
Effects of intraoperative non-steroidal anti-inflammatory drugs on early postoperative pulmonary complications following lung resection: a retrospective propensity score-matched study
Highlight box
Key findings
• The use of intraoperative non-steroidal anti-inflammatory drugs (NSAIDs) decreased the incidence of postoperative pneumonia and respiratory failure, lowered intensive care unit admission rate and shortened the postoperative hospital stay.
What is known and what is new?
• Many perioperative factors will affect the occurrence of post-pneumonectomy complications in lung cancer patients, such as anesthesia procedures, anesthetic drugs, adjuvant drugs, intubation methods, intraoperative management, etc.
• In previous studies, there was a lack of evidence for the links of between intraoperative NSAIDs use and patients’ short-term outcomes after lung resection.
• This study aimed to explore the efficacy of intraoperative NSAID on postoperative pulmonary complications (PPCs) in patients with non-small cell lung cancer after surgery.
What is the implication, and what should change now?
• Intraoperative use of NSAID may improve the short-term prognosis and reduce the incidence of PPCs. Further research is warranted to confirm the impact of intraoperative NSAID use on PPCs.
Introduction
Non-small cell lung cancer (NSCLC) is a relatively common type of lung cancer, accounting for approximately 85% of all lung cancers, and surgical intervention is still the first-line treatment for it (1). However, surgical procedures inevitably cause a sharp inflammatory response due to local tissue damage, which may be related to the occurrence of postoperative complications, especially postoperative pulmonary complications (PPCs), which are associated with high morbidity and mortality (2,3). Surgical invasion causes the production and secretion of inflammatory cytokines, prostaglandins, and vascular endothelial growth factor (VEGF), which are responsible for regulating various physiological functions during wound healing (4). Despite the continuous evolution of surgical techniques, postoperative pulmonary complications remain a primary concern in patients undergoing pneumonectomy (5).
Additionally, in case of thoracic surgery, the pain is severe and can further promote the development of postoperative complications (6). Therefore, pain relief is of particular significance for the reduction of postoperative pulmonary and cardiac complications. Because of the difficulty in pain control, a multimodal analgesic strategy has been suggested for a long time, which is now the cornerstone of pain management and offers the possibility of reducing opioid requirements and side effects (7). It is worth noting that non-steroidal anti-inflammatory drugs (NSAIDs) are an important part of this strategy. NSAIDs have a variety of pharmacological effects, including anti-inflammatory and analgesic effects, on the one hand through good analgesic effects to better promote sputum excretion and early out-of-bed activities, on the other hand through anti-inflammatory effects to inhibit systemic and local inflammatory response (8). All these can promote the recovery of postoperative lung function. In consideration of these clinical actions, studying the effects of NSAIDs on PPCs after pneumonectomy might yield beneficial results and be an important component of achieving enhanced recovery after surgery (ERAS). Nevertheless, to date, whether NSAIDs lower PPCs following lung resection has never been exclusively reported. The goal of this study was to examine the efficacy of intraoperative NSAIDs for the prevention of PPCs after surgery in patients with NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2007/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by Peking University Cancer Hospital Clinical Research Ethics Committee (approval No. 2019YJZ22-GZ02) and individual consent for this retrospective analysis was waived.
Using the hospital’s electronic medical records system, we retrospectively reviewed clinical data of NSCLC patients who had undergone lung resection between January 1 and May 31, 2019. The exclusion criteria were: (I) combined with other primary malignant tumors; (II) recurrent lung tumor; (III) complicated with infectious diseases and respiratory infections; (IV) no follow-up or data missing. The requirement for written informed consent was waived because of the study’s retrospective design.
Surgery and anesthesia methods
General anesthesia induction included double-lumen endotracheal tube placement for unilateral lung ventilation. Lung-protective ventilation parameters were implemented intraoperatively, with tidal volumes maintained at 4–6 mL/kg ideal body weight and positive end-expiratory pressure (PEEP) set at 2–5 cmH2O. Induction was performed through intravenous administration of propofol, sufentanil and cisatracurium. Maintenance of anesthesia consisted of continuous remifentanil infusion combined with either inhalational sevoflurane or inhalational sevoflurane plus infusion of propofol; Muscle relaxation was maintained through intermittent bolus dosing of cisatracurium. Neuromuscular blockade was reversed with neostigmine [0.04–0.07 mg/kg intravenous injection (IV)] and atropine at surgical closure. Although intraoperative neuromuscular monitoring was not routinely utilized, quantitative train-of-four (TOF) assessment (≥0.9) was mandatory prior to extubation. Patients were extubated in the post-anesthesia care unit (PACU) upon achieving full consciousness, spontaneous breathing, and TOF ≥0.9, followed by transfer to the general ward with continuous oxygenation monitoring. Lung resections were performed via standardized posterolateral thoracoscopy, with lobectomy and systematic mediastinal lymphadenectomy as the baseline protocol. Surgical variations (pneumonectomy for central tumors; segmentectomy in compromised pulmonary function) and lymph node management (dissection/sampling) were dynamically selected based on tumor characteristics and intraoperative findings. Patient-controlled intravenous analgesia with sufentanil was used for postoperative analgesia.
