| N0 | 1,000 (44.5) |
| N1 | 689 (30.7) |
| N2 | 391 (17.4) |
| N3 | 167 (7.4) |
| LVI | |
| Negative | 1,878 (83.6) |
| Positive | 369 (16.4) |
| PNI | |
| Negative | 1,843 (82.0) |
| Positive | 404 (18.0) |
| Postoperative adjuvant therapy | |
| Yes | 1,143 (50.9) |
| No | 1,104 (49.1) |
Data are presented as n (%), mean ± standard deviation [range] or median [range]. LVI, lymphovascular invasion; MIE, minimally invasive esophagectomy; PNI, perineural invasion.
PNI status
PNI was present in 18.0% (404/2,247) of patients. PNI exhibited significant associations with gender (P<0.001), differentiation grade (P<0.001), pT-stage (P<0.001), pN-stage (P<0.001), and LVI (P<0.001). However, it demonstrated no statistical correlation with age (P=0.76), tumor location (P=0.49), recurrence (P=0.07) and metastasis (P=0.50). In the multivariate analysis, PNI retained correlations with gender (P=0.002), differentiation grade (P<0.001), pT-stage (P<0.001), and LVI (P<0.001) (Table 2).
Table 2
| Variables | With PNI (n=404) | Without PNI (n=1,843) | P value | |
|---|---|---|---|---|
| Univariable | Multivariate | |||
| Age (years) | 0.76 | – | ||
| ≤62 | 215 (53.2) | 965 (52.4) | ||
| >62 | 189 (46.8) | 878 (47.6) | ||
| Sex | <0.001* | 0.002* | ||
| Male | 356 (88.1) | 1,472 (79.9) | ||
| Female | 48 (11.9) | 371 (20.1) | ||
| BMI (kg/m2) | 0.33 | – | ||
| <18.5 | 51 (12.6) | 256 (13.9) | ||
| 18.5–24 | 306 (75.7) | 1,331 (72.2) | ||
| >24 | 47 (11.6) | 256 (13.9) | ||
| Tumor site | 0.49 | – | ||
| Upper thoracic portion | 93 (23.0) | 427 (23.2) | ||
| Middle thoracic portion | 211 (52.2) | 1,009 (54.7) | ||
| Lower thoracic portion | 100 (24.8) | 407 (22.1) | ||
| Differentiation | <0.001* | <0.001* | ||
| High | 30 (7.4) | 363 (19.7) | ||
| Middle | 207 (51.2) | 730 (39.6) | ||
| Low | 167 (41.3) | 750 (40.7) | ||
| pT-stage | <0.001* | <0.001* | ||
| T1–2 | 45 (11.1) | 655 (35.5) | ||
| T3 | 359 (88.9) | 1,188 (64.5) | ||
| pN-stage | <0.001* | 0.16 | ||
| N0 | 141 (34.9) | 859 (46.6) | ||
| N1 | 124 (30.7) | 565 (30.7) | ||
| N2 | 101 (25.0) | 290 (15.7) | ||
| N3 | 38 (9.4) | 129 (7.0) | ||
| LVI | <0.001* | <0.001* | ||
| Negative | 284 (70.3) | 1,594 (86.5) | ||
| Positive | 120 (29.7) | 249 (13.5) | ||
| Recurrence | 0.07 | – | ||
| Yes | 62 (15.3) | 302 (16.4) | ||
| No | 342 (84.7) | 1,541 (83.6) | ||
| Metastasis | 0.50 | – | ||
| Yes | 48 (11.9) | 242 (13.1) | ||
| No | 356 (88.1) | 1,601 (86.9) | ||
Data are presented as n (%). *, significant differences. BMI, body mass index; ESCC, esophageal squamous cell carcinoma; LVI, lymphovascular invasion; PNI, perineural invasion.
Survival analysis
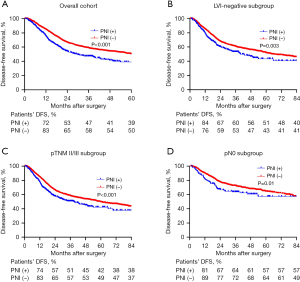
The median follow-up duration in this study was 36.7 months. The 1-year, 3-year, and 5-year OS rates were 87%, 58%, and 40%, respectively. The 1-year, 3-year, and 5-year DFS rates were 81%, 51%, and 40%, respectively. The median OS for patients with PNI-positive was 31 months, whereas for patients with PNI-negative, it was 58 months (P<0.001) (Figure 2A). To explore the effect of PNI in different clinical subgroups of patients, we did further analysis in LVI-negative, pII/III-stage, and pN0 subgroups. The results showed that the OS rate of PNI-positive patients was generally decreased in different subgroups of patients (Figure 2B-2D). Additionally, a significant disparity existed in the median DFS between PNI-negative and positive patients (median OS: 60 vs. 29 months, P<0.001) (Figure 3A). Also, we did subgroup analysis about DFS and got comparable results (Figure 3B-3D).

