
Comparative evaluation of deep learning-based and conventional reconstruction techniques for image quality enhancement in low-dose chest computed tomography
Highlight box
Key findings
• This study developed and evaluated a new deep learning (DL) image reconstruction method and validated its performance among patients with pulmonary diseases.
What is known and what is new?
• Iterative reconstruction (IR) and DL algorithms can reduce noise and provide better image quality in low-dose computed tomography images.
• Our newly developed DL algorithm obtained similar image quality to that of IR techniques under a ultra-low radiation dose.
What is the implication, and what should change now?
• The DL algorithm proved to provide better image quality and may thus be a viable technique for computed tomography use in the future.
Introduction
In clinical practice, computed tomography (CT) serves as a routine tool for radiologic examinations, but cumulative radiation exposure is a challenge for public health, especially in patients who require multiple repetitive scans (1). Low-dose CT (LDCT) scanning was first proposed in 1990 (2) and has been widely used in disease screenings and diagnosis for high-risk populations. The recent NELSON studies demonstrated that LDCT screening can significantly reduce lung cancer mortality (3-6). Additionally, a study funded by the National Heart, Lung, and Blood Institute used LDCT in patients with lymphangiomyomatosis (LAM) or other lung diseases who required frequent CT follow-up to monitor disease progression (7). During the coronavirus disease 2019 (COVID-19) pandemic, CT also served as a key tool in the early stage of the pandemic. However, repeated follow-up examinations could cause significant increases in cumulative radiation damage among patients with COVID-19. Therefore, effective and fast dose-reduction reconstruction techniques proved highly valuable during the global pandemic.
Dose reduction achieved by decreasing the tube voltage or current may greatly affect image quality. The latest generation of iterative reconstruction (IR) techniques can compensate for the undesirable of effects low-dose scans. Although IR can be a good choice for LDCT images, it changes the texture of reconstructed images and is sometimes slow during highly iterative processes, which further limits the use of IR methods (8-10). Deep learning (DL) techniques represent an innovation in computer vision, and convolutional neural networks (CNNs) have been demonstrated to be capable of providing excellent noise suppression in LDCT image reconstruction (11-15).
In this study, we developed a new DL algorithm to reduce noise and improve image quality in LDCT. A single-center investigation in patients with pulmonary diseases was designed to validate the effectiveness of algorithm, and its performance was compared to that of with several mainstream IR techniques from commercial manufacturers. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-589/rc).
Methods
Study design and participants
This study was originally designed for a setting of early lung disease screening using LDCT. As a part of the study, a retrospective image quality study was performed between November 2020 and July 2023, and a total of 108 participants were consecutively enrolled; however, 18 of them were excluded due artifacts or issues in collecting scans. Among the remaining 90 patients, 45 persons for physical check-up underwent low-dose chest CT, and 45 outpatients who underwent conventional chest CT (Figure 1). Raw data from LDCT scans were reconstructed via IR and filtered back projection (FBP). The DL algorithm took the low-dose FBP as input and output the denoised images. Raw data from standard-dose CT (SDCT) scans were reconstructed by FBP as the gold standard. Image quality was assessed using signal-to-noise ratio (SNR), absolute noise, contrast-to-noise ratio (CNR), mean squared error (MSE), and image structural similarity (SSIM). Moreover, two experienced radiologists (10 years experience in radiology) subjectively and blindly evaluated the results using a 5-point scale.
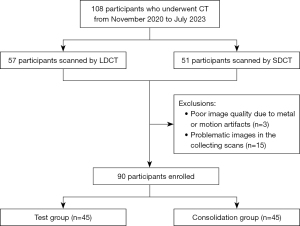
The exclusion criteria of patient enrollment were as follows: (I) metastatic tumors or radiation-related lung injuries; (II) age under 18 years or over 80 years; (III) pregnancy; and (IV) any other contraindications to CT examinations. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (ethics review approval No. 2020-184) and informed consents were waived because this is a retrospective study in which all personal information of the subjects has been de-identified, and no prior intervention was conducted during the subjects’ examinations.