Data collection
Collected parameters spanned: demographics [age, sex, body mass index (BMI)]; preoperative status [comorbidities, American Society of Anesthesiologists (ASA) class, tumor location, imaging-confirmed nodal involvement, smoking history]; anesthesia protocols [technique selection, anesthetic agents, fluid management, opioid-equivalent dosage (fentanyl), adjuvant NSAIDs]; operative metrics (resection type, lymphadenectomy scope, operative time, blood loss); postoperative outcomes [pulmonary/non-pulmonary complications, histopathology, intensive care unit (ICU) admission rate, reintubation events, hospitalization duration]. The fentanyl equivalents conversion is sufentanil 0.1ug or remifentanil 1ug for 1ug fentanyl (9).
Follow-up and outcomes
All patients were followed up until the date of discharge. The primary outcome was PPCs within postoperative 7 days and the secondary outcomes were intraoperative management (duration of surgery, total fluid volume, blood loss, propofol administration, fentanyl equivalents, glucocorticoids administration), cardiac complications (new-onset arrhythmia, myocardial ischemia and heart failure), ICU admission rate, reintubation rate and postoperative hospital stay (PHS) defined as the days from surgery to discharge. Myocardial ischemia was defined as troponin elevation exceeding the 99th percentile of reference values with new or presumed new significant septum terminal (ST) segment or T wave changes or development of pathological Q waves on electrocardiograph (ECG) (10).
According to the European Perioperative Clinical Outcome (EPCO) definitions (10), the postoperative pulmonary complications (PPCs) encompassed the following conditions:
- Pneumonia, characterized by newly developed or progressive radiographic infiltrates coupled with ≥2 of three clinical indicators: core temperature exceeding 38 ℃, abnormal leukocyte count (either elevated or reduced), and presence of purulent respiratory secretions.
- Symptomatic pleural effusion necessitating invasive drainage procedures.
- Clinically significant atelectasis requiring therapeutic bronchoscopy intervention.
- Pneumothorax demanding needle decompression or chest tube placement.
- Respiratory failure defined by postoperative hypoxemia [partial pressure of oxygen (PaO2) <60 mmHg on arterial blood gas analysis] or refractory oxygen desaturation [peripheral oxygen saturation (SpO2) <90% despite supplemental O2 administration], including cases requiring non-invasive ventilation support.
- Acute respiratory distress syndrome (ARDS) meeting Berlin diagnostic criteria.
- Radiologically confirmed pulmonary embolism.
The diagnosis of PPCs incorporated both clinical manifestations [including productive cough, pyrexia (>38 ℃), hypoxemia (oxygen saturation <90%), and dyspnea] and radiological evidence from postoperative chest imaging modalities such as X-ray or computed tomography (CT), which revealed pathological findings including pleural effusion, atelectasis, pulmonary consolidation, or infiltrates (11).
Statistical analysis
All statistical analyses were performed using IBM Statistics SPSS Version 26 (IBM Corp., Armonk, NY, USA). Since the continuous variables in this study were non-normally distributed data, these variables were presented as median [interquartile range (IQR)] and compared with the independent samples Mann-Whitney U test. Categorical variables were presented as numbers (%) and analyzed with the Pearson’s Chi-squared test or Fisher’s exact test as appropriate. To minimize bias caused by the retrospective nature of this study, the patients who received intraoperative NSAIDs (NSAIDs group) were matched to those who did not (control group) using a propensity score matching (PSM). The potential confounders and risk factors, including age, gender, BMI, preoperative complications, smoking status, ASA classification, pathological type, lymph node metastasis, surgical procedure, mediastinal lymph node dissection, and anesthesia technique, were adjusted to compensate for the propensity score. Through 1:1 nearest-neighbor matching with a 0.02 standard deviation caliper, two adequately balanced groups were generated for further analysis. A 2-tailed P value of <0.05 was considered significant.
Results
Of the 336 eligible patients, 306 were eventually included for analysis, with 139 in the NSAIDs group and 167 in the control group before PSM (Figure 1). Before matching, there were significant differences in surgical procedure, mediastinal lymph node dissection, and anesthesia technique between the two groups, whereas, after PSM, there were 119 patients in each group, with no significant differences in baseline characteristics between the groups (Table 1).