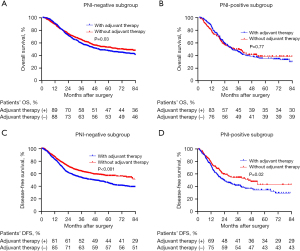
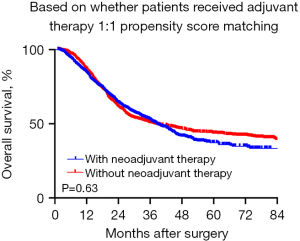
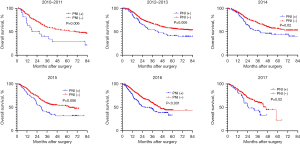
However, in the subgroup analysis of PNI-negative patients, postoperative adjuvant therapy not only failed to confer survival benefits but also resulted in a reduction in OS (median OS: 60 vs. 50 months, P=0.03) (Figure 4A). Conversely, in the survival analysis of PNI-positive patients’ subgroup, no statistically significant difference was observed (median OS: 32 vs. 30 months, P=0.77) (Figure 4B). Concerning DFS, in the survival analysis of PNI-negative patients’ subgroup, the DFS period was significantly shortened among patients with EC who underwent postoperative adjuvant therapy (median DFS: 60 vs. 45 months, P<0.001) (Figure 4C). Interestingly, a similar phenomenon was observed in the subgroup analysis of PNI-positive patients, with a median DFS of 45 months compared to 23 months (P=0.02) (Figure 4D). We performed propensity score matching based on whether patients received postoperative adjuvant therapy. The results indicated that adjuvant therapy did not confer a survival benefit (Figure 5). Furthermore, to assess whether PNI was temporally associated with patient survival outcomes, we conducted a subgroup analysis using year as a stratifying factor. The findings revealed that PNI remained a consistent prognostic factor across different years (Figure 6).
Univariate analysis indicated that OS was correlated with gender (P<0.001), age (P=0.01), BMI (P<0.001), differentiation grade (P<0.001), MIA (P=0.02), surgical approach (P=0.004), LVI (P<0.001), PNI (P<0.001), pT-stage (P<0.001), and pN-stage (P<0.001), but not with tumor location (P=0.27). In the multivariable Cox analysis of OS, age (P<0.001), gender (P=0.002), BMI (P=0.005), differentiation grade (P=0.002), pT-stage (P<0.001), pN-stage (P<0.001), and LVI (P=0.006) retained significance as independent prognostic factors (Table 3).
Table 3
| Variables | OS | DFS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||||
| Age (years) | 0.01 | <0.001* | 0.73 | ||||||||||||
| ≤62 | Ref | Ref | Ref | – | – | – | |||||||||
| >62 | 1.161 | 1.033–1.305 | 1.301 | 1.156–1.465 | 0.978 | 0.865–1.106 | 0.73 | – | – | – | |||||
| Sex | <0.001 | 0.002* | <0.001 | 0.003* | |||||||||||
| Male | Ref | Ref | Ref | Ref | |||||||||||
| Female | 0.657 | 0.557–0.774 | 0.763 | 0.645–0.902 | 0.663 | 0.559–0.787 | <0.001 | 0.768 | 0.646–0.915 | ||||||
| BMI (kg/m2) | <0.001 | 0.005* | <0.001 | 0.002* | |||||||||||
| <18.5 | Ref | Ref | Ref | ||||||||||||
| 18.5–24 | 0.678 | 0.581–0.792 | <0.001 | 0.787 | 0.671–0.922 | 0.634 | 0.541–0.744 | <0.001 | 0.751 | 0.638–0.884 | |||||
| >24 | 0.637 | 0.510–0.794 | <0.001 | 0.794 | 0.633–0.994 | 0.674 | 0.538–0.845 | <0.001 | 0.865 | 0.688–1.089 | |||||