CT protocols and image acquisition
Non-contrast chest CT examinations were assigned for all patients approximately with three scanners being used to minimize bias of imaging devices and procedures (Brilliance iCT, Philips, Amsterdam, the Netherlands; Aquilion CXL, Canon, Tokyo, Japan; Revolution CT, GE HealthCare, Chicago, IL, USA) at the First Affiliated Hospital of Soochow University. For SDCT, the scanning conditions adopted for the iCT device were as follows: 20 kVp; detector collimation, 128 × 0.625 mm; pitch, 0.992; rotation time, 0.5 s; slice thickness, 1.0 mm; and dose right index; 21. The scanning conditions for the Aquilion CXL device were as follows: 120 kVp; collimation, 64 × 0.5 mm; pitch, 0.992; rotation time, 0.5 s; and slice thickness, 1.0 mm. The scanning conditions for the revolution CT were as follows: 120 kVp; collimation, 80 mm; pitch, 0.828; rotation time, 0.5 s; and slice thickness, 1.25 mm. All the SDCT images were reconstructed with FBP as the gold standard for the low-dose scan.
Patients undergoing LDCT scans had the tube current reduced to 20 mA, and the tube voltages and other technical settings remained unchanged. The LDCT data collected from iCT were reconstructed via iterative model reconstruction (IMR) technique (noise reduction level 1). The LDCT collected from the revolution CT were reconstructed with adaptive statistical iterative reconstruction-V (ASIR-V) at 60%. The LDCT collected from Aquilion CT were reconstructed by adaptive iterative dose reduction 3D (AIDR-3D). Meanwhile, the LDCT images reconstructed by FBP were used as the DL algorithm input afterwards.
Image reconstruction base on convolutional neural network (CNN)
In the first stage of DL algorithm training, low-dose phantom data reconstructed by FBP (high noisy) of 10 subjects were inputted into the CNN, which was designed to reduce image noise and generate the de-noised results. Meanwhile, SDCT images of 10 subjects were input into CNN training and used as standard images. In the second stage, LDCT images and SDCT images were joint trained, with human and phantom data used as training samples. Thus, noise can be well-suppressed by the CNN for low-dose human data.
Image quality assessment
IR reconstructions and the DL outcomes were compared using both objective and subjective quantitative assessments. For objective quantitative assessment, CT image quality was evaluated via SNR, CNR, MSE, SSIM index, and absolute noise. For subjective assessments, two experienced radiologists independently scored the IR and DL images.
To calculate SNR, CNR, and absolute noise, radiologist sketched three circular regions of interest (ROIs) with same area (250 mm2) on the upper, middle, and lower lobe of the lung for each sample. The CT values and standard deviations (SDs) of the three ROIs were respectively averaged as signal intensity the tissue (SItissiue) and mean absolute noise (16). A CT value of −1,000 (air) was used as the background signal (SIbackground). SNR was calculated as |SItissiue|/SD, and CNR was calculated as (SItissiue − SIbackground)/SD.
FBP reconstructions of SDCT, which were used as the gold standard, were calculated using MSE and SSIM as follows:
where g(i,j) is the grayscale value at pixel position (i,j) of FBP reconstructions of SDCT; f(i,j) is the corresponding IR or DL reconstructions of LDCT; M and N are the pixel height and width of CT image, respectively; and l, c, and s are the luminance function, contrast function, and structure function, respectively.
Subjective assessments of images were based on image sharpness, image noise, and diagnostic acceptability of radiologists. A 5-point scale was used with decrement a of 1 point for each score from the highest one (17). A score of 5 indicated excellent image sharpness, a clear margin, and minimal image noise on small anatomic structures, enabling full diagnostic confidence. Meanwhile, a score of 1 indicated poor image quality with severe noise or artifacts affecting the visualization of tissue, which was diagnostically unusable. Two radiologists, each with 5 years of experience in chest CT diagnosis, independently and randomly read and rated all CT images without knowing the specific reconstruction methods. All ratings were performed on the axial CT images in a window setting of width of 1,500 Hounsfield units (HU) and a center of −650 HU.
