
Association between red blood cell distribution width to albumin ratio (RAR) and long-term prognosis of patients with sudden cardiac arrest
Highlight box
Key findings
• The higher the red blood cell distribution width to albumin ratio (RAR), the higher the mortality rates at 28 days, 90 days, 1 year and 5 years.
• A clinical model based on RAR for predicting the prognosis of patients with sudden cardiac arrest was constructed.
What is known and what is new?
• RAR has been established as a novel comprehensive inflammatory-nutritional assessment indicator.
• The RAR has demonstrated significant prognostic value in sepsis, heart failure, myocardial infarction, and chronic renal insufficiency.
• Red blood cell distribution width (RDW) and albumin are significantly associated with long-term mortality rate in cardiac arrest patients.
• A composite index RAR was used to predict patients’ long-term survival outcomes
What is the implication, and what should change now?
• In the early management of post-cardiac arrest patients, attention should be paid to easily overlooked indicators such as albumin levels and RDW.
Introduction
Cardiac arrest (CA) is one of the most dangerous conditions in clinical practice, typically defined as the absence of a palpable pulse. It can be classified based on the occurrence location (in-hospital or out-of-hospital) and the amenability to defibrillation (1). Research indicates that in the US, over 290,000 adults experience in-hospital CAs annually, with the most common causes being cardiac (50–60%) followed by respiratory insufficiency (15–40%). In 2017, there were approximately 9 to 10 cases of in-hospital CA per 1,000 hospitalized patients in the US (2), while in the UK in 2013, there were 1.6 cases of in-hospital CA per 1,000 admissions, with a discharge survival rate of only 18% (3).
Post-cardiac arrest syndrome (PCAS) is a condition characterized by brain injury due to systemic ischemia during CA and reperfusion injury during cardiopulmonary resuscitation, leading to cerebral ischemia and hypoxia; myocardial ischemia causing cardiac dysfunction; and systemic reperfusion injury. PCAS significantly affects the survival rates of the patients post-cardiopulmonary resuscitation. Although there are recommended treatment strategies to prevent PCAS, their efficacy remains uncertain (4). Early prediction of survival rates in patients post-CA is crucial.
Previous studies have shown that the red blood cell distribution width to albumin ratio (RAR) has good predictive value for overall mortality in patients with conditions such as tumors, burns, and chronic kidney disease (CKD) (5-8). However, the predictive function of RAR for in-hospital and long-term all-cause mortality among patients following CA has not been adequately studied, therefore, this is a key focus of our study. Additionally, this study aims to establish and validate a prediction model using this indicator. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2030/rc).
Methods
Data sources
The retrospective study utilized data withdrawn from Medical Information Mart for Intensive Care IV (MIMIC-IV) database, from 2008 to 2019, a total of 315,460 hospitalized patients from the Beth Israel Deaconess Medical Center in Boston and the Massachusetts General Hospital were enrolled in the study. Our authors have been given access to the database (Author Certification Number: J.L. ID: 11841874). Data files for the MIMIC-IV database can be downloaded from PhysioNet (https://physionet.org/content/mimiciv/2.2/). Given the anonymized nature of patient data and the absence of identifiable private information in the database, this research is considered exempt from the need for informed consent and ethical application. The study adheres to the principles outlined in the Declaration of Helsinki and its subsequent amendments.
Study population
This study included individuals who met the following criteria: (I) diagnosed with sudden CA; (II) aged 18 years or older; (III) intensive care unit (ICU) hospitalization duration of at least 24 hours. Exclusion criteria were defined as follows: (I) patients without records of RDW or albumin; (II) patients with more than 20% missing data in the inclusion criteria; (III) combined with polycythemia vera, multiple myeloma, secondary polycythemia and myelofibrosis.
Data extraction
We extracted the index from the MIMIC-IV database, according to CA’s International Classification of Diseases (ICD)-9 and ICD-10 codes (the diagnostic codes for CA are as follows: ‘Z8674’, ‘4275’, ‘I469’, ‘V1253’, ‘I462’, ‘I468’, ‘I97711’, ‘I97710’, ‘I97121’, ‘I97120’): age, gender, body mass index (BMI), heart rate (HR), mean arterial pressure (MAP), respiratory rate (RR), pulse oximetry [saturation of peripheral oxygen (SpO2)], hypertension, diabetes, heart failure, myocardial infarction, malignant tumour, CKD, acute kidney injury (AKI), cirrhosis, anemia, hepatitis, pneumonia, hospital stay time; clinical score scales: sequential organ failure assessment (SOFA), simplified acute physiology score II (SAPS II), acute physiology score III (APS III), oxford acute severity of illness score (OASIS), Glasgow coma scale (GCS), systemic inflammatory response syndrome (SIRS), Charlson comorbidity index (CCI); laboratory indicators (select the first measurement during hospitalization): white blood cell count (WBC), red blood cell count (RBC), hemoglobin value (Hb), platelet count (PLT), red blood cell distribution width (RDW), hematocrit, serum albumin (ALB), serum potassium, blood glucose (Glu), pH value, blood lactate (Lac), international normalized ratio (INR), total bilirubin, alanine aminotransferase, aspartate aminotransferase, serum creatinine; clinical treatment: whether accepting cardiac pulmonary resuscitation (CPR),whether using epinephrine, dobutamine, dopamine, norepinephrine, phenylephrine and milrinone; prognostic indicators: in-hospital mortality, 28-day mortality, 90-day mortality, 1-year mortality, 5-year mortality and survival time. The exposure factor RAR was calculated as RDW (%) divided by albumin (g/dL). Data extraction was performed using Navicat Premium (version 15.0). Continuous numerical variables with missing values that were not the main study indicator are filled in by the mean of the data in this column. Covariates with missing values ≥20% were excluded.
Study endpoints
The primary endpoint of this study is the 1-year mortality rate, while secondary endpoints include the 5-year, 28-day, 90-day and in-hospital mortality rates.
Statistical analysis
In this study, according to the tripartite number of RAR, all participants were roughly categorized into three groups (Q1: <4.51, Q2: 4.51–5.77, Q3: >5.77) for analysis. Continuous data conforming to normality are presented as mean ± standard deviation (SD), while continuous data that did not conform to a normal distribution were presented as median (lower quartile, upper quartile). The differences of every group were compared employing t-tests or non-parametric tests. Frequencies (percentages) were applied to represent the categorical index, and Chi-squared tests or Fisher’s exact tests were used to analyze categorical variables.
Cox proportional risk regression models were employed to analyze factors associated with in-hospital, 28-day, 90-day, 1-year and 5-year mortality rates, expressed as hazard ratio (95% confidence interval) [HR (95% CI)]. The RAR quantiles were used to divide the participants into three groups, and the first tertile group was selected as the reference group.