| Tumor location | 0.27 | 0.06 | |||||||||||||
| Upper | Ref | – | – | – | Ref | – | – | – | |||||||
| Middle | 1.109 | 0.956–1.287 | 0.17 | – | – | – | 1.140 | 0.975–1.332 | 0.10 | – | – | – | |||
| Lower | 1.145 | 0.962–1.323 | 0.13 | – | – | – | 1.240 | 1.034–1.487 | 0.02 | – | – | – | |||
| MIA | 0.02 | 0.10 | <0.001 | 0.03* | |||||||||||
| MIE | Ref | Ref | Ref | Ref | |||||||||||
| Hybrid | 1.156 | 0.937–1.427 | 0.18 | 1.261 | 1.021–1.559 | 1.252 | 1.005–1.561 | 0.045 | 1.358 | 1.088–1.694 | |||||
| Open | 1.197 | 1.054–1.358 | 0.005 | 1.039 | 0.912–1.185 | 1.324 | 1.154–1.754 | <0.001 | 1.061 | 0.908–1.239 | |||||
| Surgical approach | 0.004 | 0.09 | <0.001 | 0.046* | |||||||||||
| Sweet | Ref | Ref | Ref | Ref | |||||||||||
| Ivor-Lewis | 0.671 | 0.425–1.058 | 0.09 | 1.180 | 1.018–1.369 | 0.713 | 0.440–1.153 | <0.001 | 0.763 | 0.314–1.854 | |||||
| McKeown | 0.812 | 0.512–1.288 | 0.38 | 1.194 | 0.492–2.899 | 0.932 | 0.573–1.517 | 0.17 | 0.922 | 0.379–2.242 | |||||
| Differentiation | <0.001 | 0.002* | <0.001 | 0.002* | |||||||||||
| High | Ref | Ref | Ref | Ref | |||||||||||
| Middle | 1.438 | 1.197–1.727 | <0.001 | 1.197 | 0.991–1.445 | 1.465 | 1.208–1.776 | <0.001 | 1.248 | 1.025–1.520 | |||||
| Low | 1.642 | 1.369–1.970 | <0.001 | 1.384 | 1.147–1.670 | 1.648 | 1.361–1.995 | <0.001 | 1.418 | 1.165–1.727 | |||||
| pT-stage | <0.001 | <0.001* | <0.001 | <0.001* | |||||||||||
| T1–2 | Ref | Ref | Ref | Ref | |||||||||||
| T3 | 1.814 | 1.578–2.085 | <0.001 | 1.463 | 1.245–1.718 | 1.818 | 1.570–2.105 | <0.001 | 1.423 | 1.203–1.683 | |||||
| pN-stage | <0.001 | <0.001* | <0.001 | <0.001* | |||||||||||
| N0 | Ref | Ref | Ref | Ref | |||||||||||
| N1 | 1.797 | 1.551–2.082 | <0.001 | 0.900 | 0.499–1.625 | 1.744 | 1.497–2.033 | <0.001 | 1.017 | 0.574–1.800 | |||||
| N2 | 3.155 | 2.694–3.695 | <0.001 | 1.436 | 0.780–2.646 | 2.858 | 2.423–3.371 | <0.001 | 1.523 | 0.841–2.759 | |||||
| N3 | 4.100 | 3.357–5.009 | <0.001 | 1.517 | 0.451–5.104 | 3.721 | 3.015–4.591 | <0.001 | 2.053 | 0.603–6.996 | |||||
| PNI | 0.050 | <0.001 | 0.046* | ||||||||||||
| Negative | Ref | Ref | Ref | Ref | |||||||||||
| Positive | 1.485 | 1.287–1.715 | <0.001 | 1.161 | 1.000–1.348 | 1.459 | 1.255–1.697 | <0.001 | 1.174 | 1.003–1.373 | |||||
| LVI | 0.006* | <0.001 | 0.11 | ||||||||||||
| Negative | Ref | Ref | Ref | Ref | |||||||||||
| Positive | 1.771 | 1.535–2.044 | <0.001 | 1.234 | 1.062–1.435 | 1.626 | 1.397–1.892 | <0.001 | 1.137 | 0.970–1.333 | |||||
*, significant differences in multivariate analysis. BMI, body mass index; CI, confidence interval; ESCC, esophageal squamous cell carcinoma; DFS, disease-free survival; HR, hazard ratio; LVI, lymphovascular invasion; MIA, minimal invasive approach; MIE, minimally invasive esophagectomy; OS, overall survival; PNI, perineural invasion; Ref, reference.