Radiation dose measurements
The radiation dose of CT scan was estimated in terms of the dose-length product (DLP) and volume CT dose index (CTDIvol). The effective dose (ED) was calculated with a tissue conversion coefficient (k) for the chest of 0.014 according to following equation (18,19): effective dose = DLP × k. Dose reduction = (ED1 − ED2) / ED1 * 100%, ED1 indicates the average effective dose in SDCT, ED2 indicates the average effective dose in LDCT.
Statistical analysis
All statistical analyses were performed with SPSS software version 25 (IBM Corp., Armonk, NY, USA). A P value <0.05 indicated statistical significance. The quantitative results are expressed as mean ± SD or as the median and interquartile range. The differences in SNR, CNR, MSE, SSIM, absolute noise, and radiation dose between the DL and IR results were analyzed using a paired t-test when the data following a normal distribution or the Wilcoxon signed-rank test while the data was non-normality. Subgroup analysis was performed via the Mann-Whitney test (non-normally distributed data) or the paired t-test (normally distributed data). The difference in radiologists’ subjective scores between DL and IR was analyzed via the Wilcoxon signed-rank test.
Results
Patient characteristics
The demographics of the enrolled patients are presented in Table 1. The mean age of the study population was 44.8±13.4 years, and there were 44 males and 46 females. The mean height and weight of the study population were 162.57±22.53 cm and 67.88±14.13 kg. The average BMI of the patients was 29.51±25.75 kg/m2.
Table 1
| Characteristics | Values |
|---|---|
| Age (years), median [interquartile range] | 45 [33–55] |
| Gender, n (%) | |
| Male | 44 (48.9) |
| Female | 46 (51.1) |
| Height (cm), mean ± SD | 162.57±22.53 |
| Weight (kg), mean ± SD | 67.88±14.13 |
SD, standard deviation.
Difference in image quality between IR and DL reconstruction
The Wilcoxon signed-rank test showed a significantly higher SNR in the DL results (median 23.7; IQR, 20.4–29.8) than in the IR results (median 16.4; IQR, 13.3–20.5) in the LDCT examination. (P<0.001). The median MSE of the DL results (median 0.0024; IQR, 0.0014–0.0025) was significantly lower than that of the IR results (median 0.0025; IQR, 0.0014–0.0025) (P=0.006). The median SSIM value was not significantly different between DL (median 0.945; IQR, 0.916–0.957) and IR (median 0.944; IQR, 0.918–0.956) (P=0.09). Moreover, the DL method produced a 30% lower mean absolute noise as compared to that of IR (P<0.001). In addition, the paired t test showed that DL had a significantly higher mean CNR value (3.318±0.787) as compared to that of IR (2.218±0.710) (P<0.001). These results are summarized in Table 2.
Table 2
| Parameter | Iterative reconstruction | DL reconstruction | P value |
|---|---|---|---|
| CNR | 2.218±0.710 | 3.318±0.787 | <0.001 |
| SNR | 16.4 (13.3–20.5) | 23.7 (20.4–29.8) | <0.001 |
| SSIM | 0.944 (0.918–0.956) | 0.945 (0.916–0.957) | 0.088 |
| MSE | 0.0025 (0.0014–0.0027) | 0.0024 (0.0014–0.0025) | 0.006 |
| Noise (HU) | 69.93 (55.56–86.96) | 49.26 (38.76–56.82) | <0.001 |
Data of CNR is expressed as mean ± SD, SNR, SSIM, MSE and Noise are expressed as median (IQR). CNR, contrast-to-noise ratio; DL, deep learning; HU, Hounsfield unit; IQR, interquartile range; IR, iterative reconstruction; MSE, mean squared error; SD, standard deviation; SNR, signal-to-noise ratio; SSIM, structural similarity index measure.