The following models were used for covariate adjustments: the crude model only included RAR, and model 1 corrected for age, sex, BMI, and statistically significant indicators in general vital signs [HR, RR, mean blood pressure (MBP)] based on the original model. Model 2 adjusted for comorbidities (hypertension, diabetes, heart failure, myocardial infarction, malignancy, renal failure, AKI, cirrhosis, anemia, hepatitis, pneumonia), clinical scores (SOFA, SAPS II, APS III, OASIS, GCS, SIRS, CCI), and whether or not CPR was performed on the basis of model 1. Model 3 adjusted laboratory indicators (WBC, RBC, Hb, PLT, RDW, hematocrit, ALB, serum potassium, blood Glu, pH value, blood Lac, INR, total bilirubin, alanine aminotransferase, aspartate aminotransferase, serum creatinine) and the use of various vasoactive drugs (epinephrine, dobutamine, dopamine, norepinephrine, phenylephrine, milrinone). A restricted cubic spline fit was utilized to analyze the association between RAR and the mortality rates. Kaplan-Meier (KM) curves were used to compare the survival rates across different RAR levels. Using least absolute shrinkage and selection operator (Lasso) regression, select the optimal variables for model construction based on a Lambda of one standard error. Statistical analyses and graphical representations were conducted using R (version 4.4.1), GraphPad Prism (version 10.1.2), and IBM SPSS (version 25). A two-tailed P value <0.05 was considered significant.
Subgroup analysis
Subgroup analysis was employed to investigate potential disparities in correlations across various subgroups, encompassing gender, hypertension, diabetes, heart failure, myocardial infarction, malignancy, renal failure, AKI, cirrhosis, anemia, hepatitis, pneumonia, whether accepting CPR, whether using epinephrine, dobutamine, dopamine, norepinephrine, phenylephrine, milrinone.
Construction of prognostic models
Lasso regression was used to filter all the independent variables, and the independent variables corresponding to the Lambda coefficient under double standard error were selected as the independent variables required to construct the model. All patients were divided into 8:2 into modeling group and test group, and the clinical prognosis model of CA patients was constructed by using the COX proportional hazards regression model. The model performance was evaluated in the modeling group and the test group, and the quality of the prediction model was judged according to the discrimination, calibration and clinical utility.
Results
Population and baseline characteristics and difference of RAR between survival group and death group in patients with CA
This study finally included 1,673 CA patients from the MIMIC-IV database (Figure 1). Participants were divided into three groups, according to the tripartite number of RAR (<4.51: n=557, 4.51–5.77: n=560, >5.77: n=556), with demographic characteristics (Table 1). In patients with CA, those in Q2 and Q3 group with high RAR levels had higher median ages (67 and 68 years), heart rates (88 and 92 bpm), and compared to the Q1 and Q2 groups, patients in Q3 group had a higher proportion of male patients (42.1%). Complication-wise, the Q3 group had lower rates of hypertension and myocardial infarction but higher rates of diabetes, malignant tumor, CKD, AKI, cirrhosis, anemia, and hepatitis compared to the Q1 and Q2 groups. Significant differences were observed in various laboratory indicators. Clinical scoring showed the median SOFA score, APS III score, SAPS II score, and OASIS score in the Q3 group were 9, 63, 49, and 39, respectively, all higher than those in the other two groups. In terms of clinical treatment, adrenaline and epinephrine usage was higher in the high RAR group. Regarding the clinical prognosis of patients with CA, the in-hospital mortality rates, 28-day mortality rates, 90-day mortality rates, 1-year mortality rates, and 5-year mortality rates and proportions in the Q3 group were 312 (56.1%), 292 (52.5%), 365 (65.6%), 412 (74.1%), and 438 (78.8%), respectively, all significantly higher than those in the lower RAR value groups. The increasing trend was progressive, with the proportion of patients in the Q3 group being higher than that in the Q2 group and the Q1 group. All these differences were statistically significant (P<0.05).

Table 1
| Variables | Overall (n=1,673) | Q1 (<4.51) (n=557) | Q2 (4.51–5.77) (n=560) | Q3 (>5.77) (n=556) | P value |
|---|---|---|---|---|---|
| Age (years) | 66 [56, 77] | 63 [52, 74] | 67 [58, 79.25] | 68 [57, 78] | <0.001 |
| Gender | 0.001 | ||||
| Male | 614 (36.7) | 176 (31.6) | 204 (36.4) | 234 (42.1) | |
| Female | 1,059 (63.3) | 381 (68.4) | 356 (63.6) | 322 (57.9) | |
| BMI (kg/m2) | 28.15 [24.17, 32.95] | 28.52 [24.54, 33.02] | 28.17 [24.20, 33.18] | 27.66 [23.49, 32.64] | 0.07 |
| Heart rate (bpm) | 88 [75, 104] | 85 [73, 98] | 88 [74, 103] | 92 [78, 111] | <0.001 |
| MBP (mmHg) | 83 [71, 95] | 85 [74, 100] | 81 [70, 94] | 81 [68.75, 91] | <0.001 |
| Respiratory rate (bpm) | 20 [16, 24] | 19 [16, 24] | 20 [16, 24] | 20 [17, 25] | 0.003 |
| SpO2 (%) | 98 [95, 100] | 98 [96, 100] | 98 [95, 100] | 98 [95, 100] | 0.25 |
| Hypertension | 0.001 | ||||
| No | 1,122 (67.1) | 340 (61.0) | 387 (69.1) | 395 (71.0) | |
| Yes | 551 (32.9) | 217 (39.0) | 173 (30.9) | 161 (29.0) | |
| Diabetes | <0.001 | ||||
| No | 1,054 (63.0) | 389 (69.8) | 342 (61.1) | 323 (58.1) | |
| Yes | 619 (37.0) | 168 (30.2) | 218 (38.9) | 233 (41.9) | |
| Heart failure | 0.45 | ||||
| No | 900 (53.8) | 311 (55.8) | 292 (52.1) | 297 (53.4) | |
| Yes | 773 (46.2) | 246 (44.2) | 268 (47.9) | 259 (46.6) | |
| Myocardial infarction | <0.001 | ||||
| No | 1,314 (78.5) | 406 (72.9) | 446 (79.6) | 462 (83.1) | |
| Yes | 359 (21.5) | 151 (27.1) | 114 (20.4) | 94 (16.9) | |