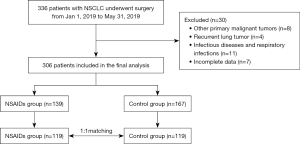
Table 1
| Characteristics | Before matching (n=306) | After matching (n=238) | |||||
|---|---|---|---|---|---|---|---|
| NSAIDs group (n=139) |
Control group (n=167) |
P value | NSAIDs group (n=119) |
Control group (n=119) |
P value | ||
| Age, years | 0.35 | 0.89 | |||||
| <65 | 87 (62.6) | 113 (67.7) | 76 (63.9) | 77 (64.7) | |||
| ≥65 | 52 (37.4) | 54 (32.3) | 43 (36.1) | 42 (35.3) | |||
| Gender | 0.66 | >0.99 | |||||
| Female | 84 (60.4) | 105 (62.9) | 71 (59.7) | 71 (59.7) | |||
| Male | 55 (39.6) | 62 (37.1) | 48 (40.3) | 48 (40.3) | |||
| BMI, kg/m2 | 0.91 | 0.60 | |||||
| <18.5 | 2 (1.4) | 4 (2.4) | 1 (0.8) | 3 (2.5) | |||
| 18.5–24.9 | 73 (52.5) | 84 (50.3) | 65 (54.6) | 57 (47.9) | |||
| 25.0–27.9 | 43 (30.9) | 55 (32.9) | 38 (31.9) | 42 (35.3) | |||
| ≥28.0 | 21 (15.1) | 24 (14.4) | 15 (12.6) | 17 (14.3) | |||
| Preoperative complications | |||||||
| COPD | 0.76 | 0.52 | |||||
| No | 132 (95.0) | 157 (94.0) | 115 (96.6) | 113 (95.0) | |||
| Yes | 7 (5.0) | 10 (6.0) | 4 (3.4) | 6 (5.0) | |||
| Coronary heart disease | 0.79 | >0.99 | |||||
| No | 134 (96.4) | 160 (95.8) | 115 (96.6) | 115 (96.6) | |||
| Yes | 5 (3.6) | 7 (4.2) | 4 (3.4) | 4 (3.4) | |||
| Hypertension | 0.66 | 0.78 | |||||
| No | 95 (68.3) | 118 (70.7) | 80 (67.2) | 82 (68.9) | |||
| Yes | 44 (31.7) | 49 (29.3) | 39 (32.8) | 37 (31.1) | |||
| Diabetes mellitus | 0.07 | 0.84 | |||||
| No | 118 (84.9) | 153 (91.6) | 104 (87.4) | 105 (88.2) | |||
| Yes | 21 (15.1) | 14 (8.4) | 15 (12.6) | 14 (11.8) | |||
| Renal dysfunction | 0.53 | 0.65 | |||||
| No | 135 (97.1) | 164 (98.2) | 117 (98.3) | 116 (97.5) | |||
| Yes | 4 (2.9) | 3 (1.8) | 2 (1.7) | 3 (2.5) | |||
| Liver dysfunction | 0.66 | 0.64 | |||||
| No | 130 (93.5) | 154 (92.2) | 110 (92.4) | 108 (90.8) | |||
| Yes | 9 (6.5) | 13 (7.8) | 9 (7.6) | 11 (9.2) | |||
| Smoking history | 0.17 | 0.89 | |||||
| No | 87 (62.6) | 117 (70.1) | 75 (63.0) | 74 (62.2) | |||
| Yes | 52 (37.4) | 50 (29.9) | 44 (37.0) | 45 (37.8) | |||
| ASA | 0.55 | 0.71 | |||||
| I | 11 (7.9) | 10 (6.0) | 10 (8.4) | 10 (8.4) | |||
| II | 126 (90.6) | 152 (91.0) | 107 (89.9) | 105 (88.2) | |||
| III | 2 (1.4) | 5 (3.0) | 2 (1.7) | 4 (3.4) | |||
| Pathological type | 0.08 | >0.99 | |||||
| Adenocarcinoma | 118 (84.9) | 140 (83.8) | 98 (82.4) | 98 (82.4) | |||
| Non-adenocarcinoma | 21 (15.1) | 27 (16.2) | 21 (17.6) | 21 (17.6) | |||
| Lymph node metastasis | 0.07 | 0.31 | |||||
| No | 123 (88.5) | 135 (80.8) | 103 (86.6) | 108 (90.8) | |||
| Yes | 16 (11.5) | 32 (19.2) | 16 (13.4) | 11 (9.2) | |||
| Surgical procedure | 0.03 | >0.99 | |||||
| Segmentectomy | 84 (60.4) | 122 (73.1) | 79 (66.4) | 79 (66.4) | |||
| Lobectomy | 52 (37.4) | 42 (25.1) | 37 (31.1) | 37 (31.1) | |||
| Total pneumectomy | 3 (2.2) | 3 (1.8) | 3 (2.5) | 3 (2.5) | |||
| Mediastinal lymph node dissection | 0.03 | 0.77 | |||||
| No | 44 (31.7) | 35 (21.0) | 32 (26.9) | 34 (28.6) | |||
| Yes | 95 (68.3) | 132 (79.0) | 87 (73.1) | 85 (71.4) | |||
| Anesthesia technique | 0.046 | 0.55 | |||||
| Inhalation anesthesia | 27 (19.4) | 49 (29.3) | 27 (22.7) | 31 (26.1) | |||
| Intravenous-inhalation anesthesia | 112 (80.6) | 118 (70.7) | 92 (77.3) | 88 (73.9) | |||
Data are presented as numbers (%) and compared with the independent samples Mann-Whitney U test, the Pearson’s chi-squared test or Fisher’s exact test as appropriate. ASA, American Society of Anesthesiologists; BMI, body mass index; COPD, chronic obstructive pulmonary disease; NSAIDs, non-steroidal anti-inflammatory drugs.