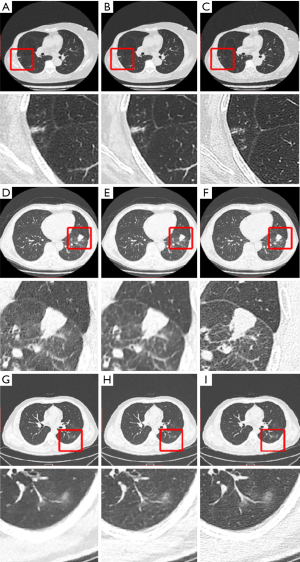
Additionally, the three conventional IR methods (ASIR-V, IMR and AIDR-3D) were individually compared with the proposed DL algorithm (Table 3). In the comparison between ASIR-V and DL, DL had a higher SNR, CNR, and SSIM, along with a lower MSE and lower noise level (P<0.001). In the comparison between AIDR-3D and DL, DL had a significantly lower noise level (as reflected in SNR and absolute noise) but a significantly higher CNR, SSIM, and MSE. In the comparison between IMR with DL, DL had a significantly higher CNR (P=0.047) but a significantly lower SSIM (P=0.004), but no significant differences were found for the other parameters. Compared with AIDR-3D (Figure 2A) reconstruction, DL (Figure 2B) produced sharper edges and clearer textures of tissues with lower noise magnitude. Compared with SDCT + FBP (Figure 2C) reconstruction, DL (Figure 2B) produced a lower noise magnitude, but the internal details of the lesion were slightly unclear. Compared with ASIR-V (Figure 2D) reconstruction, DL (Figure 2E) also produced sharper edges and clearer textures of tissues with lower noise magnitude. Compared with SDCT + FBP (Figure 2F) reconstruction, DL (Figure 2E) produced a lower noise magnitude, but the small bronchial trees were not clearly visualized. Compared with IMR (Figure 2G) reconstruction, DL (Figure 2H) retained slightly more noise. Compare with SDCT + FBP (Figure 2I) reconstruction, DL (Figure 2H) produced a smoother image with lower noise. Compare with SDCT + FBP (Figure 2C,2F,2I) reconstructions, no diagnostic information was lost by either IR or DL reconstructions in LDCT. (Figure 2A,2B,2D,2E,2G,2H).
Table 3
| Manufacturer | Parameter | Iterative reconstruction | DL reconstruction | P value |
|---|---|---|---|---|
| GE Revolution CT (n=29) | SNR | 12.98±1.60 | 25.15±7.66 | <0.001 |
| CNR | 1.82±0.52 | 3.30±0.63 | <0.001 | |
| MSE | 0.0027±0.0020 | 0.0024±0.0017 | <0.001 | |
| SSIM | 0.920±0.053 | 0.930±0.045 | <0.001 | |
| Noise (HU) | 92.76±18.26 | 51.28±17.38 | <0.001 | |
| Toshiba Aquilion CXL (n=28) | SNR | 17.53±2.15 | 30.85±10.24 | <0.001 |
| CNR | 2.29±0.78 | 3.71±1.08 | <0.001 | |
| MSE | 0.0021±0.0011 | 0.0022±0.0011 | <0.001 | |
| SSIM | 0.932±0.033 | 0.931±0.034 | 0.015 | |
| Noise (HU) | 68.05±14.19 | 42.14±16.16 | <0.001 | |
| Philips iCT (n=33) | SNR | 22.13±3.07 | 21.88±4.21 | 0.714 |
| CNR | 2.70±0.55 | 2.95±0.36 | 0.047 | |
| MSE | 0.0026±0.0023 | 0.0026±0.0023 | 0.781 | |
| SSIM | 0.927±0.045 | 0.925±0.059 | 0.004 | |
| Noise (HU) | 52.82±9.25 | 56.02±15.46 | 0.111 |
Data are presented as the mean ± SD. CNR, contrast-to-noise ratio; CT, computed tomography; DL, deep learning; HU, Hounsfield unit; MSE, mean squared error; SD, standard deviation; SNR, signal-to-noise ratio; SSIM, structural similarity index measure.