In the univariate analysis, DFS exhibited significant associations with gender (P<0.001), BMI (P<0.001), differentiation grade (P<0.001), MIA (P<0.001), surgical approach (P<0.001), LVI (P<0.001), PNI (P<0.001), pT-stage (P<0.001), and pN-stage (P<0.001), but not with age (P=0.73) or tumor location (P=0.06). In the multivariable survival analysis, PNI as an independent prognostic factor (HR: 1.174, 95% CI: 1.003–1.373, P=0.046), while LVI did not retain significance (HR: 1.137, 95% CI: 0.970–1.333, P=0.11) (Table 3).
Discussion
Previous study has recognized PNI as an adverse prognostic factor for EC patients (19), leading to controversy due to conflicting results in recent study (17). Through a retrospective study of a large sample, we established that PNI affects patient survival outcomes and PNI is an independent prognostic risk factor for DFS in ESCC patients.
Our results indicated that LVI can serve as an independent prognostic factor for OS, whereas PNI as an independent prognostic factor for DFS. We found that PNI-negative patients had a significant survival advantage than PNI-positive patients after excluding lymph node metastasis or LVI-positive. We considered that PNI was similar to LVI, representing a mode of micro-metastasis for tumors. To date, some studies on PNI have no consensus, with different studies reaching varying conclusions regarding OS and DFS. The differences in these studies mainly suggested that PNI may be an incidental phenomenon in tumor progression (17), and PNI is not an independent prognostic factor for OS and DFS (20). Some studies have suggested that PNI can be a prognostic factor (21,22). It has been shown that for lymph node-negative patients, PNI is an independent prognostic factor for both OS and DFS (13). Clearly, our data are more convincing, representing the largest sample to date (n=2,247), and includes all resectable ESCC patients. Of course, we also expect larger sample study to confirm the results of this cohort.
The prevalence of PNI-positive is particularly common in tumors with higher malignancy (23-25), indicating its highly aggressive and propensity for recurrence. Previous studies have consistently associated PNI with higher stage, LVI and lymph node metastasis (13,17,21). Moreover, our data demonstrated that PNI is closely associated with gender and differentiation but not correlated with age or tumor site. Some studies had suggested no significant association between PNI and recurrence (17,26), and our data confirmed these viewpoints. Additionally, our results demonstrated that PNI is also not correlated with distant metastasis, inconsistent with a previous study (27). Hsu et al. concluded that PNI could be used as a prognostic factor for ypII/III-stage ESCC patients (28), and our subgroup analysis confirmed that in the pII/III stage ESCC patients, the status of PNI also affected OS.
To reduce local recurrence and distant metastasis in ESCC patients with high-risk factors, postoperative adjuvant therapy is often employed. For PNI-positive EC patients, previous studies have shown that postoperative adjuvant therapy improves OS rates (16,21,29-31). In Japan, postoperative adjuvant therapy is the standard first-line therapy for patients whose pathologic diagnosis confirms lymph node metastasis (32). In China, postoperative adjuvant therapy is not recommended for T1–3N0M0 patients (33). The National Comprehensive Cancer Network (NCCN) guidelines (2024.Version 1, https://www.nccn.org/guidelines/guideline) recommend postoperative chemotherapy for esophageal adenocarcinoma patients with high-risk features such as LVI or PNI positive. However, there are no recommendations for ESCC. Our data showed that postoperative adjuvant therapy did not provide a survival benefit for ESCC patients, and that patients who received postoperative adjuvant therapy had shorter OS, but this result needs to be interpreted with caution. We propensity-matched patients according to whether the patients received adjuvant therapy or not, and there were differences in baseline information between the two groups, such as gender, surgical method, and tumor location, as shown in Table S1. This finding may explain the discrepancy between the retrospective data and the conclusions of clinical studies. Moreover, we analyzed subgroups of patients according to the PNI status, for PNI-positive patients who had achieved R0 resection, postoperative adjuvant therapy did not enhance the OS, but for PNI-negative ESCC patients, postoperative adjuvant therapy not only failed to confer survival benefits but also resulted in a reduction in OS, which is inconsistent with previous studies (22,29). To further explore the significance of PNI in different subgroups of patients, we observed significant differences in patients with stage pN0, pII/III and LVI negative, consistent with previous studies (13,28). Hsu et al. concluded that PNI could be used as a prognostic factor for ypII/III-stage ESCC patients (28), and our subgroup analysis confirmed that in the pII/III stage ESCC patients, the status of PNI also affected OS. This suggests that PNI can be a prognostic factor in some specific subgroups. Currently, prospective studies on postoperative adjuvant therapy for right thoracic three-incision or two-incision approaches in China have not yet been published, and we eagerly await the results of prospective trials.