After 1:1 propensity score matching, the intraoperative data, including duration of surgery, total fluid volume, blood loss, propofol administration, and glucocorticoids administration, were not significantly different between the two groups, while the intraoperative fentanyl equivalents were significantly lower in the NSAIDs group than in the control group (P=0.01) (Table 2).
Table 2
| Characteristics | NSAIDs group (n=119) | Control group (n=119) | Z/χ2 | P value |
|---|---|---|---|---|
| Duration of surgery, min | 172.0 (124.0–209.0) | 163.0 (127.0–202.0) | 0.603 | 0.55 |
| Total intraoperative fluid volume, mL | 1,600.0 (1,500.0–2,050.0) | 1,750.0 (1,300.0–2,200.0) | 0.327 | 0.74 |
| Intraoperative blood loss, mL | 50.0 (20.0–100.0) | 50.0 (30.0–100.0) | 1.067 | 0.29 |
| Intraoperative propofol administration, mg/kg | 4.1 (2.8–9.5) | 4.8 (3.0–13.0) | 1.763 | 0.08 |
| Intraoperative fentanyl equivalents, μg/kg | 18.8 (9.5–31.2) | 25.9 (12.3–37.6) | 2.449 | 0.01 |
| Intraoperative glucocorticoids administration | 78 (65.6) | 76 (63.9) | 0.074 | 0.79 |
Data are shown as median (interquartile range) or number (%). P<0.05 was considered statistically significant. NSAIDs, non-steroidal anti-inflammatory drugs.
After matching, there was no significant difference in overall PPCs between two groups (24.4% vs. 34.5%, P=0.09; Table 3). In terms of major PPCs, the frequencies of pneumonia and respiratory failure by postoperative day (POD) 7 were significantly lower in the NSAIDs group than those in the control group (P<0.05) (Table 3). The incidence of other PPCs by POD 7 did not differ significantly between the two groups (P>0.05) (Table 3). The NSAIDs cohort exhibited a significantly reduced rate of postoperative myocardial ischemia compared to controls (1.7% vs. 7.6%; P=0.03) (Table 4). Postoperative ICU transfers occurred significantly less frequently in the NSAIDs group compared to controls (2.5% vs. 10.1%, P=0.02) (Table 4). NSAIDs recipients had shorter hospitalization durations compared to controls (median 5.0 days, IQR 4.0–6.0 vs. 4.0–7.0 days, P=0.03) (Table 4).
Table 3
| Variables | NSAIDs group (n=119) | Control group (n=119) | χ2 | P value |
|---|---|---|---|---|
| PPCs | 29 (24.4) | 41 (34.5) | 2.914 | 0.09 |
| Pneumonia | 7 (5.9) | 16 (13.5) | 3.898 | 0.048 |
| Pleural effusion | 10 (8.4) | 15 (12.6) | 0.710 | 0.39 |
| Pneumothorax | 12 (10.1) | 10 (8.4) | 0.200 | 0.65 |
| Pulmonary embolism | 0 | 4 (3.4) | 0.122 | 0.06 |
| Atelectasis | 6 (5.0) | 4 (3.4) | 0.418 | 0.52 |
| Respiratory failure | 1 (0.8) | 7 (5.9) | 0.066 | 0.03 |
| ARDS | 0 | 0 | – | – |
Data are shown as numbers (%). P<0.05 was considered statistically significant. ARDS, acute respiratory distress syndrome; NSAIDs, non-steroidal anti-inflammatory drugs; PPCs, postoperative pulmonary complications.