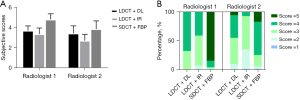
The subjective scores of three reconstructions, LDCT + IR, LDCT + DL, and SDCT + FBP, from the human observers are presented in Figure 3. The average subjective score of the two radiologists was 4.317±0.837 for SDCT + FBP (gold standard), 3.542±0.637 for LDCT + DL, and 3.021±0.689 for LDCT + IR. The Wilcoxon signed-rank test showed that there was no significant difference in the subjective scores between the DL and IR reconstructions on LDCT (P=0.24). Moreover, there was no significant difference between LDCT + DL and SDCT + FBP (P=0.11). The dustribution of scores 1-5 from high to low was SDCT + FBP (score of 5), LDCT + DL (scores of 4–5) and LDCT + IR (mainly scores of 1–3). The interrater agreement of the two radiologists for LDCT + DL, LDCT + IR and SDCT + FBP was 0.348, 0.224, and 0.271, respectively. All scores were normalized by z-scores in the analysis.
With regards to radiation dose reduction, the mean CTDIvol of all SDCT scans was 12.71±9.17 mGy, the mean DLP was 487.4±336.5 mGy·cm, and the mean effective dose was 6.82±4.71 mSv. For LDCT, the mean CTDIvol and DLP were 1.09±0.34 mGy and 42.03±14.4 mGy·cm, respectively, with the mean effective dose being reduced to 0.59±0.2 mSv. The DL algorithm enabled a mean dose reduction of 91% from the dosage level of the SDCT.
Discussion
In this study, a new DL image reconstruction algorithm was developed and compared with three mainstream IR methods. Our results showed that DL achieved a greater noise reduction and produced better image quality than did IR in low-radiation scans in clinical practice.
In CT imaging process, FBP reconstruction was a simple, fast, and reliable technique that served as the gold standard for traditional CT image reconstruction algorithms. However, poor image quality under insufficient data conditions and limited noise resistance capability revealed its inherent limitations. The problem of image texture deterioration commonly appears in IR-based LDCT reconstructions (20), but few studies have applied artificial intelligence to address this. Recently, the performance of the DL image reconstruction (DLIR) technique introduced by GE HealthCare was verified on phantoms, but further validation in clinical setting by radiologists and technologists remains lacking (14). In contrast to these studies, we assessed the performance of a newly developed DL algorithm in a clinical setting of LDCT screening for lung diseases and further compared it to the latest-generation IR techniques from mainstream vendors. To verify the clinical reliability of the DL algorithm, the image quality was assessed and compared with that of IR by radiologists.
The proposed DL method achieved higher CNR, SNR, and SSIM values and lower MSE as compared with IR in chest LDCT. DL produced a significantly higher SNR and CNR, indicating a substantial reduction in noise and sharper contrast of images. MSE was used to assess the image fidelity by revealing the distortion in pixel values between DL or IR reconstruction and the gold standard (SDCT). The lower MSE produced by DL indicated that it may better restore the original image information from SDCT to LDCT. In this study, the SSIM, which measures the image’s structural information and similarity, revealed that both IR and DL could maintain an image structures close to that of the reference SDCT. IR reconstructs images in a different manner from that of deep neural networks (DNNs) and have several disadvantages. First, based on linear and statistical models, IR estimates and modifies reconstructions in a low-level feature domain, rather than taking the whole-chain image properties of the pixels into account. In contrast, DNNs utilize multiple convolutional layers to capture both low-level and high-level features and operate pixels locally and globally to comprehensively enhance image details; moreover, they employ a series of nonlinear activation functions to achieve a better fitting effect. This more comprehensive feature extraction may explain why DL better reduced the noises as compared to IR while preserving the structural information of the image.