Certainly, there are several limitations in this study: first, it is a single-center retrospective study. Second, the population did not include patients who received neoadjuvant therapy. Third, there may be variations in the surgical procedures, lymph node dissection, and postoperative adjuvant therapy practices among different treatment groups. Fourth, from 2010 to 2017, neoadjuvant therapy was not widely adopted in China, resulting in a lack of data on the relationship between PNI and neoadjuvant therapy with prognosis.
Conclusions
In conclusion, PNI demonstrates a robust correlation with LVI and acts as an independent predictor of DFS in resectable ESCC patients. Despite the absence of a direct association between PNI-positive and recurrence or metastasis, its prognostic significance should not be underestimated. For patients with PNI-negative ESCC who have undergone R0 resection, our study showed that postoperative adjuvant therapy did not provide a significant survival benefit for patients. While our study offers valuable insights for clinical practice, further research is warranted to validate our findings and extend them to a larger and more diverse patient population, thereby enabling the development of more personalized and effective treatment strategies.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2043/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2043/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2043/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2043/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Sichuan Cancer Hospital (No. SCCHEC-02-2022-050). Consent was waived by the Ethics Committee due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent 2022;2:1-9. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma: The NEOCRTEC5010 Randomized Clinical Trial. JAMA Surg 2021;156:721-9. [Crossref] [PubMed]
- Inada M, Nishimura Y, Ishikawa K, et al. Comparing the 7th and 8th editions of the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system for esophageal squamous cell carcinoma treated by definitive radiotherapy. Esophagus 2019;16:371-6.
- Sudo N, Ichikawa H, Muneoka Y, et al. Clinical Utility of ypTNM Stage Grouping in the 8th Edition of the American Joint Committee on Cancer TNM Staging System for Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2021;28:650-60.
- Guo XF, Mao T, Gu ZT, et al. Clinical study on postoperative recurrence in patients with pN0 esophageal squamous cell carcinoma. J Cardiothorac Surg 2014;9:150. [Crossref] [PubMed]
- Li P, He HQ, Zhu CM, et al. The prognostic significance of lymphovascular invasion in patients with resectable gastric cancer: a large retrospective study from Southern China. BMC Cancer 2015;15:370. [Crossref] [PubMed]
- Huang Q, Luo K, Chen C, et al. Identification and Validation of Lymphovascular Invasion as a Prognostic and Staging Factor in Node-Negative Esophageal Squamous Cell Carcinoma. J Thorac Oncol 2016;11:583-92. [Crossref] [PubMed]
- Ma Y, Yao X, Li Z, et al. The role of vascular invasion and lymphatic invasion in predicting recurrent thoracic oesophageal squamous cell carcinoma. World J Surg Oncol 2022;20:12. [Crossref] [PubMed]
- Woodham BL, Chmelo J, Donohoe CL, et al. Prognostic Significance of Lymphatic, Venous and Perineural Invasion After Neoadjuvant Chemotherapy in Patients with Gastric Adenocarcinoma. Ann Surg Oncol 2020;27:3296-304. [Crossref] [PubMed]
- Zhang H, Chen X, Wang S, et al. Poorer prognosis associated with simultaneous lymphatic and vascular invasion in patients with squamous carcinoma of the thoracic oesophagus. Eur J Cardiothorac Surg 2017;52:378-84. [Crossref] [PubMed]
- Faiz Z, Huijgen LJW, Alqethami HJ, et al. Prevalence and Prognostic Significance of Extramural Venous Invasion in Patients with Locally Advanced Esophageal Cancer. Ann Surg Oncol 2018;25:1588-97. [Crossref] [PubMed]
- Ma Y, Chen J, Yao X, et al. Patterns and prognostic predictive value of perineural invasion in esophageal squamous cell carcinoma. BMC Cancer 2022;22:1287. [Crossref] [PubMed]
- Yang J, Lu Z, Li L, et al. Relationship of lymphovascular invasion with lymph node metastasis and prognosis in superficial esophageal carcinoma: systematic review and meta-analysis. BMC Cancer 2020;20:176. [Crossref] [PubMed]
- Tankel J, Söderström H, Reizine E, et al. Change in Density Not Size of Esophageal Adenocarcinoma During Neoadjuvant Chemotherapy Is Associated with Improved Survival Outcomes. J Gastrointest Surg 2022;26:2417-25. [Crossref] [PubMed]
- Xie C, Chen Z, Xu J, et al. Influence of Lymphangio vascular (V) and perineural (N) invasion on survival of patients with resected esophageal squamous cell carcinoma (ESCC): a single-center retrospective study. PeerJ 2022;10:e12974. [Crossref] [PubMed]
- Zhang L, Shao J, Liu Z, et al. Occurrence and Prognostic Value of Perineural Invasion in Esophageal Squamous Cell Cancer: A Retrospective Study. Ann Surg Oncol 2022;29:586-97. [Crossref] [PubMed]
- Batsakis JG. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol 1985;94:426-7.