Table 4
| Variables | NSAIDs group (n=119) | Control group (n=119) | Z/χ2 | P value |
|---|---|---|---|---|
| New-onset arrhythmia | 5 (4.2) | 4 (3.4) | 1.000 | 0.50 |
| Myocardial ischemia | 2 (1.7) | 9 (7.6) | 0.59 | 0.03 |
| Heart failure | 0 | 0 | – | – |
| ICU admission rate | 3 (2.5) | 12 (10.1) | 0.030 | 0.02 |
| Reintubation rate | 1 (0.8) | 6 (5.0) | 0.119 | 0.06 |
| PHS | 5.0 (4.0–6.0) | 5.0 (4.0–7.0) | 2.206 | 0.03 |
Data are shown as medians (interquartile range) or numbers (%). P<0.05 was considered statistically significant. ICU, intensive care unit; NSAIDs, non-steroidal anti-inflammatory drugs; PHS, postoperative hospital stay.
Discussion
Owing to the efficacy in reducing pain and inflammation, NSAIDs are amongst the most popularly used medicines, confirming their position in the World Health Organization (WHO)’s Model List of Essential Medicines (12). Intraoperative NSAIDs are commonly used to improve analgesia and reduce opiate consumption (13). In this study, flurbiprofen Axetil [a nonselective cyclooxygenase inhibitor (COX) inhibitor with high binding affinity to the site of lesion] was the only NSAID used during surgery, in doses of approximately 50–100 mg (14). Flurbiprofen axetil is a prodrug of flurbiprofen and suppresses the COX production in the spinal cord and peripheral pathways, leading to a reduction in prostaglandin synthesis (15). Although the COX-2 selectivity of flurbiprofen axetil was not high, it was widely used as an adjunct to general anesthesia (16). Researchers have demonstrated that perioperative use of flurbiprofen axetil was associated with decreases in serum concentrations of inflammatory cytokines and growth factors in blood and better prognosis of patients after surgery (14,15,17). Our prior research also showed that the intraoperative administration of flurbiprofen axetil prolonged postoperative survival, possibly due to relieved immunosuppression action in patients after cancer surgery (18). Considering the widespread use of NSAIDs during the intraoperative period, their effects on outcomes after lung surgery are indeed worthy of further investigation.
In this retrospective study, 336 patients with primary NSCLC after surgical resection were followed up, and 306 were eventually included for analysis. The incidence of PPCs associated with pulmonary resection for NSCLC has been reported to be 9–37% (19). Following PSM, both cohorts consisted of 119 patients each, with postoperative pulmonary complications occurring in 24.4% of NSAIDs-treated patients compared to 34.5% in the control group by postoperative day 7. In the case of the overall incidence of PPCs, although the frequency of PPCs in patients treated with intraoperative NSAIDs administration was lower, there was no significant difference between two groups (P=0.09). However, when major PPCs were analyzed separately, the results in this study showed that intraoperative NSAIDs administration might have an influence on different postoperative complications after lung resection.
As a predominant subtype of PPCs, postoperative pneumonia demonstrates significant associations with elevated healthcare expenditures and mortality risk following pulmonary resection for lung cancer, with reported incidence rates spanning 3.1% to 19% across clinical cohorts (20). The possible causes of postoperative pneumonia are mainly as follows: the invasiveness of the surgical procedure, the aspiration caused by the increased gastric pressure in the pre-oxygenation stage, the contamination secondary to intubation, and the injury of lung tissue induced by mechanical ventilation (21). Furthermore, other perioperative factors have also been reported to influence the development of postoperative pneumonia, such as age, physical status of ASA, intraoperative blood loss, and anesthesia-related drugs (22). In the present study, the incidence of early postoperative pneumonia was higher in the control group than that in the NSAIDs group (13.4% vs. 5.9%, P=0.048), which suggests that the use of NSAIDs during operation may influence the development of postoperative pneumonia to a certain extent. We speculated that the possible explanations involved postoperative inflammatory conditions and immunosuppression. As NSAIDs, one of its effects is to reverse the increased expression of inflammatory molecules and reduce the levels of inflammatory cytokines such as interleukin-10, interleukin-6 and tumor necrosis factor-α, as the earlier researches elucidated (23-25), which might contribute to alleviate the postoperative inflammatory response of the lungs, thereby reducing the occurrence of postoperative pneumonia. Moreover, it is well known that opioids have the immunosuppressive function (26), but the objective of intraoperative NSAIDs use for multimodal analgesia is to minimize perioperative opiate consumption, which leads to a reduction in the incidence of lung infections due to immunosuppression.