In the subjective evaluations, no significant differences were observed between LD + DL, LD + IR, and SD + FBP, indicating that DL outcomes were visually and diagnostically consistent with those of the gold standard and IR. Furthermore, the proportion of good scores (defined as score ≥4) was higher in the DL images than in the IR images for both radiologist 1 (DL: 67.6%; IR: 40.8%) and radiologist 2 (DL: 45%; IR: radiologist 2); meanwhile, DL produced a lower percentage of poor scores (defined as scores ≤2) than did IR for both radiologist 1 (DL: 0%; IR: 7%); and radiologist 2 (DL: 9.9%; IR: 35.2%). This suggested that DL likely produces more high-quality images than does IR.
Radiation dosage is another critical factor that has been considered in the context of IR techniques. In one study, IMR achieved a radiation dose 60–80% lower than that of SDCT in a phantom test under optimized conditions (17); meanwhile, ASIR-V was reported to enable an average radiation reduction of about 59.8% as compared to FBP in abdominal CT (21), and AIDR-3D was demonstrated to reduce radiation exposure to 50% in chest CT examination (22). Our proposed DL method showed a decrease in radiation exposure up to 91% in clinical conditions, with good image quality and clinical acceptability. ASIR-V can achieve a reconstruction speed of up to 25 frames/s in phantom tests (23), and IMR processes reconstructions at a speed of 3.58 frames/s and a slice thickness of 0.45 mm for chest CT (17). Our proposed DL algorithm had a speed of 60 frames/s (under a resolution of 512×512 pixels) and can be implemented in routine CT scans.
Certain limitations to this study could have influenced the results. First, the BMI of the study population was not accounted for, which might have introduced a degree of bias. Second, the 5-point scoring system adopted in previous studies largely relies on individual visual perception and preference which may vary across different settings or centers. A multicenter study with a blinded and cross-over rating design should be conducted to account for this. Third, the effect of the DL algorithm on lesion detection and morphology was not assessed in patients, which should be further analyzed. Nonetheless, the use of the DL algorithm with LDCT is worth promoting, as its scanning conditions are within the parameters of commonly used CT devices, and it can reduce radiation dose to a large extent, thus better protecting patients.
Conclusions
Our proposed DL algorithm, which was validated in patients with lung disease and healthy participants in the screening for pulmonary illness, substantially improved image quality as compared with mainstream IR methods. The novel method also yielded a greater proportion of high-quality CT images than did the IR reconstructions in the radiologists’ ratings. The DL algorithm can accommodate a radiation dose reduction of up to 91%, provide reliable and speedy denoising of chest LDCT images, and retain good image quality amenable to clinicians’ needs.
Acknowledgments
We would like to thank the radiologists Yujin Wu and Guangyu Hao from the Department of Radiology, The First Affiliated Hospital of Soochow University, for helping with the image assessment.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-589/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-589/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-589/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-589/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (Ethics Review Approval No. 2020-184) and informed consents were waived because this is a retrospective study in which all personal information of the subjects has been de-identified, and no prior intervention was conducted during the subjects’ examinations.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Frush DP, Frija G, Allen B, et al. CT radiation exposure and cancer risk: from knowing to acting. Pediatr Radiol 2024;54:1407-9. [Crossref] [PubMed]