| Malignant tumour | 0.001 | ||||
| No | 1,483 (88.6) | 509 (91.4) | 504 (90.0) | 470 (84.5) | |
| Yes | 190 (11.4) | 48 (8.6) | 56 (10.0) | 86 (15.5) | |
| CKD | <0.001 | ||||
| No | 1,233 (73.7) | 458 (82.2) | 402 (71.8) | 373 (67.1) | |
| Yes | 440 (26.3) | 99 (17.8) | 158 (28.2) | 183 (32.9) | |
| AKI | <0.001 | ||||
| No | 741 (44.3) | 309 (55.5) | 239 (42.7) | 193 (34.7) | |
| Yes | 932 (55.7) | 248 (44.5) | 321 (57.3) | 363 (65.3) | |
| Cirrhosis | <0.001 | ||||
| No | 1,554 (92.9) | 546 (98.0) | 515 (92.0) | 493 (88.7) | |
| Yes | 119 (7.1) | 11 (2.0) | 45 (8.0) | 63 (11.3) | |
| Anaemia | <0.001 | ||||
| No | 762 (45.5) | 331 (59.4) | 237 (42.3) | 194 (34.9) | |
| Yes | 911 (54.5) | 226 (40.6) | 323 (57.7) | 362 (65.1) | |
| Hepatitis | 0.001 | ||||
| No | 1,604 (95.9) | 545 (97.8) | 539 (96.2) | 520 (93.5) | |
| Yes | 69 (4.1) | 12 (2.2) | 21 (3.8) | 36 (6.5) | |
| Pneumonia | 0.06 | ||||
| No | 934 (55.8) | 333 (59.8) | 297 (53.0) | 304 (54.7) | |
| Yes | 739 (44.2) | 224 (40.2) | 263 (47.0) | 252 (45.3) | |
| WBC (×109/L) | 12.80 [8.70, 18.50] | 13.40 [9.40, 18.70] | 12.50 [8.70, 18.40] | 12.45 [8.10, 18.50] | 0.14 |
| RBC (×109/L) | 3.62 [3.02, 4.29] | 4.12 [3.50, 4.67] | 3.56 [3.03, 4.13] | 3.18 [2.71, 3.78] | <0.001 |
| Platelet count (×109/L) | 198 [142, 265] | 210 [162, 264] | 191.50 [140, 258.25] | 189 [118.75, 277] | <0.001 |
| Hemoglobin (g/L) | 10.80 [8.90, 12.70] | 12.60 [10.90, 14.20] | 10.60 [8.90, 12.10] | 9.30 [8, 10.80] | <0.001 |
| RDW (%) | 14.90 [13.60, 16.70] | 13.60 [13, 14.40] | 15 [14.10, 16.22] | 17 [15.38, 19.10] | <0.001 |
| Hematocrit (%) | 33.30 [28, 39.10] | 38 [33.20, 42.60] | 32.70 [28.20, 37.12] | 29.10 [25.58, 34.23] | <0.001 |
| Albumin (g/dL) | 3 [2.60, 3.40] | 3.50 [3.30, 3.80] | 3 [2.80, 3.20] | 2.40 [2.10, 2.70] | <0.001 |
| Potassium (mEq/L) | 4.20 [3.80, 4.80] | 4.20 [3.80, 4.60] | 4.20 [3.80, 4.80] | 4.30 [3.80, 4.93] | 0.17 |
| Glucose (mg/dL) | 154 [116, 221] | 153 [118, 221] | 161 [121.75, 230.25] | 146.50 [109, 211.25] | 0.001 |
| pH | 7.32 [7.24, 7.39] | 7.31 [7.25, 7.38] | 7.32 [7.24, 7.40] | 7.32 [7.22, 7.39] | 0.65 |
| Lactate (mmol/L) | 2.60 [1.60, 4.30] | 2.40 [1.40, 3.59] | 2.85 [1.70, 4.90] | 2.80 [1.60, 4.82] | <0.001 |
| INR | 1.30 [1.20, 1.70] | 1.20 [1.10, 1.40] | 1.40 [1.20, 1.80] | 1.50 [1.30, 1.92] | <0.001 |
| Total bilirubin (mg/dL) | 0.60 [0.40, 1.10] | 0.50 [0.40, 0.80] | 0.70 [0.40, 1.29] | 0.70 [0.40, 1.40] | <0.001 |
| ALT (IU/L) | 54 [23, 174] | 70 [30, 189] | 55 [23, 195] | 41.50 [20, 126.75] | <0.001 |
| AST (IU/L) | 84 [35, 248] | 97 [39, 290] | 89.50 [35, 272.75] | 67 [31, 176.75] | 0.001 |
| Creatinine (mg/dL) | 1.30 [0.90, 2.20] | 1.10 [0.90, 1.60] | 1.40 [0.90, 2.30] | 1.50 [0.90, 2.73] | <0.001 |
| SOFA | 8 [5, 11] | 6 [3, 9] | 8 [5, 11] | 9 [6, 11] | <0.001 |
| APS III | 56 [41, 76] | 47 [34, 67] | 57 [42, 77] | 63 [48, 84] | <0.001 |
| SIRS | 3 [2, 4] | 3 [2, 3] | 3 [2, 4] | 3 [2, 4] | <0.001 |
| SAPS II | 44 [34, 56] | 38 [29, 50] | 45 [36, 56.25] | 49 [39, 60] | <0.001 |
| OASIS | 37 [31, 44] | 34 [28, 41] | 38 [31, 44] | 39 [33, 45] | <0.001 |
| GCS | 15 [14, 15] | 15 [14, 15] | 15 [14, 15] | 15 [14, 15] | 0.78 |
| CCI | 6 [3, 8] | 4 [2, 7] | 6 [4, 8] | 6 [4, 8] | <0.001 |
| CPR | 0.08 | ||||
| No | 1,439 (86.0) | 494 (88.7) | 473 (84.5) | 472 (84.9) | |
| Yes | 234 (14.0) | 63 (11.3) | 87 (15.5) | 84 (15.1) | |
| Epinephrine | 0.007 | ||||
| No | 1,413 (84.5) | 489 (87.8) | 474 (84.6) | 450 (80.9) | |
| Yes | 260 (15.5) | 68 (12.2) | 86 (15.4) | 106 (19.1) | |
| Dobutamine | 0.56 | ||||
| No | 1,588 (94.9) | 533 (95.7) | 528 (94.3) | 527 (94.8) | |
| Yes | 85 (5.1) | 24 (4.3) | 32 (5.7) | 29 (5.2) | |
| Dopamine | 0.003 | ||||
| No | 1,494 (89.3) | 506 (90.8) | 480 (85.7) | 508 (91.4) | |
| Yes | 179 (10.7) | 51 (9.2) | 80 (14.3) | 48 (8.6) | |
| Norepinephrine | <0.001 | ||||
| No | 821 (49.1) | 327 (58.7) | 266 (47.5) | 228 (41.0) | |
| Yes | 852 (50.9) | 230 (41.3) | 294 (52.5) | 328 (59.0) | |
| Phenylephrine | <0.001 | ||||
| No | 1,292 (77.2) | 463 (83.1) | 414 (73.9) | 415 (74.6) | |
| Yes | 381 (22.8) | 94 (16.9) | 146 (26.1) | 141 (25.4) | |
| Milrinone | 0.32 | ||||
| No | 1,598 (95.5) | 530 (95.2) | 531 (94.8) | 537 (96.6) | |
| Yes | 75 (4.5) | 27 (4.8) | 29 (5.2) | 19 (3.4) | |
| In-hospital morality | <0.001 | ||||
| No | 901 (53.9) | 365 (65.5) | 292 (52.1) | 244 (43.9) | |
| Yes | 772 (46.1) | 192 (34.5) | 268 (47.9) | 312 (56.1) | |
| 28-day morality | <0.001 | ||||
| No | 919 (54.9) | 362 (65.0) | 293 (52.3) | 264 (47.5) | |
| Yes | 754 (45.1) | 195 (35.0) | 267 (47.7) | 292 (52.5) | |
| 90-day morality | <0.001 | ||||
| No | 774 (46.3) | 332 (59.6) | 251 (44.8) | 191 (34.4) | |
| Yes | 899 (53.7) | 225 (40.4) | 309 (55.2) | 365 (65.6) | |
| 1-year morality | <0.001 | ||||
| No | 657 (39.3) | 305 (54.8) | 208 (37.1) | 144 (25.9) | |
| Yes | 1,016 (60.7) | 252 (45.2) | 352 (62.9) | 412 (74.1) | |
| 5-year morality | <0.001 | ||||
| No | 590 (35.3) | 289 (51.9) | 183 (32.7) | 118 (21.2) | |
| Yes | 1,083 (64.7) | 268 (48.1) | 377 (67.3) | 438 (78.8) |
Data are presented as median [IQR] or n (%). AKI, acute kidney injury; ALT, alanine transaminase; APS III, acute physiology score III; AST, aspartate transaminase; BMI, body mass index; CCI, Charlson comorbidity index; CKD, chronic kidney disease; CPR, cardiac pulmonary resuscitation; GCS, Glasgow coma scale; INR, international normalized ratio; IQR, interquartile range; MBP, mean blood pressure; OASIS, oxford acute severity of illness score; RBC, red blood cell; RDW, red blood cell distribution width; SAPS II, simplified Acute Physiology Score II; SIRS, Systemic Inflammatory Response Syndrome; SOFA, Sequential Organ Failure Assessment; SpO2, saturation of peripheral oxygen; WBC, white blood cell.