In addition, postoperative respiratory failure was another notable postoperative pulmonary complication in this cohort. Following post anesthesia care unit (PACU) care, post-surgical patients who remain in the hospital continue to be at risk of respiratory failure. Several factors may contribute to this complication, such as the effects of anesthesia, the influence of the surgical site, pneumonia, atelectasis, impaired lung function, fluid transfer and imbalance, airway conditions, etc. (27,28). The earlier study has shown that respiratory failure occurred in 9.6% of patients with post-pneumonectomy and confers a higher risk of 90-day mortality (29). In this study, 8 of 238 matched patients developed respiratory failure due to POD 7 (3.4%), which is lower than the incidence described above and may be attributed to the heterogeneity in the study population. The incidence of respiratory failure in the NSAIDs and control groups was 0.8% and 5.9%, respectively, and the difference between the two groups was significant (P=0.03). Notably, in the NSAIDS group, intraoperative fentanyl equivalents were lower than in the control group (P=0.02), indicating lower overall opioid consumption in the NSAIDS group. The published literatures have identified the potential associations between opioid administration and postoperative respiratory depression (30,31). Because opioid administration remains common practice intraoperatively, opioid-induced respiratory depression (OIRD) is often an under-diagnosed cause of postoperative respiratory depression, which leads to hypoxia and hypercapnia (32). However, the onset of respiratory failure is characterized by the respiratory system’s inability to either oxygenate the bloodstream sufficiently (leading to hypoxemia) or remove metabolic carbon dioxide (causing hypercapnia), and the coexistence of these dysfunctions often precipitates clinical deterioration (33). Ohnuma et al. found NSAIDs were associated with decreased occurrence of postoperative respiratory failure and with lower opioid consumption when used alone or in combination with acetaminophen, similar to our results (34). Also, postoperative pneumonia has been identified as risk factor for respiratory failure (35). In the present study, the higher frequency of postoperative pneumonia in control group has made contribution to the occurrence of respiratory failure partly. Postoperative respiratory failure is a challenging and relatively common complication that necessarily leads to increased ICU occupancy, longer hospital stays, and higher costs. This is also reflected in our results.
In respect of the non-pulmonary complications, findings from this study revealed that the use of intraoperative NSAIDs decreased the incidence of postoperative myocardial ischemia. The difference was significant between two groups (P=0.03). Myocardial ischemia occurs in 8% of adults undergoing major noncardiac surgery and is diagnosed with an elevated postoperative troponin measurement (36). The majority (90%) of postoperative myocardial ischemic events occur early after surgery and are asymptomatic (37). In case of the surgery itself, noncardiac surgery is associated with platelet activation, and coronary-artery thrombus may be a mechanism of perioperative myocardial ischemic or infarction (38,39). Aspirin, a typical nonsteroidal anti-inflammatory drug, inhibits platelet aggregation, and the perioperative administration of aspirin may prevent major vascular complications by inhibiting thrombus formation (40). Nevertheless, most of the information available is derived from trials focusing on preoperative low-dose administration of aspirin (41-43). As for the postoperative myocardial ischemic events, the clinical influence associated with intraoperative NSAIDs use remains unclear. To our knowledge, there is also a lack of literatures providing clear evidence that flurbiprofen axetil improves myocardial ischemia or infarction. We hypothesized that the anticoagulant effects of NSAIDs might be responsible for the current results in the present study. Given biases caused by the small retrospective sample size, the results of this study need to be cautiously considered and confirmed by a large perioperative trial.
Besides the postoperative complications, the present study demonstrated that there were higher ICU admission rates (P=0.02) and longer PHS (P=0.03) in the control group than in the NSAIDS group. Obviously, in the control group, the increased incidence of pulmonary complications (pneumonia and respiratory failure), or non-pulmonary complications (myocardial ischemia), all brought about these results, greatly increasing the medical costs and aggravating the economic burden of patients (44).
There were also some limitations in the study. Firstly, this was a single-center retrospective study. Given the relatively small sample size, the results of this study need to be interpreted with caution. Secondly, some potentially critical perioperative data, such as ventilator parameters and pulmonary function test, were not included in the analysis due to the retrospective design. The absence of data may have an impact on the results to some extent. Thirdly, our results may be influenced by bias from the variation in the standard practice of clinicians, which may affect the incidence of the PPCs. Finally, there are several categories of NSAIDs based on the pharmacological mechanism, however, only flurbiprofen axetil was investigated in this study. Biases in different NSAIDs could also affect the final analysis. Therefore, large samples and prospective designs are needed to verify the underlying mechanisms of the effect of intraoperative NSAIDs on PPCs.