- Bai L, Yan L, Guo Y, et al. Perineural Invasion Is a Significant Indicator of High Malignant Degree and Poor Prognosis in Esophageal Cancer: A Systematic Review and Meta-Analysis. Front Oncol 2022;12:816270. [Crossref] [PubMed]
- Zhou J, Yang Y, Zhang H, et al. Lymphovascular and Perineural Invasion After Neoadjuvant Therapy in Esophageal Squamous Carcinoma. Ann Thorac Surg 2023;115:1386-94. [Crossref] [PubMed]
- Kim HE, Park SY, Kim H, et al. Prognostic effect of perineural invasion in surgically treated esophageal squamous cell carcinoma. Thorac Cancer 2021;12:1605-12. [Crossref] [PubMed]
- Ning ZH, Zhao W, Li XD, et al. The status of perineural invasion predicts the outcomes of postoperative radiotherapy in locally advanced esophageal squamous cell carcinoma. Int J Clin Exp Pathol 2015;8:6881-90.
- Hayakawa Y, Sakitani K, Konishi M, et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 2017;31:21-34. [Crossref] [PubMed]
- Peterson SC, Eberl M, Vagnozzi AN, et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 2015;16:400-12. [Crossref] [PubMed]
- Faulkner S, Jobling P, March B, et al. Tumor Neurobiology and the War of Nerves in Cancer. Cancer Discov 2019;9:702-10. [Crossref] [PubMed]
- Merritt RE, Abdel-Rasoul M, Souza DM, et al. Nomograms for predicting overall and recurrence-free survival after trimodality therapy for esophageal adenocarcinoma. J Surg Oncol 2021;123:881-90. [Crossref] [PubMed]
- Hsu PK, Chien LI, Wang LC, et al. Lymphovascular invasion and extracapsular invasion are risk factors for distant recurrence after preoperative chemoradiotherapy and oesophagectomy in patients with oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg 2017;51:1188-94. [Crossref] [PubMed]
- Hsu PK, Chien LI, Lin CH, et al. Impact of perineural invasion as a histopathological prognostic factor in ypStage II/III oesophageal squamous cell carcinoma†. Eur J Cardiothorac Surg 2019;55:927-33. [Crossref] [PubMed]
- Tsai CY, Yeh CJ, Chao YK, et al. Perineural invasion through the sheath in posttherapy esophagectomy specimens predicts poor survival in patients with esophageal squamous cell carcinoma. Eur J Surg Oncol 2017;43:1970-6. [Crossref] [PubMed]
- Gao A, Wang L, Li J, et al. Prognostic Value of Perineural Invasion in Esophageal and Esophagogastric Junction Carcinoma: A Meta-Analysis. Dis Markers 2016;2016:7340180. [Crossref] [PubMed]
- Guo YN, Tian DP, Gong QY, et al. Perineural Invasion is a Better Prognostic Indicator than Lymphovascular Invasion and a Potential Adjuvant Therapy Indicator for pN0M0 Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2020;27:4371-81. [Crossref] [PubMed]
- Kitagawa Y, Ishihara R, Ishikawa H, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus 2023;20:343-72.
- Chinese National Cancer Center. Chinese Society for Thoracic and Cardiovascular Surgery; Chinese Society for Diseases of the Esophagus. Chinese Guidelines on Perioperative Management of Resectable Esophageal Cancer (2023 edition). Zhonghua Yi Xue Za Zhi 2023;103:2552-70. [Crossref] [PubMed]