- Naidich DP, Marshall CH, Gribbin C, et al. Low-dose CT of the lungs: preliminary observations. Radiology 1990;175:729-31. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- De Luca GR, Diciotti S, Mascalchi M. The Pivotal Role of Baseline LDCT for Lung Cancer Screening in the Era of Artificial Intelligence. Arch Bronconeumol 2024; Epub ahead of print. [Crossref]
- Zhong D, Sidorenkov G, Jacobs C, et al. Lung Nodule Management in Low-Dose CT Screening for Lung Cancer: Lessons from the NELSON Trial. Radiology 2024;313:e240535. [Crossref] [PubMed]
- Henschke C, Huber R, Jiang L, et al. Perspective on Management of Low-Dose Computed Tomography Findings on Low-Dose Computed Tomography Examinations for Lung Cancer Screening. From the International Association for the Study of Lung Cancer Early Detection and Screening Committee. J Thorac Oncol 2024;19:565-80. [Crossref] [PubMed]
- Pires DC, Arueira Chaves L, Dantas Cardoso CH, et al. Effects of low dose computed tomography (LDCT) on lung cancer screening on incidence and mortality in regions with high tuberculosis prevalence: A systematic review. PLoS One 2024;19:e0308106. [Crossref] [PubMed]
- Ott JG, Becce F, Monnin P, et al. Update on the non-prewhitening model observer in computed tomography for the assessment of the adaptive statistical and model-based iterative reconstruction algorithms. Phys Med Biol 2014;59:4047-64. [Crossref] [PubMed]
- Zhang J, Gong W, Ye L, et al. A Review of deep learning methods for denoising of medical low-dose CT images. Comput Biol Med 2024;171:108112. [Crossref] [PubMed]
- Greffier J, Frandon J, Pereira F, et al. Optimization of radiation dose for CT detection of lytic and sclerotic bone lesions: a phantom study. Eur Radiol 2020;30:1075-8. [Crossref] [PubMed]
- Sun Y, Liu X, Cong P, et al. Digital radiography image denoising using a generative adversarial network. J Xray Sci Technol 2018;26:523-34. [Crossref] [PubMed]
- Chen H, Li Q, Zhou L, et al. Deep learning-based algorithms for low-dose CT imaging: A review. Eur J Radiol 2024;172:111355. [Crossref] [PubMed]
- Yi X, Babyn P. Sharpness-Aware Low-Dose CT Denoising Using Conditional Generative Adversarial Network. J Digit Imaging 2018;31:655-69. [Crossref] [PubMed]
- Greffier J, Hamard A, Pereira F, et al. Image quality and dose reduction opportunity of deep learning image reconstruction algorithm for CT: a phantom study. Eur Radiol 2020;30:3951-9. [Crossref] [PubMed]
- Yang Q, Yan P, Zhang Y, et al. Low-Dose CT Image Denoising Using a Generative Adversarial Network With Wasserstein Distance and Perceptual Loss. IEEE Trans Med Imaging 2018;37:1348-57. [Crossref] [PubMed]
- Stiller W. Basics of iterative reconstruction methods in computed tomography: A vendor-independent overview. Eur J Radiol 2018;109:147-154. [Crossref] [PubMed]
- Laqmani A, Regier M, Veldhoen S, et al. Improved image quality and low radiation dose with hybrid iterative reconstruction with 80 kV CT pulmonary angiography. Eur J Radiol 2014;83:1962-9. [Crossref] [PubMed]
- Christner JA, Kofler JM, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning. AJR Am J Roentgenol 2010;194:881-9. [Crossref] [PubMed]
- Tong X, Wang S, Cheng Q, et al. Effect of fully automatic classification model from different tube voltage images on bone density screening: A self-controlled study. Eur J Radiol 2024;177:111521. [Crossref] [PubMed]
- Geyer LL, Schoepf UJ, Meinel FG, et al. State of the Art: Iterative CT Reconstruction Techniques. Radiology 2015;276:339-57. [Crossref] [PubMed]
- Kwon H, Cho J, Oh J, et al. The adaptive statistical iterative reconstruction-V technique for radiation dose reduction in abdominal CT: comparison with the adaptive statistical iterative reconstruction technique. Br J Radiol 2015;88:20150463. [Crossref] [PubMed]
- Yamashiro T, Miyara T, Honda O, et al. Adaptive Iterative Dose Reduction Using Three Dimensional Processing (AIDR3D) improves chest CT image quality and reduces radiation exposure. PLoS One 2014;9:e105735. [Crossref] [PubMed]
- Lim K, Kwon H, Cho J, et al. Initial phantom study comparing image quality in computed tomography using adaptive statistical iterative reconstruction and new adaptive statistical iterative reconstruction v. J Comput Assist Tomogr 2015;39:443-8. [Crossref] [PubMed]