Conclusions
Taken together, although intraoperative NSAIDs did not reduce overall PPCs development, the lower frequencies of postoperative pneumonia, respiratory failure and ICU admission as well as shortened hospital stay implicated that the use of NSAIDs during operation is an extremely simple medical act that appears to be associated with a better prognosis for patients with NSCLC. These observations warrant prospective clinical studies.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2007/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2007/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2007/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2007/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by Peking University Cancer Hospital Clinical Research Ethics Committee (approval No. 2019YJZ22-GZ02) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mithoowani H, Febbraro M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr Oncol 2022;29:1828-39. [Crossref] [PubMed]
- Finnerty CC, Mabvuure NT, Ali A, et al. The surgically induced stress response. JPEN J Parenter Enteral Nutr 2013;37:21S-9S. [Crossref] [PubMed]
- Sameed M, Choi H, Auron M, et al. Preoperative Pulmonary Risk Assessment. Respir Care 2021;66:1150-66. [Crossref] [PubMed]
- Ceelen W, Pattyn P, Mareel M. Surgery, wound healing, and metastasis: recent insights and clinical implications. Crit Rev Oncol Hematol 2014;89:16-26. [Crossref] [PubMed]
- Pu CY, Batarseh H, Zafron ML, et al. Effects of Preoperative Breathing Exercise on Postoperative Outcomes for Patients With Lung Cancer Undergoing Curative Intent Lung Resection: A Meta-analysis. Arch Phys Med Rehabil 2021;102:2416-2427.e4. [Crossref] [PubMed]
- Yeung JH, Gates S, Naidu BV, et al. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev 2016;2:CD009121. [Crossref] [PubMed]
- Wick EC, Grant MC, Wu CL. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: A Review. JAMA Surg 2017;152:691-7. [Crossref] [PubMed]
- Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol 2020;180:114147. [Crossref] [PubMed]
- Zhu W, Zhu L, Li S, et al. Anesthetic predictors for postoperative pneumonia in patients with non-small cell lung cancer. J Thorac Dis 2024;16:3204-12. [Crossref] [PubMed]
- Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015;32:88-105. [Crossref] [PubMed]
- Huang Q, Rauniyar R, Yang J, et al. Risk stratification of postoperative pulmonary complications in elderly patients undergoing lung cancer resection: a propensity score-matched study. J Thorac Dis 2023;15:3908-18. [Crossref] [PubMed]
- Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol 2020;180:114147. [Crossref] [PubMed]
- Gazendam A, Ekhtiari S, et al. Effect of a Postoperative Multimodal Opioid-Sparing Protocol vs Standard Opioid Prescribing on Postoperative Opioid Consumption After Knee or Shoulder Arthroscopy: A Randomized Clinical Trial. JAMA 2022;328:1326-35. [Crossref] [PubMed]
- Zhao X, Ji L. Flurbiprofen axetil: Analgesic effect and adverse reaction. Pak J Pharm Sci 2018;31:1163-7.
- Wan Z, Chu C, Zhou R, et al. Effects of Oxycodone Combined With Flurbiprofen Axetil on Postoperative Analgesia and Immune Function in Patients Undergoing Radical Resection of Colorectal Cancer. Clin Pharmacol Drug Dev 2021;10:251-9. [Crossref] [PubMed]
- Sakamaki K, Watanabe K, Woo T, et al. Multicentre randomised phase II study of the perioperative administration of flurbiprofen axetil in patients with non-small cell lung cancer: study protocol of the FLAX Study. BMJ Open 2020;10:e040969. [Crossref] [PubMed]
- Wen Y, Wang M, Yang J, et al. A Comparison of Fentanyl and Flurbiprofen Axetil on Serum VEGF-C, TNF-α, and IL-1ß Concentrations in Women Undergoing Surgery for Breast Cancer. Pain Pract 2015;15:530-7. [Crossref] [PubMed]
- Huang WW, Zhu WZ, Mu DL, et al. Perioperative Management May Improve Long-term Survival in Patients After Lung Cancer Surgery: A Retrospective Cohort Study. Anesth Analg 2018;126:1666-74. [Crossref] [PubMed]
- Motono N, Ishikawa M, Iwai S, et al. Analysis of risk factors for postoperative complications in non-small cell lung cancer: comparison with the Japanese National Clinical Database risk calculator. BMC Surg 2022;22:180. [Crossref] [PubMed]
- Zhou J, Wu D, Zheng Q, et al. A Clinical Prediction Model for Postoperative Pneumonia After Lung Cancer Surgery. J Surg Res 2023;284:62-9. [Crossref] [PubMed]
- Lai G, Guo N, Jiang Y, et al. Duration of one-lung ventilation as a risk factor for postoperative pulmonary complications after McKeown esophagectomy. Tumori 2020;106:47-54. [Crossref] [PubMed]