Association between RAR and the morality of patients in CA
The bar graphs depicting the differences in RAR values between the in-hospital, 28-day, 90-day, 1-year, and 5-year survival groups and the deceased groups were plotted separately. As shown in Figure 2, the RAR values in the deceased group were significantly higher than those in the survival group.

Cox regression analysis was used to analyze the relationship between RAR and outcomes in CA patients, with results shown in Table 2. We examined the association between RAR as both a continuous and categorical variable with 90-day, 28-day, 1-year, 5-year and in-hospital mortality. Four models were established: the Crude model without adjusting any covariates. Model 1 corrected for age, sex, BMI, and statistically significant indicators in general vital signs (HR, RR, MBP) based on the original model. Model 2 adjusted for comorbidities (hypertension, diabetes, heart failure, myocardial infarction, malignancy, renal failure, AKI, cirrhosis, anemia, hepatitis, pneumonia), clinical scores (SOFA, SAPS II, APS III, OASIS, GCS, SIRS, CCI), and whether or not CPR was performed on the basis of model 1. Model 3 adjusted laboratory indicators (WBC, RBC, Hb, PLT, RDW, hematocrit, ALB, serum potassium, blood Glu, pH value, blood Lac, INR, total bilirubin, alanine aminotransferase, aspartate aminotransferase, serum creatinine) and the use of various vasoactive drugs (epinephrine, dobutamine, dopamine, norepinephrine, phenylephrine, milrinone). When RAR is treated as a continuous numerical variable in model 3, adjusted for all covariates, the HR (95% CI) for RAR in relation to in-hospital mortality were 1.01 (0.98–1.04), for 28-day mortality were 1.02 (1.00–1.05), for 90-day mortality were 1.03 (1.01–1.05), for 1-year mortality were 1.03 (1.01–1.05), and for 5-year mortality were 1.03 (1.01–1.05). Specifically, the risk ratios for 90-day, 1-year, and 5-year mortality were statistically significant (P<0.05). When treating RAR as a categorical variable in model 3, with the Q1 group as the reference, in the analysis of 1-year mortality, compared to the Q1 group, the HR (95% CI) for the Q2 group were 1.27 (1.06–1.52) (P=0.009), and for the Q3 group were 1.42 (1.17–1.72) (P<0.001), both indicating risk factors. The risk ratios increased incrementally with higher RAR values. In the analysis of 5-year mortality, compared to the Q1 group, the HR (95% CI) for the Q2 and Q3 groups were 1.29 (1.09–1.53) (P=0.004) and 1.44 (1.19–1.74) (P<0.001), respectively. Similarly, the above patterns were observed in the analyses of 28- and 90-day mortality rates, with these patterns becoming more significant in longer-term prognostic assessments compared to the 28-day mortality rates. However, these patterns were not observed in the analysis of in-hospital mortality rates.
Table 2
| Variables | Crude model | Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| In-hospital morality | |||||||||||
| RAR | 1.02 (1.00–1.04) | 0.076 | 1.01 (0.99–1.04) | 0.252 | 1.00 (0.98–1.03) | 0.812 | 1.01 (0.98–1.04) | 0.38 | |||
| Group | |||||||||||
| Q1 | Ref | Ref | Ref | Ref | |||||||
| Q2 | 1.24 (1.03–1.49) | 0.025 | 1.18 (0.98–1.42) | 0.083 | 1.09 (0.89–1.32) | 0.404 | 1.09 (0.89–1.34) | 0.39 | |||
| Q3 | 1.18 (0.98–1.41) | 0.075 | 1.08 (0.90–1.31) | 0.402 | 0.95 (0.78–1.17) | 0.649 | 1.01 (0.81–1.26) | 0.95 | |||
| P for trend | 0.066 | <0.001 | <0.001 | <0.001 | |||||||
| 28-day morality | |||||||||||
| RAR | 1.04 (1.02–1.05) | <0.001 | 1.03 (1.01–1.05) | 0.001 | 1.01 (0.99–1.04) | 0.247 | 1.02 (1.00–1.05) | 0.09 | |||
| Group | |||||||||||
| Q1 | Ref | Ref | Ref | Ref | |||||||
| Q2 | 1.48 (1.23–1.77) | <0.001 | 1.39 (1.16–1.68) | <0.001 | 1.23 (1.01–1.49) | 0.039 | 1.26 (1.03–1.55) | 0.03 | |||
| Q3 | 1.62 (1.35–1.94) | <0.001 | 1.47 (1.22–1.77) | <0.001 | 1.19 (0.97–1.46) | 0.095 | 1.28 (1.02–1.60) | 0.03 | |||
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| 90-day morality | |||||||||||
| RAR | 1.04 (1.03–1.05) | <0.001 | 1.04 (1.02–1.06) | <0.001 | 1.02 (1.00–1.04) | 0.024 | 1.03 (1.01–1.05) | 0.01 | |||
| Group | |||||||||||
| Q1 | Ref | Ref | Ref | Ref | |||||||
| Q2 | 1.53 (1.28–1.81) | <0.001 | 1.43 (1.20–1.70)) | <0.001 | 1.23 (1.03–1.48) | 0.023 | 1.24 (1.02–1.49) | 0.03 | |||
| Q3 | 1.85 (1.57–2.19) | <0.001 | 1.67 (1.41–1.98) | <0.001 | 1.32 (1.10–1.59) | 0.003 | 1.38 (1.12–1.69) | 0.002 | |||
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| 1-year morality | |||||||||||
| RAR | 1.04 (1.03–1.05) | <0.001 | 1.04 (1.03–1.06) | <0.001 | 1.03 (1.01–1.05) | 0.007 | 1.03 (1.01–1.05) | 0.01 | |||
| Group | |||||||||||
| Q1 | Ref | Ref | Ref | Ref | |||||||
| Q2 | 1.60 (1.36–1.88) | <0.001 | 1.50 (1.28–1.77) | <0.001 | 1.29 (1.09–1.53) | 0.004 | 1.27 (1.06–1.52) | 0.009 | |||
| Q3 | 1.99 (1.70–2.32) | <0.001 | 1.81 (1.54–2.13) | <0.001 | 1.42 (1.19–1.69) | <0.001 | 1.42 (1.17–1.72) | <0.001 | |||
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| 5-year morality | |||||||||||
| RAR | 1.04 (1.03–1.05) | <0.001 | 1.05 (1.03–1.06) | <0.001 | 1.03 (1.01–1.05) | <0.001 | 1.03 (1.01–1.05) | 0.002 | |||
| Group | |||||||||||
| Q1 | Ref | Ref | Ref | Ref | |||||||
| Q2 | 1.64 (1.40–1.92) | <0.001 | 1.55 (1.32–1.81) | <0.001 | 1.33 (1.13–1.57) | <0.001 | 1.29 (1.09–1.53) | 0.004 | |||
| Q3 | 2.07 (1.77–2.41) | <0.001 | 1.90 (1.62–2.22) | <0.001 | 1.48 (1.25–1.76) | <0.001 | 1.44 (1.19–1.74) | <0.001 | |||
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
CI, confidence interval; HR, hazard ratio; RAR, red blood cell distribution width to albumin ratio; RDW, red blood cell distribution width.
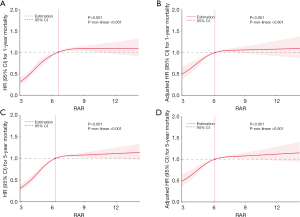
KM survival curves (Figure 3) further illustrated observably higher 1- and 5-year mortality rates in the high RAR group compared to the low RAR group. The same pattern appears in the KM survival curve of 28- and 90-day mortality (Figure S1). Additionally, restricted cubic spline analyses for RAR and mortality at 1 and 5 years revealed a nonlinear relationship (Figure 4), as well as 28 and 90 days (Figures S2,S3).

Subgroup analysis
Building on model 3 constructed in the Cox proportional hazards regression model, we conducted subgroup analyses to further investigate whether age, in-hospital treatment modalities, and various comorbidities could interfere with the conclusions drawn earlier. In the subgroup analyses of RAR and 1-year mortality rates (Table 3) as well as 5-year mortality rates (Table 4), it was observed that the aforementioned conclusions did not apply to the subgroups of heart failure, AKI, and whether cardiopulmonary resuscitation was performed. An interaction effect was present, indicating that in patients with higher RAR values, their 1- and 5-year mortality rates were higher, a conclusion that only applied to groups other than those predefined for heart failure, AKI, and whether cardiopulmonary resuscitation was performed in this study. In the subgroup analysis of 90-day mortality rates, apart from the aforementioned subgroups, the use of norepinephrine may also exhibit an interaction effect on the results (Table S1). In the subgroup analysis of RAR and 28-day mortality rates (Table S2), it was observed that the above patterns did not apply to the subgroups of heart failure and AKI.
Table 3
| Variables | No. of patients | HR (95% CI) | P for interaction | ||
|---|---|---|---|---|---|
| Q1 (<4.5) | Q2 (4.5–5.7) | Q3 (>5.7) | |||
| Gender | 0.17 | ||||
| Male | 1,059 | Ref | 1.05 (0.78–1.42) | 1.36 (0.99–1.88) | |
| Female | 614 | Ref | 1.39 (1.11–1.75) | 1.49 (1.16–1.91) | |
| Hypertension | 0.15 | ||||
| No | 551 | Ref | 1.20 (0.88–1.62) | 1.30 (0.92–1.83) | |
| Yes | 1,122 | Ref | 1.33 (1.06–1.66) | 1.52 (1.20–1.94) | |
| Diabetes | 0.41 | ||||
| No | 1,054 | Ref | 1.37 (1.09–1.72) | 1.49 (1.15–1.92) | |
| Yes | 619 | Ref | 1.20 (0.89–1.61) | 1.40 (1.02–1.91) | |
| Heart failure | 0.009 | ||||
| No | 900 | Ref | 1.14 (0.90–1.46) | 1.39 (1.06–1.81) | |
| Yes | 773 | Ref | 1.42 (1.08–1.87) | 1.50 (1.11–2.02) | |
| Myocardial infarction | 0.64 | ||||
| No | 1,314 | Ref | 1.25 (1.03–1.53) | 1.44 (1.16–1.79) | |
| Yes | 359 | Ref | 1.24 (0.81–1.88) | 1.21 (0.75–1.96) | |
| Malignant tumour | 0.27 | ||||
| No | 1,483 | Ref | 1.19 (0.99–1.44) | 1.40 (1.13–1.72) | |
| Yes | 190 | Ref | 2.44 (1.30–4.59) | 2.36 (1.16–4.83) | |
| CKD | 0.45 | ||||
| No | 1,233 | Ref | 1.25 (1.02–1.54) | 1.48 (1.17–1.86) | |
| Yes | 440 | Ref | 1.44 (0.97–2.14) | 1.37 (0.92–2.05) | |
| AKI | <0.001 | ||||
| No | 741 | Ref | 1.43 (1.07–1.89) | 2.05 (1.49–2.84) | |
| Yes | 932 | Ref | 1.14 (0.90–1.44) | 1.13 (0.88–1.45) | |
| Cirrhosis | 0.68 | ||||
| No | 1,554 | Ref | 1.31 (1.09–1.58) | 1.46 (1.19–1.78) | |
| Yes | 119 | Ref | 0.56 (0.17–1.79) | 1.13 (0.39–3.27) | |
| Anaemia | 0.33 | ||||
| No | 911 | Ref | 1.52 (1.17–1.98) | 1.72 (1.30–2.27) | |
| Yes | 762 | Ref | 0.99 (0.76–1.28) | 1.30 (0.98–1.73) | |
| Hepatitis | 0.83 | ||||
| No | 1,604 | Ref | 1.29 (1.07–1.54) | 1.45 (1.19–1.76) | |
| Yes | 69 | Ref | 1.31 (0.13–13.59) | 0.74 (0.08–6.53) | |
| Pneumonia | 0.49 | ||||
| No | 934 | Ref | 1.34 (1.04–1.73) | 1.37 (1.04–1.80) | |
| Yes | 739 | Ref | 1.07 (0.82–1.39) | 1.36 (1.03–1.80) | |
| CPR | 0.009 | ||||
| No | 1,439 | Ref | 1.35 (1.11–1.64) | 1.54 (1.24–1.91) | |
| Yes | 234 | Ref | 0.81 (0.51–1.28) | 0.80 (0.48–1.33) | |
| Epinephrine | 0.30 | ||||
| No | 1,413 | Ref | 1.22 (1.01–1.49) | 1.47 (1.19–1.82) | |
| Yes | 260 | Ref | 1.65 (0.97–2.83) | 1.32 (0.78–2.22) | |
| Dobutamine | 0.71 | ||||
| No | 1,588 | Ref | 1.29 (1.07–1.55) | 1.48 (1.21–1.81) | |
| Yes | 85 | Ref | 0.25 (0.07–0.89) | 0.15 (0.04–0.61) | |
| Dopamine | 0.78 | ||||
| No | 179 | Ref | 1.20 (0.66–2.16) | 1.08 (0.55–2.13) | |
| Yes | 1,494 | Ref | 1.30 (1.08–1.57) | 1.49 (1.21–1.82) | |
| Norepinephrine | 0.07 | ||||
| No | 821 | Ref | 1.22 (0.95–1.59) | 1.45 (1.08–1.94) | |
| Yes | 852 | Ref | 1.28 (0.99–1.66) | 1.37 (1.04–1.79) | |
| Phenylephrine | 0.20 | ||||
| No | 1,292 | Ref | 1.17 (0.95–1.43) | 1.34 (1.08–1.68) | |
| Yes | 381 | Ref | 1.60 (1.07–2.40) | 1.70 (1.11–2.62) | |
| Milrinone | 0.23 | ||||
| No | 1,598 | Ref | 1.28 (1.07–1.51) | 1.54 (1.47–1.80) | |
| Yes | 75 | Ref | 0.64 (0.08–5.37) | 0.00 (0.00–0.05) | |
AKI, acute kidney injury; CKD, chronic kidney disease; CPR, cardiac pulmonary resuscitation; CI, confidence interval; HR, hazard ratio; RAR, red blood cell distribution width to albumin ratio.