- Okamura A, Watanabe M, Mine S, et al. Spirometric Lung Age Predicts Postoperative Pneumonia After Esophagectomy. World J Surg 2016;40:2412-8. [Crossref] [PubMed]
- Raaijmakers TK, van den Bijgaart RJE, Scheffer GJ, et al. NSAIDs affect dendritic cell cytokine production. PLoS One 2022;17:e0275906. [Crossref] [PubMed]
- Nakata H, Shelby T, Wang JC, et al. Postoperative Complications Associated with Non-Steroidal Anti-Inflammatory Combinations Used Status-Post Total Hip and Knee Arthroplasty. J Clin Med 2023;12:6969. [Crossref] [PubMed]
- Vaish V, Sanyal SN. Chemopreventive effects of NSAIDs on cytokines and transcription factors during the early stages of colorectal cancer. Pharmacol Rep 2011;63:1210-21. [Crossref] [PubMed]
- Bradley A, Boland JW. Effects of Opioids on Immune and Endocrine Function in Patients with Cancer Pain. Curr Treat Options Oncol 2023;24:867-79. [Crossref] [PubMed]
- Attaallah AF, Vallejo MC, Elzamzamy OM, et al. Perioperative risk factors for postoperative respiratory failure. J Perioper Pract 2019;29:49-53. [Crossref] [PubMed]
- Imperatore F, Gritti F, Esposito R, et al. Non-Invasive Ventilation Reduces Postoperative Respiratory Failure in Patients Undergoing Bariatric Surgery: A Retrospective Analysis. Medicina (Kaunas) 2023;59:1457. [Crossref] [PubMed]
- Kidane B, Jacob N, Bruinooge A, et al. Postoperative but not intraoperative transfusions are associated with respiratory failure after pneumonectomy. Eur J Cardiothorac Surg 2020;58:1004-9. [Crossref] [PubMed]
- Gupta K, Nagappa M, Prasad A, et al. Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses. BMJ Open 2018;8:e024086. [Crossref] [PubMed]
- Khanna AK, Bergese SD, Jungquist CR, et al. Prediction of Opioid-Induced Respiratory Depression on Inpatient Wards Using Continuous Capnography and Oximetry: An International Prospective, Observational Trial. Anesth Analg 2020;131:1012-24. [Crossref] [PubMed]
- Ayad S, Khanna AK, Iqbal SU, et al. Characterisation and monitoring of postoperative respiratory depression: current approaches and future considerations. Br J Anaesth 2019;123:378-91. [Crossref] [PubMed]
- Palermo J, Tingey S, Khanna AK, et al. Evaluation and Prevention of Perioperative Respiratory Failure. J Clin Med 2024;13:5083. [Crossref] [PubMed]
- Ohnuma T, Raghunathan K, Ellis AR, et al. Effects of Acetaminophen, NSAIDs, Gabapentinoids, and Their Combinations on Postoperative Pulmonary Complications After Total Hip or Knee Arthroplasty. Pain Med 2020;21:2385-93. [Crossref] [PubMed]
- Sachdev G, Napolitano LM. Postoperative pulmonary complications: pneumonia and acute respiratory failure. Surg Clin North Am 2012;92:321-44. ix. [Crossref] [PubMed]
- Devereaux PJ, Sessler D, Lalu M. Myocardial injury after noncardiac surgery. Can J Anaesth 2022;69:561-7. [Crossref] [PubMed]
- Rajagopalan S, Ford I, Bachoo P, et al. Platelet activation, myocardial ischemic events and postoperative non-response to aspirin in patients undergoing major vascular surgery. J Thromb Haemost 2007;5:2028-35. [Crossref] [PubMed]
- Gualandro DM, Campos CA, Calderaro D, et al. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis 2012;222:191-5. [Crossref] [PubMed]
- Zheng KL, Bor WL, Vernooij LM, et al. Postoperative myocardial injury and platelet reactivity in patients undergoing vascular surgery: The platelet reactivity and postoperative myocardial injury after major vascular surgery (PROMISE) study. Thromb Res 2022;218:177-85. [Crossref] [PubMed]
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014;370:1494-503. [Crossref] [PubMed]
- Smilowitz NR, Berger JS. Perioperative Cardiovascular Risk Assessment and Management for Noncardiac Surgery: A Review. JAMA 2020;324:279-90. [Crossref] [PubMed]
- Oscarsson A, Gupta A, Fredrikson M, et al. To continue or discontinue aspirin in the perioperative period: a randomized, controlled clinical trial. Br J Anaesth 2010;104:305-12. [Crossref] [PubMed]
- Antolovic D, Rakow A, Contin P, et al. A randomised controlled pilot trial to evaluate and optimize the use of anti-platelet agents in the perioperative management in patients undergoing general and abdominal surgery--the APAP trial (ISRCTN45810007). Langenbecks Arch Surg 2012;397:297-306. [Crossref] [PubMed]
- Tsai MH, Chuang HC, Lin YT, et al. Predictors of hospital expenses and hospital stay among patients undergoing total laryngectomy: Cost effectiveness analysis. PLoS One 2020;15:e0236122. [Crossref] [PubMed]