Table 4
| Variables | No. of patients | HR (95% CI) | P for interaction | ||
|---|---|---|---|---|---|
| Q1 (<4.5) | Q2 (4.5–5.7) | Q3 (>5.7) | |||
| Gender | 0.13 | ||||
| Male | 1,059 | Ref | 1.04 (0.78–1.39) | 1.32 (0.96–1.80) | |
| Female | 614 | Ref | 1.43 (1.14–1.78) | 1.55 (1.22–1.97) | |
| Hypertension | 0.16 | ||||
| No | 551 | Ref | 1.22 (0.91–1.62) | 1.33 (0.95–1.85) | |
| Yes | 1,122 | Ref | 1.34 (1.08–1.68) | 1.55 (1.22–1.96) | |
| Diabetes | 0.17 | ||||
| No | 1,054 | Ref | 1.43 (1.15–1.78) | 1.53 (1.19–1.96) | |
| Yes | 619 | Ref | 1.16 (0.88–1.55) | 1.40 (1.03–1.90) | |
| Heart failure | 0.01 | ||||
| No | 900 | Ref | 1.18 (0.93–1.49) | 1.39 (1.07–1.81) | |
| Yes | 773 | Ref | 1.42 (1.09–1.84) | 1.54 (1.15–2.05) | |
| Myocardial infarction | 0.54 | ||||
| No | 1,314 | Ref | 1.27 (1.05–1.54) | 1.45 (1.18–1.79) | |
| Yes | 359 | Ref | 1.29 (0.86–1.94) | 1.29 (0.82–2.05) | |
| Malignant tumour | 0.36 | ||||
| No | 1,483 | Ref | 1.22 (1.02–1.47) | 1.42 (1.16–1.74) | |
| Yes | 190 | Ref | 2.26 (1.22–4.18) | 2.20 (1.09–4.42) | |
| CKD | 0.37 | ||||
| No | 1,233 | Ref | 1.27 (1.04–1.55) | 1.50 (1.20–1.87) | |
| Yes | 440 | Ref | 1.47 (1.01–2.15) | 1.41 (0.96–2.06) | |
| AKI | <0.001 | ||||
| No | 741 | Ref | 1.46 (1.11–1.91) | 2.05 (1.50–2.79) | |
| Yes | 932 | Ref | 1.17 (0.93–1.47) | 1.17 (0.92–1.50) | |
| Cirrhosis | 0.90 | ||||
| No | 1,554 | Ref | 1.32 (1.11–1.58) | 1.49 (1.22–1.81) | |
| Yes | 119 | Ref | 0.72 (0.23–2.22) | 1.40 (0.48–4.10) | |
| Anaemia | 0.37 | ||||
| No | 911 | Ref | 1.48 (1.15–1.90) | 1.66 (1.28–2.71) | |
| Yes | 762 | Ref | 1.04 (0.81–1.34) | 1.41 (1.07–1.87) | |
| Hepatitis | 0.94 | ||||
| No | 1,604 | Ref | 1.30 (1.09–1.55) | 1.46 (1.20–1.77) | |
| Yes | 69 | Ref | 0.96 (0.12–7.55) | 0.82 (0.10–6.71) | |
| Pneumonia | 0.49 | ||||
| No | 934 | Ref | 1.35 (1.06–1.72) | 1.39 (1.07–1.81) | |
| Yes | 739 | Ref | 1.10 (0.85–1.42) | 1.39 (1.06–1.82) | |
| CPR | 0.02 | ||||
| No | 1,439 | Ref | 1.36 (1.12–1.64) | 1.54 (1.26–1.90) | |
| Yes | 234 | Ref | 0.85 (0.53–1.34) | 0.88 (0.53–1.44) | |
| Epinephrine | 0.39 | ||||
| No | 1,413 | Ref | 1.24 (1.03–1.50) | 1.48 (1.21–1.82) | |
| Yes | 260 | Ref | 1.70 (1.00–2.87) | 1.44 (0.87–2.38) | |
| Dobutamine | 0.76 | ||||
| No | 1,588 | Ref | 1.30 (1.09–1.56) | 1.50 (1.23–1.82) | |
| Yes | 85 | Ref | 0.26 (0.08–0.90) | 0.19 (0.05–0.69) | |
| Dopamine | 0.80 | ||||
| No | 179 | Ref | 1.22 (0.69–2.15) | 1.20 (0.62–2.30) | |
| Yes | 1,494 | Ref | 1.32 (1.10–1.59) | 1.51 (1.24–1.85) | |
| Norepinephrine | 0.10 | ||||
| No | 821 | Ref | 1.23 (0.96–1.57) | 1.46 (1.11–1.94) | |
| Yes | 852 | Ref | 1.31 (1.02–1.68) | 1.40 (1.07–1.82) | |
| Phenylephrine | 0.33 | ||||
| No | 1,292 | Ref | 1.17 (0.96–1.42) | 1.32 (1.07–1.64) | |
| Yes | 381 | Ref | 1.68 (1.13–2.51) | 1.89 (1.24–2.89) | |
| Milrinone | 0.46 | ||||
| No | 1,598 | Ref | 1.30 (1.09–1.56) | 1.49 (1.23–1.81) | |
| Yes | 75 | Ref | 1.28 (0.18–8.97) | 0.01 (0.00–0.17) | |
AKI, acute kidney injury; CKD, chronic kidney disease; CPR, cardiac pulmonary resuscitation; CI, confidence interval; HR, hazard ratio; RAR, red blood cell distribution width to albumin ratio.
Construction of a clinical prognosis model for patients with CA
In order to facilitate clinicians in predicting the survival probability of patients with CA more conveniently during the diagnosis and treatment process, we further developed a predictive model for the clinical prognosis outcomes of patients with CA (Figure 5). The model is suitable for all patients diagnosed with CA, with the values of indicators measured for the first time after admission to the ICU.

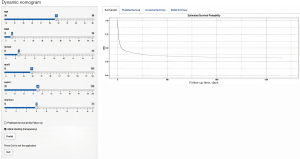
Firstly, we utilized lasso regression to select the optimal variables for the model, with the results shown in Figure 5. Six variables, including RAR, selected at one standard error were included for the subsequent model construction. Patients were divided into modeling and testing groups at a ratio of 8:2. We used Cox proportional hazards regression models to construct clinical prediction models for the survival probabilities of patients with CA at 90 days, 1 year, and 3 years, as shown in Figure 6 (https://nomogramrar.shinyapps.io/DynNomapp/). The model was evaluated in terms of discrimination, accuracy, and clinical utility. The results are presented in Figures 7-9. In the training set, the area under the receiver operating characteristic curve (ROC curve) was found to be 0.720 (95% CI: 0.693–0.747) for 90 days, 0.725 (95% CI: 0.697–0.753) for 1 year, and 0.730 (95% CI: 0.702–0.758) for 3 years. In the validation set, the AUC values were 0.710 (95% CI: 0.652–0.767) for 90 days, 0.711 (95% CI: 0.652–0.769) for 1 year, and 0.714 (95% CI: 0.655–0.773) for 3 years. In the calibration curve, it was observed that the calibration curve closely aligned with the reference line in both the modeling and testing groups, indicating minimal bias. Figure 9 demonstrates that the model provides clinically significant benefits in predicting the survival probabilities of patients with CA at 90 days, 1 year, and 3 years across various probability thresholds, showcasing its strong clinical utility.



Discussion
This study is the first investigation into the potential connection of RAR with in-hospital and long-term all-cause mortality rates following cardiac resuscitation. We observed a significant increase in 28-day, 90-day, 1-year, and 5-year death rates as RAR levels escalated, a trend consistent with prior research on RAR. Notably, a significant nonlinear relationship between RAR and long-term mortality rates was identified. Even after adjusting for relevant covariates, RAR demonstrated a significant non-linear correlation with 90-day and 1-year mortality rates, indicating its potential as a risk factor for adverse outcomes. This further confirms the strong association between elevated RAR levels and long-term clinical mortality, highlighting its importance in predicting patient prognosis. The clinical model constructed based on the RAR index for predicting the prognosis of patients with CA demonstrates good discriminative ability, accuracy, and clinical utility, aiding clinicians in making preliminary predictions regarding patients’ prognostic outcomes in the early stages.
These findings collectively suggest that RAR holds promise as a prognostic indicator for survival outcomes in CA patients. According to KM curves, RAR demonstrated its best predictive efficacy at 1 year. However, concerning in-hospital mortality, an increase in RAR following adjustments for variables like age and gender was associated with a rise in mortality rates, although statistical significance was not achieved. Thus, RAR may be a neoteric and promising biomarker for long-term mortality in CA patients.
In subgroup analyses, the impact of RAR on 90-day all-cause mortality appeared relatively stable. This stability may indicate that RAR holds greater predictive power for long-term patient outcomes, potentially offering guiding insights for the management of post-cardiac resuscitation patients and the prevention of post-resuscitation syndrome.
CA poses a global challenge, inflicting severe consequences on various organs and systems throughout the body. Survivors often endure post-resuscitation syndrome, encompassing physical and mental impairments, as well as psychological and cognitive dysfunction (9-12). Consequently, assessing the prognosis of CA patients presents a significantly intricate task for healthcare professionals. Given the lack of specific and reliable serum markers for CA, clinicians must rely on a comprehensive evaluation based on laboratory tests and imaging evidence (13,14), thereby increasing the economic burden on healthcare providers.
RAR, as a novel prognostic indicator for diseases, offers several advantages such as ease of sampling, low cost, broad applicability, and straightforward clinical use. Current clinical research indicates correlations between RAR and mortality rates across various conditions like acute myocardial infarction, atrial fibrillation, diabetes, heart failure, and so on (15-18). In septic patients, higher RAR levels are associated with increased 28- and 90-day mortality rates (19,20). In a study involving 707 post-percutaneous coronary intervention patients from the MIMIC-IV database, RAR demonstrated fine predictive capabilities for all-cause mortality post-PCI, with the highest RAR group showing the highest 90-day all-cause mortality rate [HR, 3.67 (95% CI: 1.82–7.40)] (21). Additionally, Hao et al. conducted a prospective cohort study revealing a positive correlation between higher baseline RAR levels in the general population and increased risks of all-cause mortality and cause-specific mortality (5).
Higher RAR levels may be associated with elevated RDW and decreased albumin levels. RDW is a hematological parameter reflecting the heterogeneity of RBC volume and is a readily accessible laboratory indicator (22). Existing research has unveiled associations between RDW and numerous cardiovascular, cerebrovascular, and respiratory system diseases, including their occurrence, progression, and risks of mortality (23). Woo et al. found that RDW ≥15% serves as an independent predictive factor for poorer cerebral performance category (CPC) scores upon discharge in outcomes in out-of-hospital CA survivors (24). However, the mechanisms underlying the relationship between RDW and outcomes in cardiovascular diseases remain unclear.
In physiological conditions, RDW increases with age, pregnancy, and physical activity, and is closely linked to inflammation, oxidative stress, ineffective red blood cell production, and damaged red blood cell membranes (25). CA triggers a comprehensive activation of systemic nonspecific inflammatory responses. Inflammation impacts bone marrow function, inhibiting RBC maturation and leading to increased reticulocyte count and elevated RDW. Furthermore, oxidative stress leads to an increase in RDW by shortening the lifespan of RBCs and releasing them prematurely into the peripheral circulation. This may explain the link between high RDW and negative outcomes in CA patients (26).
Albumin, a moderately sized protein produced by the liver, indicates nutritional status, as well as contributing to the colloid osmotic pressure of the human body (27,28). It plays a crucial role in oxidative stress processes and, as a negative acute-phase protein, its synthesis rate is inversely correlated with inflammatory activity (28-30). Due to its antioxidant activity, albumin can exert a protective effect by diluting blood and reducing oxidative stress (31-33).
In a study investigating the link between baseline ALB levels in critically ill patients with end-stage renal disease and CA, it was discovered that within the range of 3.26–5.6 g/dL of albumin, for every 1 g/dL increase in serum level, the risk of CA in these patients decreased by 68% (34). Furthermore, a meta-analysis by Lee et al. on the levels of ALB and prognosis in patients with CA highlighted that lower serum albumin levels were associated with a higher in-hospital mortality rate in post-resuscitation patients (35).
There are several strengths in our study. Firstly, it is the first investigation exploring the correlation between RAR and long-term mortality in CA patients. Secondly, we thoroughly considered the influence of confounding factors on various study outcomes and employed multiple models to validate the results rigorously. Nevertheless, there are also certain defects in our study. Firstly, it was a retrospective study design, which inherently carries biases and involves missing data. Therefore, prospective studies are necessary to further validate these results. Secondly, since the database does not differentiate between in-hospital and out-of-hospital CA, nor does it record baseline information such as initial rhythm, low-flow time, or the presence of witnesses, the introduction of confounding factors is unavoidable, which may impact the conclusions. Additionally, to minimize bias related to the timing of measurements, this study exclusively focused on the first RAR measurement obtained after ICU admission and did not dynamically observe the relationship between RAR and long-term prognosis or in-hospital mortality in CA patients. The presence of missing RAR data for some patients in the database resulted in a degree of selection bias in this study. We were unable to access patient admission conditions, cardiac functional grading, echocardiography results, imaging findings, and other unaccounted factors from the MIMIC-IV database, all of which could potentially impact the assessment of patient prognosis. Hence, further research is warranted to validate the findings of this study.
Conclusions
RAR can be used as a potential prognostic indicator for patients who have experienced CA and is linked to a poor clinical prognosis, with higher levels correlating to increased long-term mortality. The clinical model constructed based on RAR for predicting the prognosis of patients with CA can provide clinicians with a convenient scoring system that holds guiding significance for evaluating the prognosis of patients with CA.
Acknowledgments
Thanks to the Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center for the MIMIC project.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2030/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2030/prf
Funding: This research was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2030/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barros AJ, Enfield KB. In-Hospital Cardiac Arrest. Emerg Med Clin North Am 2023;41:455-64. [Crossref] [PubMed]
- Holmberg MJ, Ross CE, Fitzmaurice GM, et al. Annual Incidence of Adult and Pediatric In-Hospital Cardiac Arrest in the United States. Circ Cardiovasc Qual Outcomes 2019;12:e005580.
- Nolan JP, Soar J, Smith GB, et al. Incidence and outcome of in-hospital cardiac arrest in the United Kingdom National Cardiac Arrest Audit. Resuscitation 2014;85:987-92. [Crossref] [PubMed]
- Dalessio L. Post-Cardiac Arrest Syndrome. AACN Adv Crit Care 2020;31:383-93. [Crossref] [PubMed]
- Hao M, Jiang S, Tang J, et al. Ratio of Red Blood Cell Distribution Width to Albumin Level and Risk of Mortality. JAMA Netw Open 2024;7:e2413213. [Crossref] [PubMed]
- Seo YJ, Yu J, Park JY, et al. Red cell distribution width/albumin ratio and 90-day mortality after burn surgery. Burns Trauma 2022;10:tkab050. [Crossref] [PubMed]
- Kimura H, Tanaka K, Saito H, et al. Impact of red blood cell distribution width-albumin ratio on prognosis of patients with CKD. Sci Rep 2023;13:15774. [Crossref] [PubMed]
- Lu C, Long J, Liu H, et al. Red blood cell distribution width-to-albumin ratio is associated with all-cause mortality in cancer patients. J Clin Lab Anal 2022;36:e24423. [Crossref] [PubMed]
- Gräsner JT, Herlitz J, Tjelmeland IBM, et al. European Resuscitation Council Guidelines 2021: Epidemiology of cardiac arrest in Europe. Resuscitation 2021;161:61-79. [Crossref] [PubMed]
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023;147:e93-e621. [Crossref] [PubMed]
- Yaow CYL, Teoh SE, Lim WS, et al. Prevalence of anxiety, depression, and post-traumatic stress disorder after cardiac arrest: A systematic review and meta-analysis. Resuscitation 2022;170:82-91. [Crossref] [PubMed]
- Vincent A, Beck K, Thommen E, et al. Post-intensive care syndrome in out-of-hospital cardiac arrest patients: A prospective observational cohort study. PLoS One 2022;17:e0276011. [Crossref] [PubMed]
- Sandroni C, D'Arrigo S, Cacciola S, et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med 2020;46:1803-51. [Crossref] [PubMed]
- Ye L, Lu J, Yuan M, et al. Correlation between Lactate Dehydrogenase to Albumin Ratio and the Prognosis of Patients with Cardiac Arrest. Rev Cardiovasc Med 2024;25:65. [Crossref] [PubMed]
- Li D, Ruan Z, Wu B. Association of Red Blood Cell Distribution Width-Albumin Ratio for Acute Myocardial Infarction Patients with Mortality: A Retrospective Cohort Study. Clin Appl Thromb Hemost 2022;28:10760296221121286. [Crossref] [PubMed]
- Chen C, Cai J, Song B, et al. Relationship between the Ratio of Red Cell Distribution Width to Albumin and 28-Day Mortality among Chinese Patients over 80 Years with Atrial Fibrillation. Gerontology 2023;69:1471-81. [Crossref] [PubMed]
- Hong J, Hu X, Liu W, et al. Impact of red cell distribution width and red cell distribution width/albumin ratio on all-cause mortality in patients with type 2 diabetes and foot ulcers: a retrospective cohort study. Cardiovasc Diabetol 2022;21:91. [Crossref] [PubMed]
- Ni Q, Wang X, Wang J, et al. The red blood cell distribution width-albumin ratio: A promising predictor of mortality in heart failure patients - A cohort study. Clin Chim Acta 2022;527:38-46. [Crossref] [PubMed]
- Xu W, Huo J, Chen G, et al. Association between red blood cell distribution width to albumin ratio and prognosis of patients with sepsis: A retrospective cohort study. Front Nutr 2022;9:1019502. [Crossref] [PubMed]
- Ma C, Liang G, Wang B, et al. Clinical value of the red blood cell distribution width to albumin ratio in the assessment of prognosis in critically ill patients with sepsis: a retrospective analysis. J Thorac Dis 2024;16:516-29. [Crossref] [PubMed]
- Weng Y, Peng Y, Xu Y, et al. The Ratio of Red Blood Cell Distribution Width to Albumin Is Correlated With All-Cause Mortality of Patients After Percutaneous Coronary Intervention - A Retrospective Cohort Study. Front Cardiovasc Med 2022;9:869816. [Crossref] [PubMed]
- Roberts GT, El Badawi SB. Red blood cell distribution width index in some hematologic diseases. Am J Clin Pathol 1985;83:222-6. [Crossref] [PubMed]
- Haybar H, Pezeshki SMS, Saki N. Evaluation of complete blood count parameters in cardiovascular diseases: An early indicator of prognosis? Exp Mol Pathol 2019;110:104267. [Crossref] [PubMed]
- Woo SH, Lee WJ, Kim DH, et al. Initial red cell distribution width as a predictor of poor neurological outcomes in out-of-hospital cardiac arrest survivors in a prospective, multicenter observational study (the KoCARC study). Sci Rep 2020;10:17549. [Crossref] [PubMed]
- Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86-105. [Crossref] [PubMed]
- Peng Y, Guan X, Wang J, et al. Red cell distribution width is correlated with all-cause mortality of patients in the coronary care unit. J Int Med Res 2020;48:300060520941317. [Crossref] [PubMed]
- Che R, Huang X, Zhao W, et al. Low Serum Albumin level as a Predictor of Hemorrhage Transformation after Intravenous Thrombolysis in Ischemic Stroke Patients. Sci Rep 2017;7:7776. [Crossref] [PubMed]
- Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004;17:432-7. [Crossref] [PubMed]
- LeFevre ML. U.S. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161:281-90. [Crossref] [PubMed]
- Belinskaia DA, Voronina PA, Shmurak VI, et al. Serum Albumin in Health and Disease: Esterase, Antioxidant, Transporting and Signaling Properties. Int J Mol Sci 2021;22:10318. [Crossref] [PubMed]
- Belayev L, Busto R, Zhao W, et al. Effect of delayed albumin hemodilution on infarction volume and brain edema after transient middle cerebral artery occlusion in rats. J Neurosurg 1997;87:595-601. [Crossref] [PubMed]
- Reinhart WH, Nagy C. Albumin affects erythrocyte aggregation and sedimentation. Eur J Clin Invest 1995;25:523-8. [Crossref] [PubMed]
- Zoellner H, Höfler M, Beckmann R, et al. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J Cell Sci 1996;109:2571-80. [Crossref] [PubMed]
- Zeng YQ, Qin ZA, Guo ZW, et al. Non-linear relationship between basal serum albumin concentration and cardiac arrest in critically ill patients with end-stage renal disease: a cross-sectional study. BMJ Open 2022;12:e051721. [Crossref] [PubMed]
- Lee H, Lee J, Shin H, et al. Association between Early Phase Serum Albumin Levels and Outcomes of Post-Cardiac Arrest Patients: A Systematic Review and Meta-Analysis. J Pers Med 2022;12:1787. [Crossref] [PubMed]


